Primary Nocturnal Enuresis and Intelligence Levels in Children: A Meta-Analysis and Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Methodology
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Search Strategy
2.5. Reference Management
3. Data Extraction
3.1. Risk of Bias Assessment
3.2. Data Synthesis and Meta-Analysis
4. Results
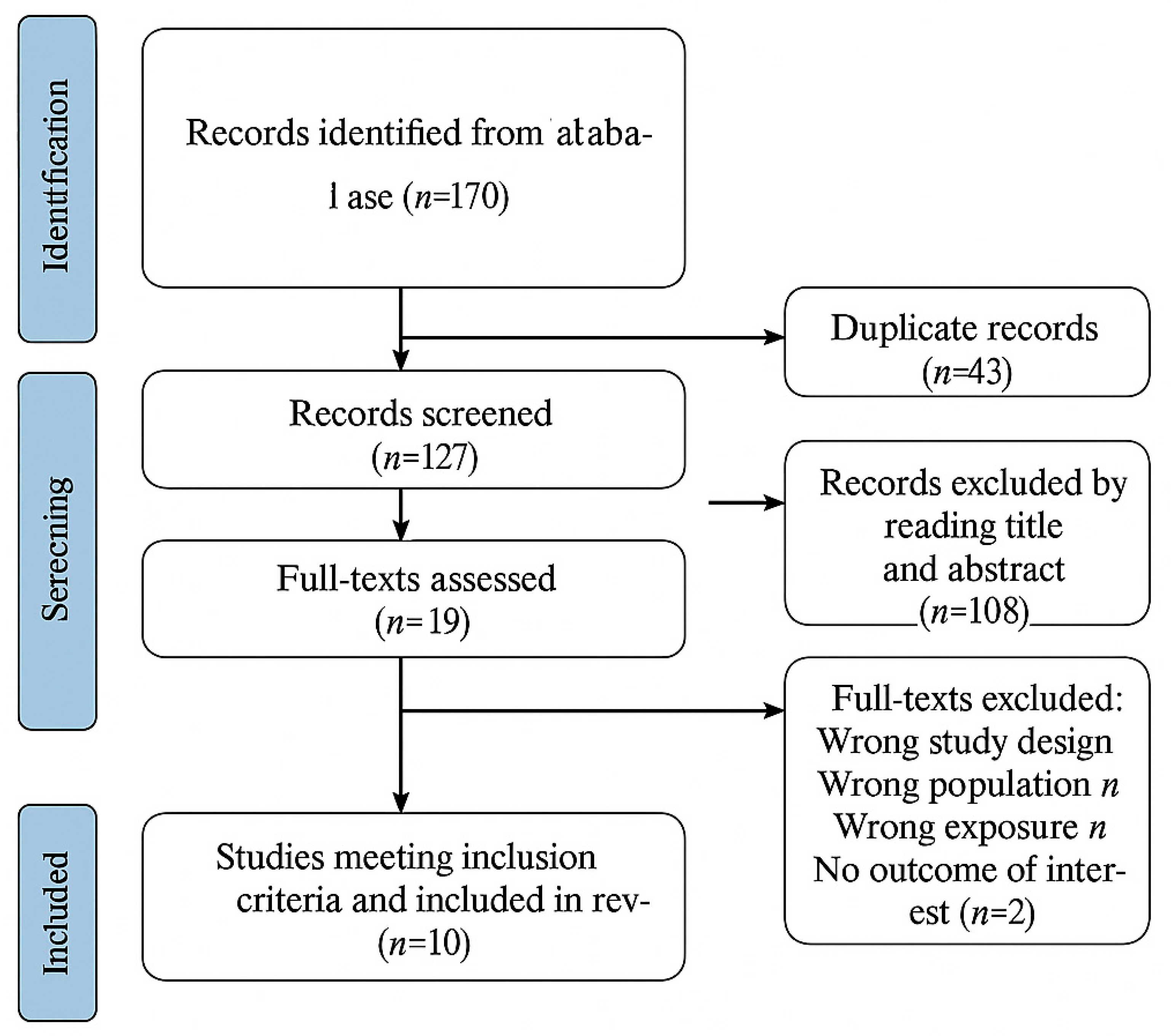
4.1. Study Selection
4.2. Descriptive Characteristics
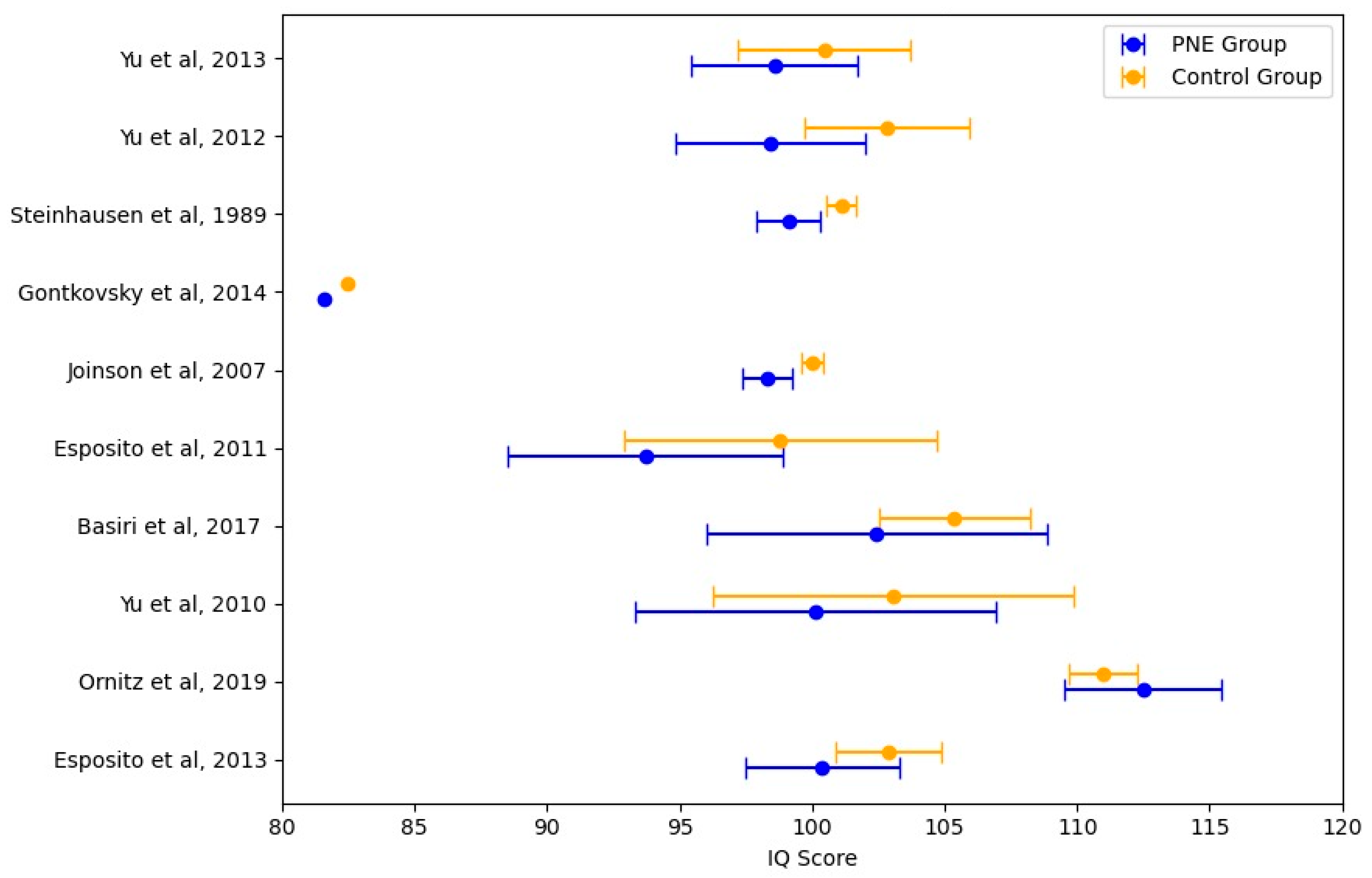
4.3. Meta-Analysis of Intelligence Quotient Scores in Children with PNE
4.4. Summary of Findings
5. Discussion
5.1. Limitations
5.2. Strengths
5.3. Clinical Implications and Future Research
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Mills, J.N. Diurnal Rhythm in Urine Flow. J. Physiol. 1951, 113, 528. [Google Scholar] [CrossRef] [PubMed]
- Nevéus, T.; Fonseca, E.; Franco, I.; Kawauchi, A.; Kovacevic, L.; Nieuwhof-Leppink, A.; Raes, A.; Tekgül, S.; Yang, S.S.; Rittig, S. Management and Treatment of Nocturnal Enuresisdan Updated Standardization Document from the International Children’s Continence Society. J. Pediatr. Urol. 2020, 16, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.F.; Bauer, S.B.; Bower, W.; Chase, J.; Franco, I.; Hoebeke, P.; Rittig, S.; Walle, J.V.; Von Gontard, A.; Wright, A.; et al. The Standardization of Terminology of Lower Urinary Tract Function in Children and Adolescents: Update Report from the Standardization Committee of the International Children’s Continence Society. Neurourol. Urodyn. 2016, 35, 471–481. [Google Scholar] [CrossRef] [PubMed]
- de Sena Oliveira, A.C.; Athanasio, B.D.S.; Mrad, F.C.D.C.; Vasconcelos, M.M.D.A.; Albuquerque, M.R.; Miranda, D.M.; Simões e Silva, A.C. Attention Deficit and Hyperactivity Disorder and Nocturnal Enuresis Co-Occurrence in the Pediatric Population: A Systematic Review and Meta-Analysis. Pediatr. Nephrol. 2021, 36, 3547–3559. [Google Scholar] [CrossRef]
- Roccella, M.; Smirni, D.; Smirni, P.; Precenzano, F.; Operto, F.F.; Lanzara, V.; Quatrosi, G.; Carotenuto, M. Parental Stress and Parental Ratings of Behavioral Problems of Enuretic Children. Front. Neurol. 2019, 10, 1054. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5-TR, 5th ed.; American Psychiatric Association Publishing: Washington, DC, USA, 2022. [Google Scholar]
