Budget Impact Analysis of the Use of Specific Biomarkers GFAP and UCH-L1 in the Management of Mild Traumatic Brain Injury in Spain
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population, Perspective, and Timelines
2.2. Structure of the Model and Comparators
2.3. Identification, Quantification, and Evaluation of Clinical Inputs
2.4. Cost Estimation
2.5. Management Time Estimation
2.6. Sensitivity Analysis
3. Results
3.1. Budgetary Impact
3.2. Impact on Management Times
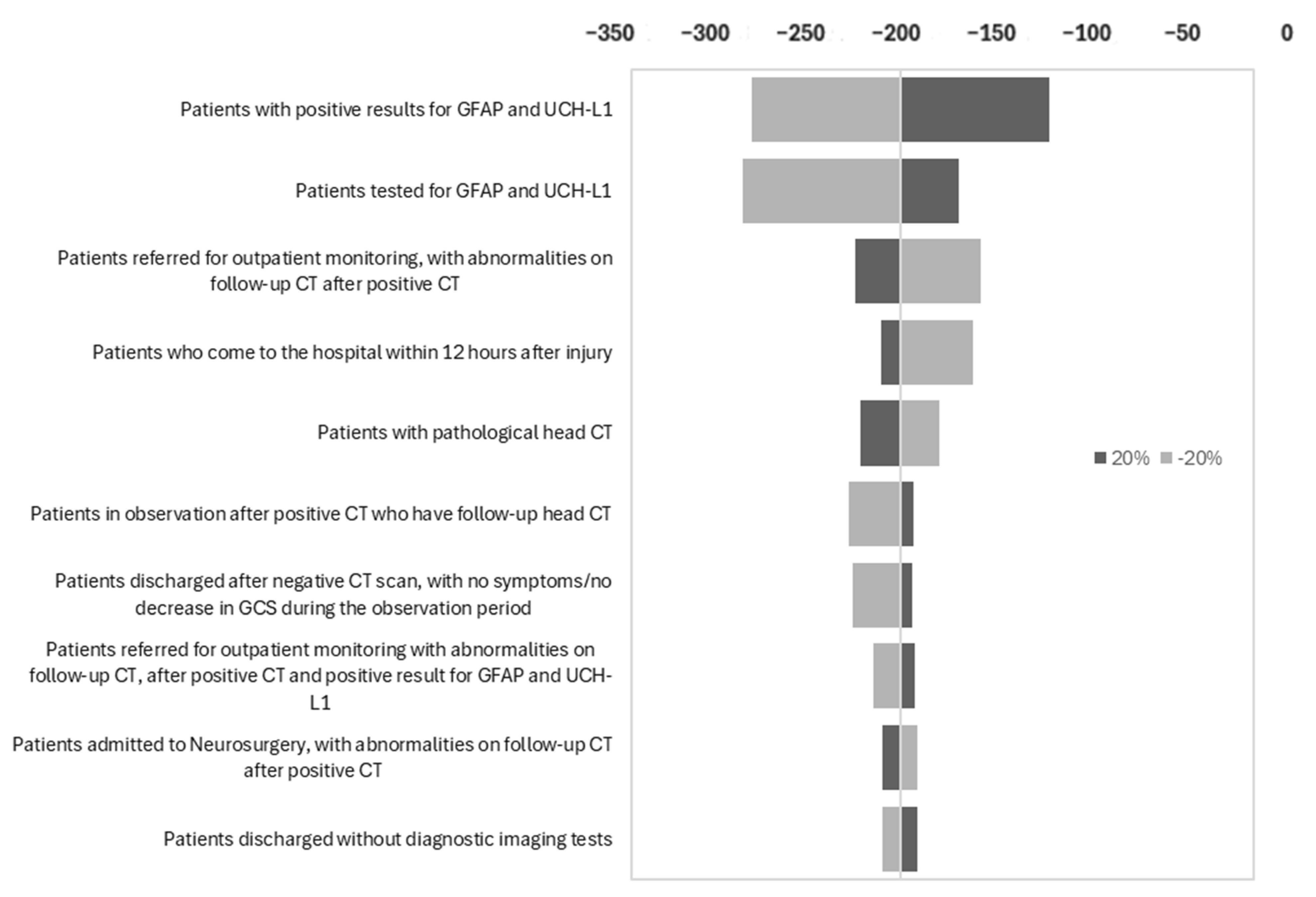
3.3. Sensitivity Analysis Results
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GFAP | Glial fibrillary acid protein |
| UCH-L1 | Ubiquitin C-terminal hydrolase L1 |
| TBI | Mild traumatic brain injury |
| LD | Linear dichroism |
| BIA | Budget impact analysis |
| CT | Computed tomography |
| GCS | Glasgow Coma Scale |
| AII | Acute intracranial injury |
| ED | Emergency departments |
| SEMES | Spanish Society of Emergency Medicine |
| ICU | Intensive care unit |
| RCP | Routine clinical practice |
| MBDS | Minimum Basic Data Set |
| RECH | Spanish Network of Hospital Costs |
| DRG | Diagnosis-Related Group |
References
- Rubiano, A.M.; Rosenfeld, J.V.; Adeleye, A.O.; Park, K.B.; Shrime, M.G.; Hung, Y.-C.; Rattani, A.; Punchak, M.; Gupta, S.; Dewan, M.C.; et al. Estimating the global incidence of traumatic brain injury. J. Neurosurg. 2018, 130, 1080–1097. [Google Scholar]
- Blennow, K.; Brody, D.L.; Kochanek, P.M.; Levin, H.; McKee, A.; Ribbers, G.M.; Yaffe, K.; Zetterberg, H. Traumatic brain injuries. Nat. Rev. Dis. Primers 2016, 2, 16084. [Google Scholar] [CrossRef]
- Maas, A.I.R.; Menon, D.K.; Adelson, P.D.; Andelic, N.; Bell, M.J.; Belli, A.; Bragge, P.; Brazinova, A.; Büki, A.; Chesnut, R.M.; et al. Traumatic brain injury: Integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017, 16, 987–1048. [Google Scholar] [CrossRef]
- Freire-Aragón, M.D.; Rodríguez-Rodríguez, A.; José Egea-Guerrero, J. Update in mild traumatic brain injury. Med. Clin. 2017, 149, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Faisal, M.; Vedin, T.; Edelhamre, M.; Forberg, J.L. Diagnostic performance of biomarker S100B and guideline adherence in routine care of mild head trauma. Scand. J. Trauma Resusc. Emerg. Med. 2023, 31, 3. [Google Scholar] [CrossRef]
