Factors Associated with Response to SGLT-2 Inhibitors and GLP-1 Receptor Agonists in Veterans with Type 2 Diabetes Mellitus †
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
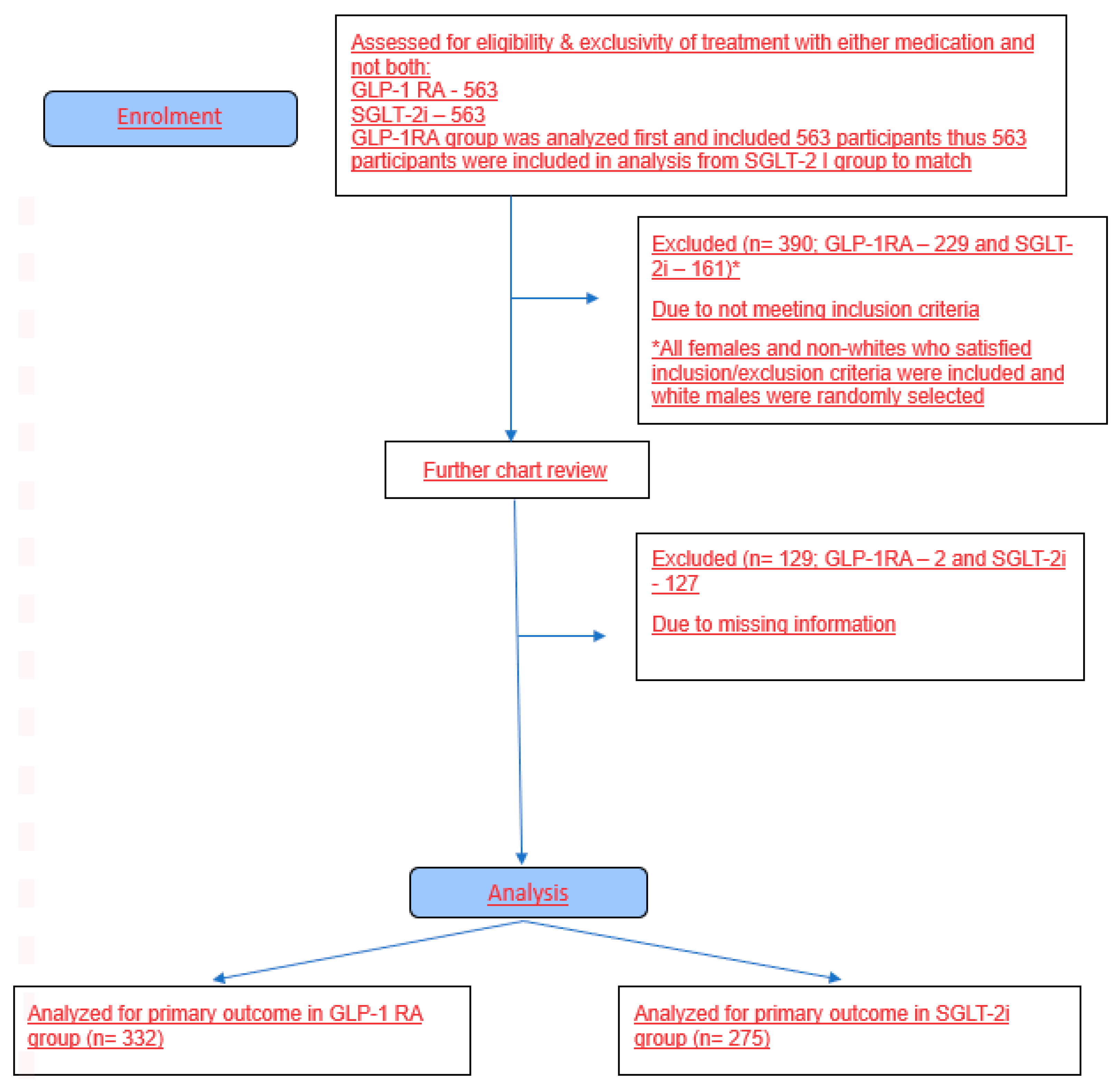
2.2. Study Population Selection
2.3. Inclusion and Exclusion Criteria Are Summarized Below
- Diagnosis of T2DM;
- Age 19–89 years;
- On treatment with a GLP-1 RA or SGLT-2i for at least 6 months and up to 12 months.
- Diagnosis of type 1 diabetes mellitus;
- Estimated baseline eGFR of <30 mL/minute/1.73 m2.
- For GLP-1 RA adverse reaction data collected: nausea, vomiting, diarrhea, constipation, and pancreatitis
- For SGLT-2i adverse reaction data collected: urinary tract infections, genitourinary fungal infection, dyslipidemia, nausea, acute renal failure, and ketoacidosis.