- Li, W.; Yang, G.; Tian, W.; Li, Y.; Zhang, L.; Wang, Y.; Hong, Y. Bibliometric and Visual Analysis of Nocturnal Enuresis from 1982 to 2022. Front. Pediatr. 2022, 10, 972751. [Google Scholar] [CrossRef]
- Liao, J.; Zhu, L.; Xie, D.; Wang, X.; Zhou, P. Improving the Quality of Life of Children and Parents with Nocturnal Enuresis: The Role of Health Education. Front. Pediatr. 2024, 12, 1464465. [Google Scholar] [CrossRef]
- Esposito, M.; Gallai, B.; Parisi, L.; Roccella, M.; Marotta, R.; Lavano, S.M.; Mazzotta, G.; Carotenuto, M. Primary Nocturnal Enuresis as a Risk Factor for Sleep Disorders: An Observational Questionnaire-Based Multicenter Study. Neuropsychiatr. Dis. Treat. 2013, 9, 437–443. [Google Scholar] [CrossRef]
- Gulisano, M.; Domini, C.; Capelli, M.; Pellico, A.; Rizzo, R. Importance of Neuropsychiatric Evaluation in Children with Primary Monosymptomatic Enuresis. J. Pediatr. Urol. 2017, 13, 36.e1–36.e6. [Google Scholar] [CrossRef]
- Esposito, M.; Gallai, B.; Parisi, L.; Roccella, M.; Marotta, R.; Lavano, S.M.; Mazzotta, G.; Patriciello, G.; Precenzano, F.; Carotenuto, M. Visuomotor Competencies and Primary Monosymptomatic Nocturnal Enuresis in Prepubertal Aged Children. Neuropsychiatr. Dis. Treat. 2013, 9, 921–926. [Google Scholar] [CrossRef]
- Dang, J.; Tang, Z. Pathogenesis and Brain Functional Imaging in Nocturnal Enuresis: A Review. Exp. Biol. Med. 2021, 246, 1483–1490. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Kong, F.; Peng, M.; Ma, H.; Liu, N.; Guo, Q. Assessment of Memory/Attention Impairment in Children with Primary Nocturnal Enuresis: A Voxel-Based Morphometry Study. Eur. J. Radiol. 2012, 81, 4119–4122. [Google Scholar] [CrossRef] [PubMed]
- Karlidag, R.; Ozisik, H.I.; Soylu, A.; Kizkin, S.; Sipahi, B.; Unal, S.; Ozcan, C. Topographic Abnormalities in Event-Related Potentials in Children with Monosyptomatic Nocturnal Enuresis. Neurourol. Urodyn. 2004, 23, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, Y.; Chen, J.; Du, X.; Di, Y.; Liu, Q.; Wang, C.; Zhang, Q. Abnormal Microstructure of Corpus Callosum in Children with Primary Nocturnal Enuresis: A DTI Study. Eur. Child. Adolesc. Psychiatry 2024, 33, 3563–3570. [Google Scholar] [CrossRef]
