Advancing Auricular Reconstruction: The Evolution and Outcomes of Auricular Reconstruction Using a Porous Polyethylene (PPE) Framework
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Study Selection
2.2. Data Collection and Analysis
3. Results
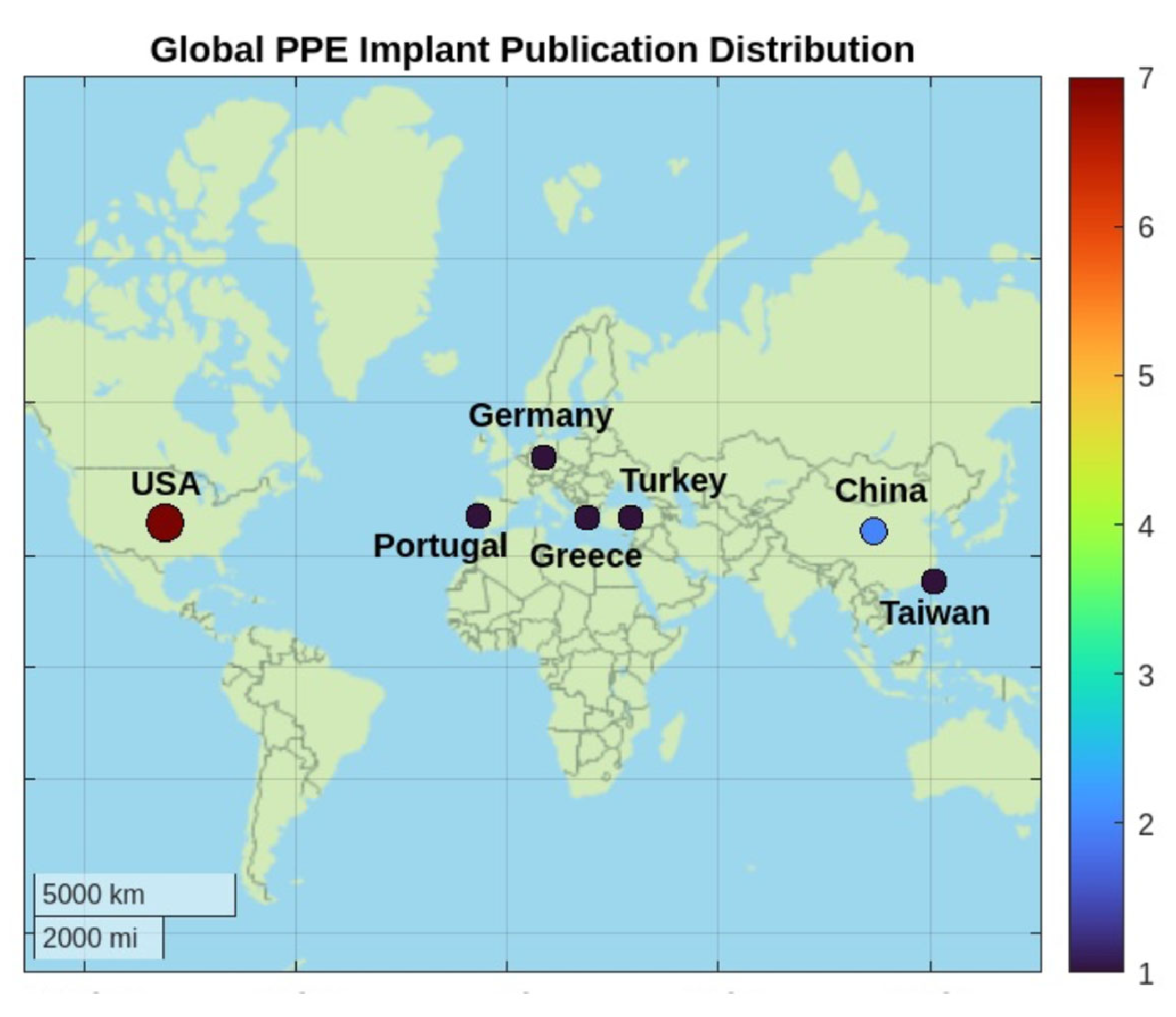
3.1. Included Studies and Origin Countries of Publication
3.2. Patient Demographics
3.3. Surgical Indications and Staging
One-Stage Approach
3.4. Alternative Flaps When the TPF and Occipital Flaps Are Not Available
Two-Stage Approach
3.5. PPE Framework Evolution
3.6. Reported Complications
3.7. Management of Complications and Secondary Procedures
3.8. Esthetic and Patient-Reported Outcomes
4. Discussion
4.1. Advantages of PPE Auricular Reconstruction
4.2. Global Adoption Trends of PPE Auricular Reconstruction
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PPE | Porous polyethylene |
| TPF | Temporoparietal fascia |
References
- Berghaus, A.; Toplak, F. Surgical Concepts for Reconstruction of the Auricle: History and Current State of the Art. Arch. Otolaryngol. Head Neck Surg. 1986, 112, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Wellisz, T.; Dougherty, W. The Role of Alloplastic Skeletal Modification in the Reconstruction of Facial Bums. Ann. Plast. Surg. 1993, 30, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, Y.; Zhuang, H.; Jiang, H.; Jiang, W.; Hu, X.; Hu, S.; Wang, S.; Pan, B. Clinical evaluation of three total ear reconstruction methods. J. Plast. Reconstr. Aesthetic Surg. 2009, 62, 1550–1554. [Google Scholar] [CrossRef] [PubMed]
- Podolsky, D.J.; Kasrai, L.; Fisher, D.M. Auricular construction. In Plastic Surgery: Volume 3: Craniofacial, Head and Neck Surgery and Pediatric Plastic Surgery; Neligan, J.E.L.P.C., Ed.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 110–137. [Google Scholar]
- Berghaus, A.; Nicoló, M.S. Milestones in the History of Ear Reconstruction. Facial Plast. Surg. 2015, 31, 563–566. [Google Scholar] [CrossRef]
- Ma, Y.; Lloyd, M.S. Systematic Review of Medpor Versus Autologous Ear Reconstruction. J. Craniofac. Surg. 2022, 33, 602–606. [Google Scholar] [CrossRef]
- Su-Genyk, P.; Quatela, O.; Quatela, V. Our Evolution of Approaches to Microtia Reconstruction. Facial Plast. Surg. Clin. N. Am. 2024, 32, 105–125. [Google Scholar] [CrossRef]
- Lewin, S.; Bishop, R.; Woerner, J.E.; Yates, D. Three Techniques for Reconstruction of Congenital Microtia: Porous Implant Ear Reconstruction, Auricular Reconstruction Using Autologous Rib, and Osseointegrated Craniofacial Implants with Auricular Prosthesis. Atlas Oral Maxillofac. Surg. Clin. N. Am. 2022, 30, 113–128. [Google Scholar] [CrossRef]
- Berghaus, A. Porous Polyethylene in Reconstructive Head and Neck Surgery. Arch. Otolaryngol. 1985, 111, 154–160. [Google Scholar] [CrossRef]
- Johns, A.L.; Lucash, R.E.; Im, D.D.; Lewin, S.L. Pre and post-operative psychological functioning in younger and older children with microtia. J. Plast. Reconstr. Aesthet. Surg. 2015, 68, 492–497. [Google Scholar] [CrossRef]