- Su, Y.S.; Schuster, J.M.; Smith, D.H.; Stein, S.C. Cost-Effectiveness of Biomarker Screening for Traumatic Brain Injury. J. Neurotrauma 2019, 36, 2083–2091. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, F.T.; Torrecilla, F.M.; Sánchez, M.Á.A.; Gómez, I.A.; Bártulos, A.V.; España, F.J.G.; Román, M.M.; Rodríguez, A.M.; Morell-García, D.; de las Heras, I.P.; et al. Traumatismo craneoencefálico leve y biomarcadores de lesión cerebral aguda. Rev. Esp. Urg. Emerg. 2024, 3, 31–36. [Google Scholar]
- Boice, J.D.; Novelline, R.A.; Larson, P.A.; Mahesh, M.; Linton, O.W.; Tenforde, T.S.; Bushberg, J.T.; Amis, E.S.; Timins, J.K.; Sierzenski, P.R.; et al. Applications of justification and optimization in medical imaging: Examples of clinical guidance for computed tomography use in emergency medicine. Ann. Emerg. Med. 2014, 11, 36–44. [Google Scholar]
- Hoffman, J.R.; Nagaraj, G.; Sharp, A.L.; Swap, C.J.; McCormick, T.; Shen, E.; Silver, M.A.; Rippberger, E.J.; Vinson, D.R. Computed Tomography Use for Adults With Head Injury: Describing Likely Avoidable Emergency Department Imaging Based on the Canadian CT Head Rule. Acad. Emerg. Med. 2017, 24, 22–30. [Google Scholar]
- Datwyler, S.A.; Bazarian, J.J.; McQuiston, B.; Jeffrey, C.A.; Caudle, K.; Chen, J.Y.; Chandran, R.; Zhang, H.; McCaw, T.; Welch, R.D. Accuracy of a rapid glial fibrillary acidic protein/ubiquitin carboxyl-terminal hydrolase L1 test for the prediction of intracranial injuries on head computed tomography after mild traumatic brain injury. Acad. Emerg. Med. 2021, 28, 1308–1317. [Google Scholar]
- Augustovski, F.; Shau, W.-Y.; Lee, K.M.; Sullivan, S.D.; Orlewska, E.; Minchin, M.; Penna, P.; Caro, J.J.; Mauskopf, J.A.; Barrios, J.-M.R. Budget impact analysis-Principles of good practice: Report of the ISPOR 2012 budget impact analysis good practice II task force. Value Health 2014, 17, 5–14. [Google Scholar]
- Carswell, C.; Drummond, M.; Petrou, S.; Greenberg, D.; Augustovski, F.; Loder, E.; Briggs, A.H.; Mauskopf, J.; Pwu, R.-F.; Chaiyakunapruk, N.; et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) 2022 Explanation and Elaboration: A Report of the ISPOR CHEERS II Good Practices Task Force. Value Health 2022, 25, 10–31. [Google Scholar]