2.4. Study Outcomes
2.5. Statistical Analysis
3. Results
3.1. Subsection: Baseline Characteristics and Results
3.2. Results: A1c Reduction, Weight Loss, and Bivariate Analysis of Baseline and Clinical Characteristics by Adequate Response
3.3. Results: Logistic Regression Model Results with Adequate Response as the Outcome
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hossain, M.J.; Al-Mamun, M.; Islam, M.R. Diabetes mellitus, the fastest growing global public health concern: Early detection should be focused. Health Sci. Rep. 2024, 7, e2004. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rowley, W.R.; Bezold, C.; Arikan, Y.; Byrne, E.; Krohe, S. Diabetes 2030: Insights from Yesterday, Today and Future Trends. Popul. Health Manag. 2017, 20, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Latif, W.; Lambrinos, K.J.; Patel, P.; Rodriguez, R. Compare and Contrast the Glucagon-Like Peptide 1 Receptor Agonists (GLP1RAs). Available online: https://www.ncbi.nlm.nih.gov/books/NBK572151/ (accessed on 25 February 2024).
- National Institute of Diabetes and Digestive and Kidney Disease. Story of Discovery: SGLT2 Inhibitors: Harnessing the Kidneys to Help Treat Diabetes. Available online: https://www.niddk.nih.gov/news/archive/2016/story-discovery-sglt2-inhibitors-harnessing-kidneys-help-treat-diabetes#:~:text=The%20first%20SGLT2%20inhibitor%20to,Jardiance%C2%AE)%20in%20August%202014 (accessed on 9 June 2016).
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Care in Diabetes—2023. Diabetes Care 2023, 46 (Suppl. S1), S140–S157. [Google Scholar] [CrossRef] [PubMed]
- Prasad-Reddy, L.; Isaacs, D. A clinical review of GLP-1 receptor agonists: Efficacy and safety in diabetes and beyond. Drugs Context 2015, 4, 212283. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tariq, S.; Ali, M.A.; Hassan Iftikhar, H.M.; Fareh Ali, M.; Shah, S.Q.A.; Perveen, F.; Zaman, T. Long-Term Cardiovascular Outcomes of Glucagon-Like Peptide-1 (GLP-1) Receptor Agonists in Type 2 Diabetes: A Systematic Review. Cureus 2024, 16, e73705. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pan, H.C.; Chen, J.Y.; Chen, H.Y.; Yeh, F.Y.; Sun, C.Y.; Huang, T.T.M.; Wu, V.C. GLP-1 receptor agonists’ impact on cardio-renal outcomes and mortality in T2D with acute kidney disease. Nat. Commun. 2024, 15, 5912. [Google Scholar] [CrossRef] [PubMed]
- McGuire, D.K.; Shih, W.J.; Cosentino, F.; Charbonnel, B.; Cherney, D.Z.I.; Dagogo-Jack, S.; Pratley, R.; Greenberg, M.; Wang, S.; Huyck, S.; et al. Association of SGLT2 Inhibitors With Cardiovascular and Kidney Outcomes in Patients With Type 2 Diabetes: A Meta-analysis. JAMA Cardiol. 2021, 6, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Rabizadeh, S.; Nakhjavani, M.; Esteghamati, A. Cardiovascular and Renal Benefits of SGLT2 Inhibitors: A Narrative Review. Int. J. Endocrinol. Metab. 2019, 17, e84353. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- American Diabetes Association Professional Practice Committee. Pharmacological Approaches to Glycemic Treatment: Standards of Care in Diabetes—2025. Diabetes Care 2025, 48 (Suppl. S1), S181–S206. [Google Scholar] [CrossRef] [PubMed]
- Dungan, K.; DeSantis, A. Glucagon-Like Peptide 1-Based Therapies for the Treatment of Type 2 Diabetes Mellitus. UpToDate. 2024. Available online: https://www.uptodate.com/contents/glucagon-like-peptide-1-based-therapies-for-the-treatment-of-type-2-diabetes-mellitus (accessed on 11 April 2025).
- Marx, N.; Husain, M.; Lehrke, M.; Verma, S.; Sattar, N. GLP-1 Receptor Agonists for the Reduction of Atherosclerotic Cardiovascular Risk in Patients With Type 2 Diabetes. Circulation 2022, 146, 1882–1894. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.M.Y.; Sattar, N.; Pop-Busui, R.; Deanfield, J.; Emerson, S.S.; Inzucchi, S.E.; Mann, J.F.E.; Marx, N.; Mulvagh, S.L.; Poulter, N.R.; et al. Cardiovascular and Kidney Outcomes and Mortality With Long-Acting Injectable and Oral Glucagon-Like Peptide 1 Receptor Agonists in Individuals With Type 2 Diabetes: A Systematic Review and Meta-analysis of Randomized Trials. Diabetes Care 2025, 48, 846–859. [Google Scholar] [CrossRef]
- Padda, I.S.; Mahtani, A.U.; Parmar, M. Sodium-Glucose Transport Protein 2 (SGLT2) Inhibitors. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK576405/ (accessed on 11 April 2025).