- Yu, B.; Guo, Q.; Fan, G.; Ma, H.; Wang, L.; Liu, N. Evaluation of Working Memory Impairment in Children with Primary Nocturnal Enuresis: Evidence from Event-Related Functional Magnetic Resonance Imaging. J. Paediatr. Child. Health 2011, 47, 429–435. [Google Scholar] [CrossRef]
- Yu, B.; Sun, H.; Ma, H.; Peng, M.; Kong, F.; Meng, F.; Liu, N.; Guo, Q. Aberrant Whole-Brain Functional Connectivity and Intelligence Structure in Children with Primary Nocturnal Enuresis. PLoS ONE 2013, 8, e51924. [Google Scholar] [CrossRef]
- Zhang, Y.; Di, Y.; Chen, J.; Du, X.; Li, J.; Liu, Q.; Wang, C.; Zhang, Q. Functional Connectivity Density of Brain in Children with Primary Nocturnal Enuresis: Results from a Resting-State FMRI Study. Eur. Child Adolesc. Psychiatry 2024, 34, 1627–1635. [Google Scholar] [CrossRef]
- Kam, J.W.Y.; Solbakk, A.K.; Endestad, T.; Meling, T.R.; Knight, R.T. Lateral Prefrontal Cortex Lesion Impairs Regulation of Internally and Externally Directed Attention. Neuroimage 2018, 175, 91–99. [Google Scholar] [CrossRef]
- Jiang, K.; Liu, L.; Pan, C.; Ge, Y.; Zheng, A.; Li, Y.; Li, Y. The Study of Functional Connectivity of Attention Cognitive Impairment in Children with Nocturnal Enuresis. Int. J. Dev. Neurosci. 2022, 82, 646–653. [Google Scholar] [CrossRef]
- Kim, M.; Cheon, K.A. Exploring the Clinical Characteristics and Comorbid Disorders of Borderline Intellectual Functioning. J. Korean Acad. Child Adolesc. Psychiatry 2024, 35, 181. [Google Scholar] [CrossRef]
- Esposito, M.; Carotenuto, M. Intellectual Disabilities and Power Spectra Analysis during Sleep: A New Perspective on Borderline Intellectual Functioning. J. Intellect. Disabil. Res. 2014, 58, 421–429. [Google Scholar] [CrossRef]
- Peterson, B.S.; Pine, D.S.; Cohen, P.; Brook, J.S. Prospective, Longitudinal Study of Tic, Obsessive-Compulsive, and Attention-Deficit/Hyperactivity Disorders in an Epidemiological Sample. In Obsessive-Compulsive Disorder and Tourette’s Syndrome, the Science of Mental Health; Routledge: New York, NY, USA, 2002; pp. 107–117. [Google Scholar] [CrossRef]