- Bastida, J.L.; Oliva, J.; Antoñanzas, F.; García-Altés, A.; Gisbert, R.; Mar, J.; Puig-Junoy, J. Propuesta de guía para la evaluación económica aplicada a las tecnologías sanitarias. Gac. Sanit. 2010, 24, 154–170. [Google Scholar] [CrossRef] [PubMed]
- Lindner, T.; Vossough, A.; Ornato, J.; O Okonkwo, D.; Bazarian, J.J.; Barzo, P.; Peacock, W.F.; A Leidel, B.; Ludington, E.; Jagoda, A.S.; et al. Serum GFAP and UCH-L1 for prediction of absence of intracranial injuries on head CT (ALERT-TBI): A multicentre observational study. Lancet Neurol. 2018, 17, 782–789. [Google Scholar]
- Ruan, S.; Noyes, K.; Bazarian, J.J. The Economic Impact of S-100B as a Pre-Head CT Screening Test on Emergency Department Management of Adult Patients with Mild Traumatic Brain Injury. J. Neurotrauma 2009, 26, 1654–1655. [Google Scholar] [CrossRef] [PubMed]
- Ministerio de Sanidad. Registro de Actividad de Atención Especializada. RAE-CMBD. Available online: https://www.sanidad.gob.es/estadEstudios/estadisticas/cmbdhome.htm (accessed on 5 September 2024).
- Grupo RECH. Red Española de Costes Hospitalarios (RECH). Available online: https://www.rechosp.org/faces/es/jsf/index.jsp (accessed on 5 September 2024).
- Dwamena, B.A.; Sapin, V.; Oris, C.; Pereira, B.; Kahouadji, S.; Puravet, A.; Bouvier, D. Can the Association of the Biomarkers GFAP and UCH-L1 Predict Intracranial Injury After Mild Traumatic Brain Injury in Adults? A Systematic Review and Meta-Analysis. Ann. Emerg. Med. 2025; Online ahead of print. [Google Scholar] [CrossRef]
- Jabaudon, M.; Evrard, B.; Rouzaire, P.; Bourgne, C.; Sapin, V.; Rouzaire, M.; Duret, T.; Pereira, B.; Bouvier, D.; Nourrisson, C. Preanalytical, analytical, gestational and pediatric aspects of the S100B immuno-assays. Clin. Chem. Lab. Med. 2016, 54, 833–842. [Google Scholar]
- Sapin, V.; Gaulmin, R.; Aubin, R.; Walrand, S.; Coste, A.; Abbot, M. Blood biomarkers of mild traumatic brain injury: State of art. Neurochirurgie 2021, 67, 249–254. [Google Scholar] [CrossRef]
- Ray, P.; Alen, J.F.; Jacquin, L.; Durand, G.; Sebbane, M.; Maignan, M.; Payen, J.-F.; Laribi, S.; Morales, J.; Pavlov, V.; et al. An automated blood test for glial fibrillary acidic protein (GFAP) and ubiquitin carboxy-terminal hydrolase L1 (UCH-L1) to predict the absence of intracranial lesions on head CT in adult patients with mild traumatic brain injury: BRAINI, a multicentre observational study in Europe. EBioMedicine 2024, 110, 105477. [Google Scholar]