- Patel, K.R.; Benchetrit, L.; Ronner, E.A.; Occhiogrosso, J.; Hadlock, T.; Shaye, D.; Quesnel, A.M.; Cohen, M.S. Development of an interdisciplinary microtia-atresia care model: A single-center 20-year experience. Laryngoscope Investig. Otolaryngol. 2022, 7, 2103–2111. [Google Scholar] [CrossRef]
- Reinisch, J. Ear Reconstruction in Young Children. Facial Plast. Surg. 2015, 31, 600–603. [Google Scholar] [CrossRef] [PubMed]
- Berghaus, A.; Stelter, K.; Naumann, A.; Hempel, J.M. Ear reconstruction with porous polyethylene implants. Adv. Otorhinolaryngol. 2010, 68, 53–64. [Google Scholar]
- Reinisch, J.; Tahiri, Y. Polyethylene Ear Reconstruction: A State-of-the-Art Surgical Journey. Plast. Reconstr. Surg. 2018, 141, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Reinisch, J.F.; Lewin, S. Ear reconstruction using a porous polyethylene framework and temporoparietal fascia flap. Facial Plast. Surg. 2009, 25, 181–189. [Google Scholar] [CrossRef]
- Reinisch, J.F.; Tahiri, Y. Complications and Management of Alloplastic Ear Reconstruction. In Modern Microtia Reconstruction: Art, Science, and New Clinical Techniques; Reinisch, J.F., Tahiri, Y., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 111–134. [Google Scholar]
- Reinisch, J.F.; van Hövell Tot Westerflier, C.V.A.; Gould, D.J.; Tahiri, Y.T. Secondary Salvage of the Unsatisfactory Microtia Reconstruction. Plast. Reconstr. Surg. 2020, 145, 1252–1261. [Google Scholar] [CrossRef] [PubMed]
- Reinisch, J.F.; van Hövell Tot Westerflier, C.V.A.; Tahiri, Y.; Yao, C.A. The Occipital Artery-Based Fascial Flap for Ear Reconstruction. Plast. Reconstr. Surg. 2019, 143, 592e–601e. [Google Scholar] [CrossRef]
- Lewin, M.L. Formation of the helix with a postauricular flap. Plast. Reconstr. Surg. (1946) 1950, 5, 432–440. [Google Scholar] [CrossRef]
- Kludt, N.A.; Vu, H. Auricular reconstruction with prolonged tissue expansion and porous polyethylene implants. Ann. Plast. Surg. 2014, 72, S14–S17. [Google Scholar] [CrossRef]
- Tahiri, Y.; Reinisch, J. Porous Polyethylene Ear Reconstruction. Clin. Plast. Surg. 2019, 46, 223–230. [Google Scholar] [CrossRef]
- Stephan, S.; Reinisch, J. Auricular Reconstruction Using Porous Polyethylene Implant Technique. Facial Plast. Surg. Clin. N. Am. 2018, 26, 69–85. [Google Scholar] [CrossRef]
- Romo, T.I.; Morris, L.G.T.; Reitzen, S.D.; Ghossaini, S.N.; Wazen, J.J.; Kohan, D. Reconstruction of Congenital Microtia-Atresia: Outcomes with the Medpor/Bone-Anchored Hearing Aid–Approach. Ann. Plast. Surg. 2009, 62, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Berghaus, A.; Braun, T. Porous polyethylene for the reconstruction of severe Cosman cleft ear deformities. Eur. J. Plast. Surg. 2012, 35, 547–550. [Google Scholar] [CrossRef]
- Simsek, T.; Eroglu, L. Auricle reconstruction with a radial forearm flap prelaminated with porous polyethylene (Medpor®) implant. Microsurgery 2012, 32, 627–630. [Google Scholar] [CrossRef] [PubMed]