- Kılıç, A.; Hacıhamdioğlu, D.Ö.; Tural, E.; Karademir, F. Evaluation of Neuropsychological Development of Children Diagnosed with Primary Monosymptomatic Nocturnal Enuresis: A Pilot Study. Turk. J. Urol. 2020, 46, 320. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.; Godfrey, C.; McInerney, P.; Soares, C. The Joanna Briggs Institute Reviewers’ Manual 2015: Methodology for JBI Scoping Reviews; The Joanna Briggs Institute: Adelaide, Australia, 2015. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Steinhausen, H.C.; Göbel, D. Enuresis in Child Psychiatric Clinic Patients. J. Am. Acad. Child. Adolesc. Psychiatry 1989, 28, 279–281. [Google Scholar] [CrossRef] [PubMed]
- Gontkovsky, S.T. Prevalence of Enuresis in a Community Sample of Children and Adolescents Referred for Outpatient Clinical Psychological Evaluation: Psychiatric Comorbidities and Association with Intellectual Functioning. J. Child. Adolesc. Ment. Health 2011, 23, 53–58. [Google Scholar] [CrossRef]
- Joinson, C.; Heron, J.; Butler, R.; Von Gontard, A.; Butler, U.; Emond, A.; Golding, J. A United Kingdom Population-Based Study of Intellectual Capacities in Children with and without Soiling, Daytime Wetting, and Bed-Wetting. Pediatrics 2007, 120, e308–e316. [Google Scholar] [CrossRef]
- Esposito, M.; Carotenuto, M.; Roccella, M. Primary Nocturnal Enuresis and Learning Disability. Minerva Pediatr. 2011, 63, 99–104. [Google Scholar]
- Basiri, A.; Bahrainian, S.A.; Khoshdel, A.; Jalaly, N.; Golshan, S.; Pakmanesh, H. Primary Nocturnal Enuresis Is Associated with Lower Intelligence Quotient Scores in Boys from Poorer Socioeconomic Status Families. Int. J. Urol. 2017, 24, 217–221. [Google Scholar] [CrossRef]
- Ornitz, E.M.; Russell, A.T.; Gabikian, P.; Gehricke, J.-G.; Guthrie, D.; Ornitz, E.M. Prepulse Inhibition of Startle, Intelligence and Familial Primary Nocturnal Enuresis. Acta Paediatr. 2000, 89, 475–481. [Google Scholar] [CrossRef]
| Study | Selection (0–4) | Comparability (0–2) | Outcome (0–3) | Total NOS Score | Quality Rating |
|---|---|---|---|---|---|
| Yu et al. [16] | 4 | 2 | 1 | 7 | High |
| Yu et al. (Second Study) [17] | 4 | 2 | 1 | 7 | High |
| Steinhausen et al. [28] | 3 | 2 | 2 | 7 | High |
| Gontkovsky [29] | 3 | 1 | 2 | 6 | Moderate |
| Joinson et al. [30] | 2 | 2 | 2 | 6 | Moderate |