- McDade, C.; Zimmer, L.; Textoris, J.; Krause, A.; Blanc, E.; Beyhaghi, H.; Pavlov, V.; Purser, M.; Earnshaw, S. Cost-Effectiveness of Blood-Based Brain Biomarkers for Screening Adults with Mild Traumatic Brain Injury in the French Health Care Setting. J. Neurotrauma 2023, 40, 706–719. [Google Scholar]
- Ladang, A.; Vavoulis, G.; Trifonidi, I.; Calluy, E.; Karagianni, K.; Mitropoulos, A.; Vlachos, K.; Cavalier, E.; Makris, K. Increased specificity of the “GFAP/UCH-L1” mTBI rule-out test by age dependent cut-offs. Clin. Chem. Lab. Med. 2024, 63, 995–1003. [Google Scholar] [CrossRef] [PubMed]
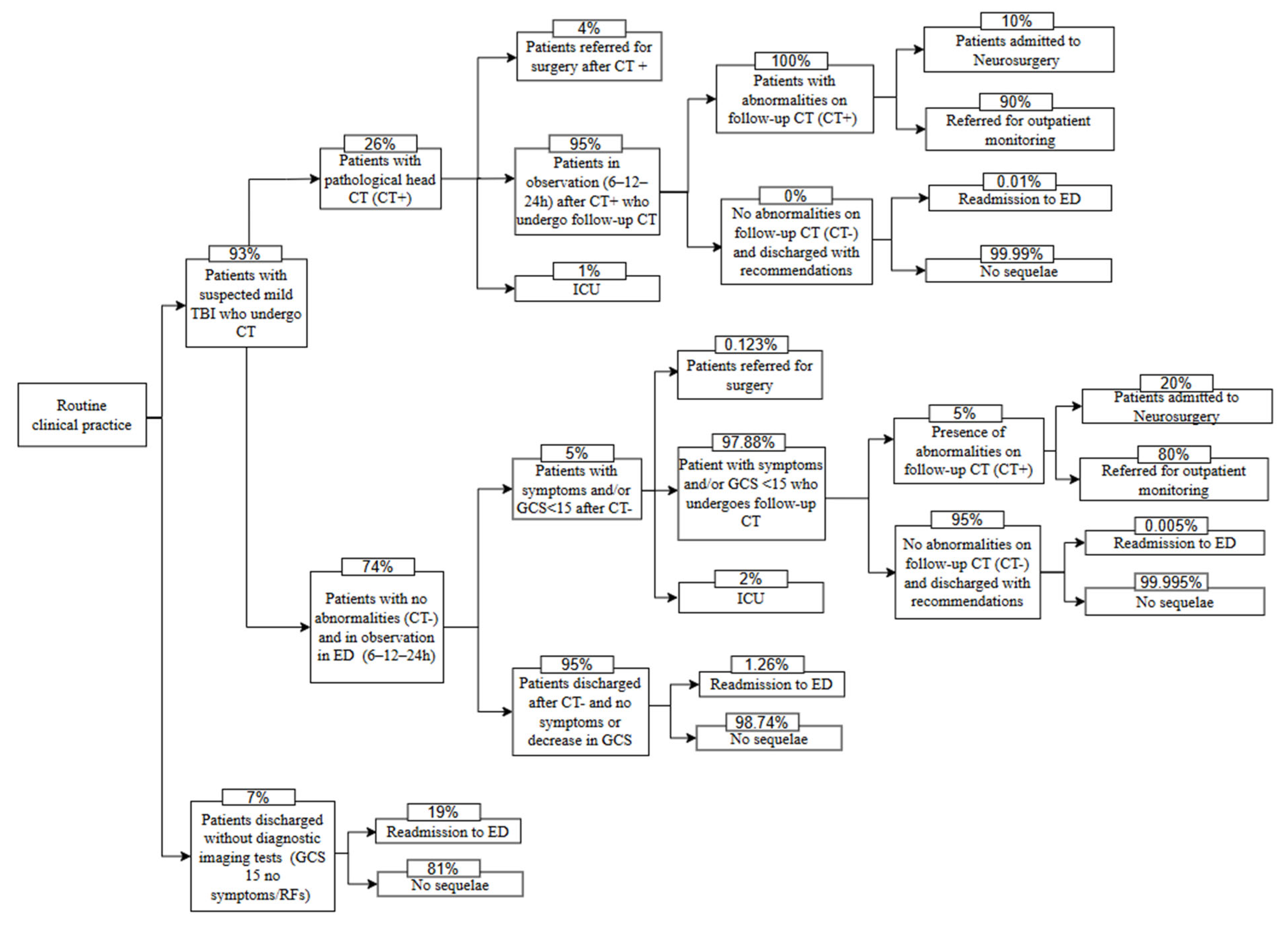
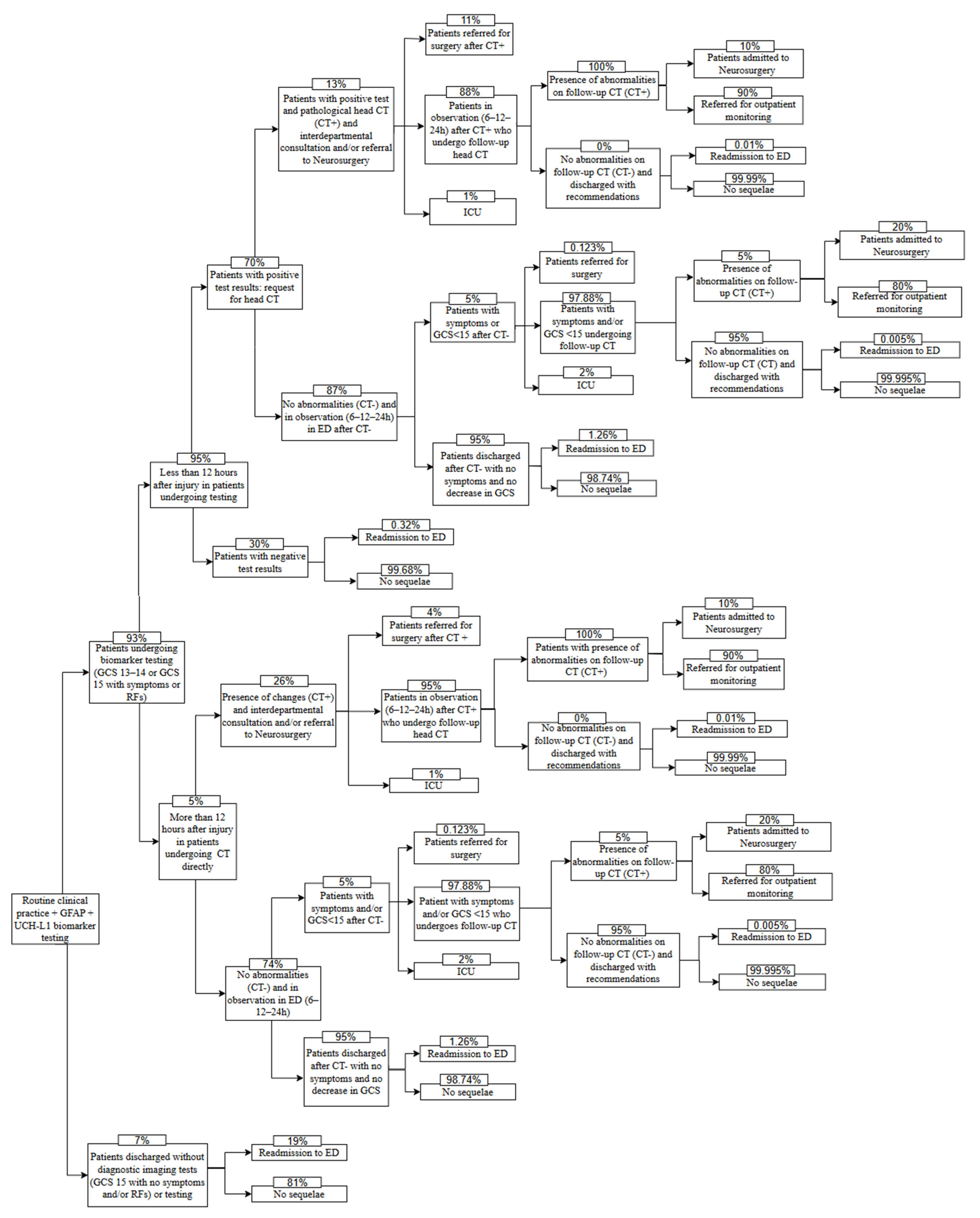
| Description | Value (%) | Source |
|---|---|---|
| Patients discharged without diagnostic imaging tests * | 7.00 | RCP experience |
| Patients readmitted to the ED after discharge without any type of test | 19.00 | RCP experience |
| Patients with pathological head CT | 26.00 | RCP experience |
| Patients discharged after negative CT scan, no symptoms/no decrease in GCS during the observation period | 95.00 | RCP experience |
| Patients referred for surgery after negative CT but with symptoms/decrease in GCS | 0.123 | Ruan et al. (2009) [15] |
| Patients admitted to the ICU after negative CT with symptoms/decrease in GCS | 2.00 | RCP experience |
| Symptomatic patients/GCS < 15 who undergo follow-up CT after negative CT | 97.88 | - ** |
| Patients with no abnormalities on follow-up CT and discharged after negative CT | 95.00 | RCP experience |
| Patients readmitted to the ED after negative CT | 1.26 | Ruan et al. (2009) [15] |
| Patients admitted to neurosurgery, with abnormalities on follow-up CT, after negative CT | 20.00 | RCP experience |
| Patients referred for outpatient monitoring, with abnormalities on follow-up CT, after negative CT | 80.00 | RCP experience |
| Patients readmitted to the ED after negative follow-up CT and negative CT | 0.005 | Ruan et al. (2009) [15] |
| Patients referred for surgery after positive CT | 4.00 | RCP experience |