- Constantine, K.K.; Gilmore, J.; Lee, K.; Leach, J., Jr. Comparison of microtia reconstruction outcomes using rib cartilage vs porous polyethylene implant. JAMA Facial Plast. Surg. 2014, 16, 240–244. [Google Scholar] [CrossRef]
- Fernandes, J.R.; Driscoll, D.N. Burn Ear Reconstruction Using Porous Polyethylene Implants and Tissue Expansion. J. Burn. Care Res. 2016, 37, e348–e352. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Ng, L.-S.; Chang, C.-S.; Lu, T.-C.; Chen, N.-H.; Chen, Z.-C. Pursuing Mirror Image Reconstruction in Unilateral Microtia: Customizing Auricular Framework by Application of Three-Dimensional Imaging and Three-Dimensional Printing. Plast. Reconstr. Surg. 2017, 139, 1433–1443. [Google Scholar] [CrossRef]
- Horta, R.; Valença-Filipe, R.; Carvalho, J.; Nascimento, R.; Silva, A.; Amarante, J. Reconstruction of a near total ear amputation with a neurosensorial radial forearm free flap prelaminated with porous polyethylene implant and delay procedure. Microsurgery 2018, 38, 203–208. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Liang, W.; Xiao, X.; Jiaqi, Z.; Jian, Z.; Zheng, S.; Meng, L.; Yuhong, C.; Chenyang, J. Ear Reconstruction with the Combination of Expanded Skin Flap and Medpor Framework: 20 Years of Experience in a Single Center. Plast. Reconstr. Surg. 2021, 148, 850–860. [Google Scholar] [CrossRef]
- Bini, A.; Derka, S.; Stavrianos, S. Hemifacial Microsomia Surgical Approach and Anotia Reconstruction: A Case Report. In Vivo 2024, 38, 2550–2556. [Google Scholar] [CrossRef]
- Gomez, G.; Sie, K.C.Y.; Osterbauer, B.; Arom, G.; Kochhar, A. Salvage of Infected Alloplastic Ear Reconstructions via Negative Pressure Wound Therapy. Laryngoscope 2024, 134, 4122–4125. [Google Scholar] [CrossRef]
- Jiang, C.; Zhao, C.; Chen, B.; Lu, L.; Sun, Y.; Yan, X.; Yi, B.; Wu, H.; Shi, R. Auricular reconstruction using Medpor combined with different hearing rehabilitation approaches for microtia. Acta Otolaryngol. 2021, 141, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Braun, T.; Gratza, S.; Becker, S.; Ilona, S.; Klaus, S.; Martin, P.; Alexander, B.; Martin, H.J. Auricular reconstruction with porous polyethylene frameworks: Outcome and patient benefit in 65 children and adults. Plast. Reconstr. Surg. 2010, 126, 1201–1212. [Google Scholar] [CrossRef]
- Lewin, S. Complications after Total Porous Implant Ear Reconstruction and Their Management. Facial Plast. Surg. 2015, 31, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.C.; Wallace, C.G.; Wai-Yee Ho, V.; Wu, C.M.; Chen, H.Y.; Chen, Z.C. Simultaneous auricular reconstruction and transcutaneous bone conduction device implantation in patients with microtia. J. Formos Med. Assoc. 2019, 118, 1202–1210. [Google Scholar] [CrossRef]
- Klassen, A.F.; Rae, C.; Bulstrode, N.W.; Berenguer, B.; Cui, C.; Fisher, D.M.; Kasrai, L.; Li, Y.; Lloyd, M.; Panchapakesan, V.; et al. An international study to develop the EAR-Q patient-reported outcome measure for children and young adults with ear conditions. J. Plast. Reconstr. Aesthetic Surg. 2021, 74, 2341–2348. [Google Scholar] [CrossRef]