- Cersosimo, A.; Drera, A.; Adamo, M.; Metra, M.; Vizzardi, E. Exploring the Cardiorenal Benefits of SGLT2i: A Comprehensive Review. Kidney Dial. 2024, 4, 184–202. [Google Scholar] [CrossRef]
- Mavrakanas, T.A.; Tsoukas, M.A.; Brophy, J.M.; Sharma, A.; Gariani, K. SGLT-2 inhibitors improve cardiovascular and renal outcomes in patients with CKD: A systematic review and meta-analysis. Sci. Rep. 2023, 13, 15922. [Google Scholar] [CrossRef] [PubMed]
- Armillotta, M.; Angeli, F.; Paolisso, P.; Belmonte, M.; Raschi, E.; Di Dalmazi, G.; Amicone, S.; Canton, L.; Fedele, D.; Suma, N.; et al. Cardiovascular therapeutic targets of sodium-glucose co-transporter 2 (SGLT2) inhibitors beyond heart failure. Pharmacol. Ther. 2025, 270, 108861. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, N.C.; Davies, M.J.; Lingvay, I.; Knop, F.K. Semaglutide for the treatment of overweight and obesity: A review. Diabetes Obes. Metab. 2023, 25, 18–35. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Green, J.B.; Everett, B.M.; Ghosh, A.; Younes, N.; Krause-Steinrauf, H.; Barzilay, J.; Desouza, C.; Inzucchi, S.E.; Pokharel, Y.; Schade, D.; et al. Cardiovascular Outcomes in GRADE (Glycemia Reduction Approaches in Type 2 Diabetes: A Comparative Effectiveness Study). Circulation 2024, 149, 993–1003. [Google Scholar] [CrossRef]
- Jung, H.N.; Jung, C.H. The Upcoming Weekly Tides (Semaglutide vs. Tirzepatide) against Obesity: STEP or SURPASS? J. Obes. Metab. Syndr. 2022, 31, 28–36. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hashmi, S.; Samson, K.; Arora, G.; Azad, S.K.H.; Awad, D.; Desouza, C.V. Factors associated with the response to SGLT2 inhibitors and GLP-1 Receptor Agonists in Veterans with Type 2 Diabetes. In Proceedings of the Endocrine Society National Conference, Boston, MA, USA, 1–4 June 2024. [Google Scholar]
| Total (n = 607) | |
|---|---|
| Medication | n (%) |
| GLP-1 RA | 332 (54.7%) |
| SGLT-2i | 275 (45.3%) |
| Age | |
| Mean (SD) | 67.4 (9.67) |
| n | 607 |
| Sex | |
| Female | 53 (8.7%) |
| Male | 554 (91.3%) |
| Race | |
| Black | 56 (9.2%) |
| Other | 14 (2.3%) |
| White | 537 (88.5%) |
| HbA1c | |
| <8.0% | 201 (33.1%) |
| 8.0% or higher | 406 (66.9%) |
| BMI | |
| <30 | 133 (22.0%) |
| 30 or higher | 472 (78.0%) |
| missing | 2 |
| Systolic BP | |
| Mean (SD) | 131.5 (18.33) |
| n | 606 |
| TG | |
| Mean (SD) | 228.1 (142.65) |
| n | 565 |
| Insulin use per day | |
| None | 292 (48.1%) |
| 100+ units | 103 (17.0%) |
| <100 | 212 (34.9%) |
| Metformin use | |
| No | 136 (22.7%) |
| Yes | 464 (77.3%) |
| missing | 7 |
| Adequate Response (Both Weight Reduction and A1c Reduction) | |||
|---|---|---|---|
| No (n = 440) | Yes (n = 167) | p-Value | |
| Medication, n (%) | <0.001 | ||
| GLP-1RA | 219 (66.0%) | 113 (34.0%) | |
| SGLT-2i | 221 (80.4%) | 54 (19.6%) | |
| Age | 0.40 † | ||
| Mean (SD) | 67.2 (9.54) | 67.9 (9.99) | |
| n | 440 | 167 | |
| Sex, n (%) | 0.89 | ||
| Female | 38 (71.7%) | 15 (28.3%) | |
| Male | 402 (72.6%) | 152 (27.4%) | |
| Race, n (%) | 0.34 | ||
| Black | 43 (76.8%) | 13 (23.2%) | |
| Other | 8 (57.1%) | 6 (42.9%) | |
| White | 389 (72.4%) | 148 (27.6%) | |