| Patients admitted to ICU after positive CT | 1.00 | RCP experience |
| Patients in observation after positive CT who have follow-up head CT | 95.00 | - ** |
| Patients with abnormalities on follow-up CT, after positive CT | 100.00 | RCP experience |
| Patients admitted to Neurosurgery, with abnormalities on follow-up CT, after positive CT | 10.00 | RCP experience |
| Patients referred for outpatient monitoring, with abnormalities on follow-up CT, after positive CT | 90.00 | RCP experience |
| Patients readmitted to the ED after positive CT and negative follow-up CT | 0.01 | Ruan et al. (2009) [15] |
| Patients tested for specific biomarkers GFAP and UCH-L1 | 93.00 | RCP experience |
| Patients who come to the hospital within 12 h after sustaining injury | 95.00 | RCP experience |
| Patients with positive results for specific biomarkers GFAP and UCH-L1 | 70.00 | RCP experience |
| Patients with negative results for specific biomarkers GFAP and UCH-L1 readmitted to the ED | 0.32 | RCP experience |
| Patients with positive results for specific biomarkers GFAP and UCH-L1 and pathological head CT | 13.00 | RCP experience |
| Patients referred for surgery after positive CT and positive result for specific biomarkers GFAP and UCH-L1 | 11.00 | RCP experience |
| Patients admitted to the ICU after positive CT and positive result for specific biomarkers GFAP and UCH-L1 | 1.00 | RCP experience |
| Patients in observation after positive CT and positive result for specific biomarkers GFAP and UCH-L1 who undergo follow-up CT | 88.00 | - ** |
| Patients with abnormalities on follow-up CT, after positive CT and positive result for specific biomarkers GFAP and UCH-L1 | 100.00 | RCP experience |
| Patients admitted to Neurosurgery with abnormalities on follow-up CT, after positive CT and positive result for specific biomarkers GFAP and UCH-L1 | 10.00 | RCP experience |
| Patients referred for outpatient monitoring with abnormalities on follow-up CT after positive CT and positive result for specific biomarkers GFAP and UCH-L1 | 90.00 | RCP experience |
| Patients readmitted to the ED after positive result for specific biomarkers GFAP and UCH-L1, positive CT, and negative follow-up CT *** | 0.01 | Ruan et al. (2009) [15] |