- Wolters, A.J.P.; de Henau, M.; Piatkowski de Grzymala, A.A. EAR-Q outcomes in healthy adults: Determining normative data. Eur. J. Plast. Surg. 2024, 47, 84. [Google Scholar] [CrossRef]
- Yang, S.L.; Zheng, J.H.; Ding, Z.; Liu, Q.Y.; Mao, G.Y.; Jin, Y.P. Combined fascial flap and expanded skin flap for enveloping Medpor framework in microtia reconstruction. Aesthetic Plast. Surg. 2009, 33, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.F.; Sun, J.M.; Li, X.D. Auricular reconstruction by soft tissue expansion techniques without skin grafting. Zhonghua Zheng Xing Wai Ke Za Zhi 2012, 28, 115–119. [Google Scholar]
- Baluch, N.; Nagata, S.; Park, C.; Wilkes, G.H.; Reinisch, J.; Kasrai, L.; Fisher, D. Auricular reconstruction for microtia: A review of available methods. Plast. Surg. (Oakv.) 2014, 22, 39–43. [Google Scholar] [CrossRef]
- Ali, K.; Trost, J.G.; Truong, T.A.; Harshbarger, R.J., 3rd. Total Ear Reconstruction Using Porous Polyethylene. Semin. Plast. Surg. 2017, 31, 161–172. [Google Scholar] [CrossRef]
- Zhang, B.; Zeng, X.; Yang, X. Clinical applications of ear reconstruction with Medpor. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2019, 44, 562–570. [Google Scholar] [PubMed]



| Results | Range | Average | |
|---|---|---|---|
| Auricles and Patients | 1718 auricles for 1036 patients | ||
| Reconstruction indications | Congenital deformities: microtia/anotia, hemifacial microsomia, Cosmon cleft auricles deformity, burn, and trauma | ||
| Age (in years) ^ Children: Adults: | __ | 3 years old to 14 years old 20 years old to 59 years old | 8.2 years old 31.7 years old |
| Gender ^ Female Male | 83 52 | Some studies reported gender category in percentage: 64% female and 36% male. | _ |
| PPE implant used | Medpor (Porex Surgical, Newnan, GA, USA) Medpor (Stryker, Kalamazoo, MI, USA) | ||
| Authors | Year | Country | Study Design | Sample Size (Patients) | Duration of Study (From-To) | Surgical Indication | Surgical Stages | Flap Type |
|---|---|---|---|---|---|---|---|---|
| Wellisz et al. [2] | 1993 | USA | Retrospective | 26 auricles for 18 patients | May 1988 through May 1992 | Burn | 2 | TPF |
| Romo et al. [23] | 2009 | USA | Prospective | 28 auricles for 25 patients | 2000 through 2006 | Microtia-atresia (grade III microtia with complete bony EAC atresia); 14 patients right atresia, 8 left atresia, and 3 bilateral atresia | 2 | TPF |
| Zhao et al. [3] | 2009 | China | Retrospective | 355 | 2002 through January 2006 | Majority is microtia, post burn or trauma | 2 | TPF |
| Berghaus et al. [24] | 2012 | Germany | Case report | 1 auricle | 2012 | Cosman cleft auricle deformity | 1 | Postauricular fascia flap |
| Simsek et al. [25] | 2012 | Turkey | Case report | 1 auricle | 2011 | Traumatic amputation of the left auricle | 2 | Radial forearm flap |
| Kludt et al. [20] | 2014 | USA | Case series | 15 patients | 2014 | All with either grade 3 or 4 microtia. | 3 | TPF |
| Constantine et al. [26] | 2014 | USA | Retrospective | 17 auricles for 17 patients | 2001 through 2012 | Microtia | 1 | TPF |