| HbA1c, n (%) | <0.001 | ||
| <8.0 | 168 (83.6%) | 33 (16.4%) | |
| 8.0 or higher | 272 (67.0%) | 134 (33.0%) | |
| BMI, n (%) | 0.01 | ||
| <30 | 108 (81.2%) | 25 (18.8%) | |
| 30 or higher | 330 (69.9%) | 142 (30.1%) | |
| n | 2 | 0 | |
| Systolic BP | 0.43 † | ||
| Mean (SD) | 131.1 (18.69) | 132.4 (17.37) | |
| n | 440 | 166 | |
| TG | 0.61 † | ||
| Mean (SD) | 226.2 (147.29) | 233.0 (129.90) | |
| n | 410 | 155 | |
| Insulin | 0.13 | ||
| None | 220 (75.3%) | 72 (24.7%) | |
| 100+ | 67 (65.0%) | 36 (35.0%) | |
| <100 | 153 (72.2%) | 59 (27.8%) | |
| Metformin, n (%) | 0.43 | ||
| No | 102 (75.0%) | 34 (25.0%) | |
| Yes | 332 (71.6%) | 132 (28.4%) | |
| Missing | 6 | 1 | |
| Unadjusted Analysis | Adjusted Analysis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Reference | Unadjusted Odds Ratio | 95% Confidence Interval | p-Value | Reference | Adjusted Odds Ratio | 95% Confidence Interval | p-Value | |||
| Medication | ||||||||||
| GLP-1RA | SGLT-2i | 2.11 | 1.45 | 3.07 | <0.001 | SGLT-2i | 1.86 | 1.14 | 3.03 | 0.01 |
| Age (in decades) | None | 1.31 | 1.03 | 1.68 | 0.03 | |||||
| Sex | ||||||||||
| Female | Male | 1.67 | 0.82 | 3.43 | 0.16 | |||||
| Race | ||||||||||
| Black | White | 0.91 | 0.39 | 2.13 | 0.44 | |||||
| Other | White | 2.29 | 0.46 | 11.48 | 0.44 | |||||
| Baseline HbA1c | ||||||||||
| 8.0 or higher | <8.0 | 2.33 | 1.44 | 3.76 | 0.001 | |||||
| Baseline BMI | ||||||||||
| 30 or higher | <30 | 1.51 | 0.87 | 2.64 | 0.15 | |||||
| Baseline Systolic BP | 1.01 | 0.91 | 1.12 | 0.93 | ||||||
| Baseline TG | 1.00 | 0.99 | 1.02 | 0.65 | ||||||
| Baseline Insulin use | ||||||||||
| 100+ | Not on insulin | 1.09 | 0.53 | 2.25 | 0.88 | |||||
| <100 | Not on insulin | 0.95 | 0.53 | 1.72 | 0.88 | |||||
| Baseline Metformin use | ||||||||||
| Yes | Not on Metformin | 1.54 | 0.95 | 2.52 | 0.08 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arora, G.; Hashmi, S.; Kaeli, S.; Azad, S.; Batra, J.; Perugu, V.D.; Davis, C.; Desouza, C.V. Factors Associated with Response to SGLT-2 Inhibitors and GLP-1 Receptor Agonists in Veterans with Type 2 Diabetes Mellitus. J. Clin. Med. 2025, 14, 4092. https://doi.org/10.3390/jcm14124092
Arora G, Hashmi S, Kaeli S, Azad S, Batra J, Perugu VD, Davis C, Desouza CV. Factors Associated with Response to SGLT-2 Inhibitors and GLP-1 Receptor Agonists in Veterans with Type 2 Diabetes Mellitus. Journal of Clinical Medicine. 2025; 14(12):4092. https://doi.org/10.3390/jcm14124092
Chicago/Turabian StyleArora, Gunjan, Sulman Hashmi, Samson Kaeli, Sarah Azad, Jaskaran Batra, Vijaya Deepika Perugu, Clifton Davis, and Cyrus V. Desouza. 2025. "Factors Associated with Response to SGLT-2 Inhibitors and GLP-1 Receptor Agonists in Veterans with Type 2 Diabetes Mellitus" Journal of Clinical Medicine 14, no. 12: 4092. https://doi.org/10.3390/jcm14124092
APA StyleArora, G., Hashmi, S., Kaeli, S., Azad, S., Batra, J., Perugu, V. D., Davis, C., & Desouza, C. V. (2025). Factors Associated with Response to SGLT-2 Inhibitors and GLP-1 Receptor Agonists in Veterans with Type 2 Diabetes Mellitus. Journal of Clinical Medicine, 14(12), 4092. https://doi.org/10.3390/jcm14124092