| Patients with positive results for specific biomarkers GFAP and UCH-L1 with symptoms/decrease in GCS after observation period and negative CT | 5.00 | RCP experience |
| Patients readmitted to ED due to TBI after negative CT and positive result for specific biomarkers GFAP and UCH-L1 *** | 1.26 | Ruan et al. (2009) [15] |
| Patients referred for surgery after negative CT and positive result for specific biomarkers GFAP and UCH-L1 with symptoms/decrease in GCS after observation period *** | 0.123 | Ruan et al. (2009) [15] |
| Patients with mild TBI admitted to ICU after negative CT and positive result for specific biomarkers GFAP and UCH-L1 but with symptoms/decrease in GCS after the observation period | 2.00 | RCP experience |
| Patients with symptoms/GCS < 15 undergoing follow-up CT after negative CT and positive result for specific biomarkers GFAP and UCH-L1 | 97.88 | - ** |
| Patients with abnormalities on follow-up CT after negative CT and positive result for specific biomarkers GFAP and UCH-L1 | 5.00 | RCP experience |
| Patients admitted to Neurosurgery with abnormalities on follow-up CT after negative CT and positive result for specific biomarkers GFAP and UCH-L1 | 20.00 | RCP experience |
| Patients referred for outpatient monitoring with abnormalities on follow-up CT after negative CT and positive result for specific biomarkers GFAP and UCH-L1 | 80.00 | RCP experience |
| Patients readmitted to the ED after negative follow-up CT, and negative CT, with positive result for specific biomarkers GFAP and UCH-L1 *** | 0.005 | Ruan et al. (2009) [15] |
| Costs | Value (EUR) |
|---|---|
| Visit for outpatient monitoring | 167 |
| Hospital stay: admission to Neurosurgery | 1240 |
| Admission to ICU | 857 |
| Neurosurgical intervention (operating room) | 1740 |
| Repeat visit to ED | 215 |
| Observation in ED | 410 |
| Specific biomarkers GFAP and UCH-L1 | 32 |
| CT | 184 |
| Standard visit to ED | 215 |
| Description of the Event | Min |
|---|---|
| Standard ED visit time (patients who do not require CT) | 98 |
| Time for request, preparation, transport, examination, and interpretation of the CT | 156 |
| Mean time of observation in the emergency department for patients requiring observation | 319 |
| Mean response time of the laboratory performing the test | 43 |