| Esposito et al. [31] | 4 | 1 | 3 | 8 | High |
| Basiri et al. [32] | 3 | 2 | 1 | 6 | Moderate |
| Yu et al. (Third Study) [13] | 2 | 2 | 2 | 6 | Moderate |
| Ornitz et al. [33] | 3 | 2 | 2 | 7 | High |
| Esposito et al. [11] (Second Study) | 4 | 1 | 3 | 8 | High |
| Author(s) | Year | Total Sample Size | PNE | Control | Diagnostic Criteria | Age at Diagnosis | Age at IQ Assessment | IQ Assessment Tool | Study Design | Ethnicity | Key Findings |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Yu et al. [17] | 2013 | 133.0 | 67 | 66 | DSM IV | 10.5 ± 1.2 | 10.1 ± 1.1 | C-WISC | Case–Control Study | China | PNE exhibit imbalances in intelligence structure and attention deficits. |
| Yu et al. [13] | 2012 | 147.0 | 75 | 72 | DSM IV | 10.4 ± 1.3 | 10.0 ± 1.2 | C-WISC | Case–Control Study | China | Voxel-based morphometry revealed differences in gray matter density between children with PNE and control subjects. |
| Steinhausen et al. [28] | 1989 | 2.792 | 386 | 2404 | Not Reported | - | - | ICD-9 | Prevalence Study | Germany | Association between enuresis and comorbid psychiatric disorder. |
| Gontkovsky et al. [29] | 2014 | 363.0 | 58 | 305 | DSM-IV-TR criteria | - | - | C-WISC | Prevalence Study | USA | Examines the frequency of enuresis and psychiatric comorbidities among children and adolescents referred for outpatient clinical psychological evaluation. |
| Joinson et al. [30] | 2007 | 6063.0 | 965 | 5098 | DSMIV | 7.5 ± 0.8 | 7.6 ± 0.9 | WISC-III | Longitudinal Cohort Study | UK | Suggests a link between combined elimination issues and reduced intellectual capacities. |
| Esposito et al. [31] | 2011 | 79.0 | 25 | 54 | DSM-IV | - | - | WISC-R | Cross-Sectional Study | Italy | Evaluates the association between PNE and learning disabilities. |
| Basiri et al. [32] | 2017 | 152.0 | 55 | 97 | DSM-IV | - | - | WISC-R | Case–Control Study | Iran | Boys with PNE from low-income districts had lower intelligence quotient scores compared to control participants, indicating a correlation between socioeconomic status, PNE, and cognitive performance. |
| Yu et al. [16] | 2010 | 28.0 | 13 | 15 | DSM-IV | - | - | C-WISC | Case–Control Study | China | Investigates brain functional abnormalities related to working memory in PNE using functional magnetic resonance imaging, revealing specific impairments in working memory processes. |