| Reinisch et al. [12] | 2015 | USA | Retrospective | 1178 auricles (earlier: 25 procedures) | March 1991 through September 2015 *1993–1995* | (62.9%) had no atresia repair, 211 (22.0%) had a prior atresia repair, and 144 (15.0%) had an atresia repair at the time of the auricular reconstruction. Bilateral microtia in 11.2%, 603 Initially, 2 (ear framework then concha and tragal reconstruction). | _ | 0 |
| 1178 auricles (recent: 487 procedures) | March 1991 through September 2015 *2008–2013* | Mostly 1 stage | Mainly TPF | |||||
| Fernandes et al. [27] | 2016 | USA | Retrospective | 17 auricles for 16 patients | 2004 through 2012 | Burn | 2 | TPF |
| Chen et al. [28] | 2017 | Taiwan and Singapore | Prospective | 6 auricles for 6 patients | January 2015 through January 2016 | Unilateral microtia with hemifacial microsomia | 0 | TPF |
| Horta et al. [29] | 2018 | Portugal | Case report | 1 auricle | 2018 | Traumatic Amputation | 2 | Radial forearm Flap |
| Wang et al. [30] | 2021 | China | Retrospective | 70 auricles for 68 patients | 1998 through 2018 | 0 | 0 | Expanded skin flap, and TPF or postauricular fascia |
| Bini et al. [31] | 2024 | Greece | Case Report | 1 auricle | 2024 | Right hemifacial microsomia and anotia | 1 | TPF |
| Gomez et al. [32] | 2024 | USA | Case reports | 2 auricles for 2 patients | 2024 | grade III microtia with atresia and left grade III | 1 | TPF |
| Authors | Sample Size (Patients) | Reported Complications | Follow-Up Period | No. of Major Complications | No. of Minor Complications | Intervention Required for Complication |
|---|---|---|---|---|---|---|
| Wellisz et al. (1993) [2] | 26 auricles for 18 patients | 2 exposures (after 4 weeks and 6 weeks); non-patent superficial temporal vessels with eschar 1 × 2 cm, and the second due to lack of complete coverage with the flap, at 6 weeks | 2 years (mean 10 months) | 2 | 0 | At 4 weeks a split thickness skin graft was applied, the second exposure, trimming of the implants |
| Romo et al. (2009) [23] | 28 auricles for 25 patients | 7 cases of minor complications, 6 wound dehiscence (<1 cm), from trauma to the region in the postoperative period. There was 1 postauricular hematoma, which was aspirated in the office. | 6 to 60 months (mean 35 months). | 0 | 7 | 2 cases further touch-up work (scar revision) |
| Zhao et al. (2009) [3] | 355 | 48 cases of exposures and 1 infection | 3 months to 5 years | 48 | 1 | Not specified |
| Berghaus et al. (2012) [24] | 1 auricle | 0 | 6 months | 0 | 0 | _ |
| Simsek et al. (2012) [25] | 1 auricle | 0 | 1 year | 0 | 0 | _ |
| Kludt et al. (2014) [20] | 15 patients | 1 exposure | from 6 months to 5 years | 1 | 0 | Exposed implant was resected and covered with a random flap |
| Constantine et al. (2014) [26] | 17 auricles for 17 patients | 2 (1 infection and 1 extrusion) | from 2 to 6 years | 2 | 0 | Underwent subsequent reconstruction with cartilage grafts |
| Reinisch et al. (2015) [12] | 1178 auricles | Of 25 procedures, there were 7 implant fractures, 11 exposures, and 1 infection | 3 years (min) | 18 | 1 | Not specified |