| Procedure | Routine Clinical Practice | Specific Biomarkers | Difference |
|---|---|---|---|
| Emergency visits | EUR 215.00 | EUR 215.00 | −EUR |
| Specific biomarkers GFAP and UCH-L1 | −EUR | EUR 28.10 | EUR 28.10 |
| CT | EUR 171.12 | EUR 122.35 | −EUR 48.77 |
| Follow-up CT | EUR 48.46 | EUR 20.29 | −EUR 28.18 |
| Observation in ED | EUR 376.34 | EUR 268.82 | −EUR 107.52 |
| Admission to Neurosurgery | EUR 28.90 | EUR 10.54 | −EUR 18.36 |
| Outpatient monitoring | EUR 34.75 | EUR 12.55 | −EUR 22.20 |
| Operating room | EUR 16.90 | EUR 16.29 | −EUR 0.61 |
| ICU | EUR 2.66 | EUR 1.28 | −EUR 1.38 |
| Readmission to ED | EUR 4.63 | EUR 4.52 | −EUR 0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moya Torrecilla, F.; Álvarez-Corral, G.; Gutiérrez Pérez, E.; Morell-Garcia, D.; Ortega Pérez, J.; Rodríguez, B.M.; Sánchez Martín, L.; Temboury Ruiz, F. Budget Impact Analysis of the Use of Specific Biomarkers GFAP and UCH-L1 in the Management of Mild Traumatic Brain Injury in Spain. J. Clin. Med. 2025, 14, 4095. https://doi.org/10.3390/jcm14124095
Moya Torrecilla F, Álvarez-Corral G, Gutiérrez Pérez E, Morell-Garcia D, Ortega Pérez J, Rodríguez BM, Sánchez Martín L, Temboury Ruiz F. Budget Impact Analysis of the Use of Specific Biomarkers GFAP and UCH-L1 in the Management of Mild Traumatic Brain Injury in Spain. Journal of Clinical Medicine. 2025; 14(12):4095. https://doi.org/10.3390/jcm14124095
Chicago/Turabian StyleMoya Torrecilla, Francisco, Gemma Álvarez-Corral, Eva Gutiérrez Pérez, Daniel Morell-Garcia, Juan Ortega Pérez, Beatriz Miriam Rodríguez, Leticia Sánchez Martín, and Francisco Temboury Ruiz. 2025. "Budget Impact Analysis of the Use of Specific Biomarkers GFAP and UCH-L1 in the Management of Mild Traumatic Brain Injury in Spain" Journal of Clinical Medicine 14, no. 12: 4095. https://doi.org/10.3390/jcm14124095
APA StyleMoya Torrecilla, F., Álvarez-Corral, G., Gutiérrez Pérez, E., Morell-Garcia, D., Ortega Pérez, J., Rodríguez, B. M., Sánchez Martín, L., & Temboury Ruiz, F. (2025). Budget Impact Analysis of the Use of Specific Biomarkers GFAP and UCH-L1 in the Management of Mild Traumatic Brain Injury in Spain. Journal of Clinical Medicine, 14(12), 4095. https://doi.org/10.3390/jcm14124095