| Ornitz et al. [33] | 2019 | 140.0 | 83 | 57 | X | Median 106 (74–135 mo) ≈ 8.8 | Median 106 (74–135 mo) ≈ 8.8 | WISC-R | Case–Control Study | Taiwan | Suggests a neurobiological link between PNE, sensory gating, and attentional processes. |
| Esposito et al. [11] | 2013 | 92.0 | 31 | 61 | ICCS | 8.14 ± 1.36 | 8.03 ± 1.44 | WISC-III | Case–Control Study | Italy | Evaluates the prevalence of fine motor coordination and visuomotor integration abnormalities in prepubertal children with PMNE, finding significant impairments in these areas. |
| Total PNE Sample | Total Control Sample | Total Sample Size | Mean IQ Difference (Control—PNE) | Pooled Standard Deviation | T-Statistic | p-Value |
|---|---|---|---|---|---|---|
| 1758 | 8229 | 9987 | 2.437777 | 13.908 | −1.165 | 0.260 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costanza, C.; Marrapodi, M.M.; Amoroso, L.; Roccella, M.; Sorrentino, M.; Gnazzo, M.; Bargiacchi, G.; Carotenuto, M. Primary Nocturnal Enuresis and Intelligence Levels in Children: A Meta-Analysis and Systematic Review. J. Clin. Med. 2025, 14, 4084. https://doi.org/10.3390/jcm14124084
Costanza C, Marrapodi MM, Amoroso L, Roccella M, Sorrentino M, Gnazzo M, Bargiacchi G, Carotenuto M. Primary Nocturnal Enuresis and Intelligence Levels in Children: A Meta-Analysis and Systematic Review. Journal of Clinical Medicine. 2025; 14(12):4084. https://doi.org/10.3390/jcm14124084
Chicago/Turabian StyleCostanza, Carola, Maria Maddalena Marrapodi, Laura Amoroso, Michele Roccella, Michele Sorrentino, Martina Gnazzo, Giuditta Bargiacchi, and Marco Carotenuto. 2025. "Primary Nocturnal Enuresis and Intelligence Levels in Children: A Meta-Analysis and Systematic Review" Journal of Clinical Medicine 14, no. 12: 4084. https://doi.org/10.3390/jcm14124084
APA StyleCostanza, C., Marrapodi, M. M., Amoroso, L., Roccella, M., Sorrentino, M., Gnazzo, M., Bargiacchi, G., & Carotenuto, M. (2025). Primary Nocturnal Enuresis and Intelligence Levels in Children: A Meta-Analysis and Systematic Review. Journal of Clinical Medicine, 14(12), 4084. https://doi.org/10.3390/jcm14124084