| Of 487 procedures, there were 7 to 42 implant fractures, 21 exposures, and 5 infections | 1.5 year (min) | ~46 | 5 | Not specified | ||
| Fernandes et al. (2016) [27] | 17 auricles for 16 patients | 2 exposures | up to 5 years. | 2 | 0 | Replacement of the PPE implant with local advancement flap, and the other exposure removal with primary closure |
| Chen et al. (2017) [28] | 6 auricles for 6 patients | 2 transitory alopecia and 1 partial exposure | 10.3 months | 0 | 1 | The hair grew up 3 to 4 months |
| Horta et al. (2018) [29] | 1 auricle | 0 | 3 to 6 months | 0 | 0 | 0 |
| Wang et al. (2021) [30] | 70 auricles for 68 patients | 16 auricles in 15 patients presented with complications (22.06%), including 9 framework exposures (13.24%), 3 infections (4.41%), 2 scar hypertrophy (4.41 %), and 2 hematomas (2.94%) | 6 months to 19 years. | 9 | 8 | Frameworks were taken out due to intractable exposure |
| Bini et al. (2024) [31] | 1 auricle | Partial exposure due to inflammation and infection | 10 months | 1 | 0 | A temporalis muscular flap along with the deep temporal fascia were used as a salvage operation and a full thickness skin graft. Auricular helix reconstruction was completed with a rotation scalp flap after tissue expansion |
| Gomez et al. (2024) [32] | 2 auricles for 2 patients | One-by-one centimeter area along the distal posterior helix was noted to have implant exposure. Ten weeks post-operatively the patients presented with copious purulent discharge from a small (one by two millimeter) area of implant exposure along the inferior aspect of the retro-auricular sulcus. | 6 months to 12 months | 2 | 0 | Antibiotics and primary wound closure, plus one week of negative pressure wound therapy (NPWT), and the other patient underwent a PPE implant replacement |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussein, S.M.; Sharaf, B.A.; Mardini, S.; Gibreel, W. Advancing Auricular Reconstruction: The Evolution and Outcomes of Auricular Reconstruction Using a Porous Polyethylene (PPE) Framework. J. Clin. Med. 2025, 14, 4116. https://doi.org/10.3390/jcm14124116
Hussein SM, Sharaf BA, Mardini S, Gibreel W. Advancing Auricular Reconstruction: The Evolution and Outcomes of Auricular Reconstruction Using a Porous Polyethylene (PPE) Framework. Journal of Clinical Medicine. 2025; 14(12):4116. https://doi.org/10.3390/jcm14124116
Chicago/Turabian StyleHussein, Sara M., Basel A. Sharaf, Samir Mardini, and Waleed Gibreel. 2025. "Advancing Auricular Reconstruction: The Evolution and Outcomes of Auricular Reconstruction Using a Porous Polyethylene (PPE) Framework" Journal of Clinical Medicine 14, no. 12: 4116. https://doi.org/10.3390/jcm14124116
APA StyleHussein, S. M., Sharaf, B. A., Mardini, S., & Gibreel, W. (2025). Advancing Auricular Reconstruction: The Evolution and Outcomes of Auricular Reconstruction Using a Porous Polyethylene (PPE) Framework. Journal of Clinical Medicine, 14(12), 4116. https://doi.org/10.3390/jcm14124116






