Focusing on Selinexor for Holding and Bridging Prior to CAR-T in Relapsed/Refractory Multiple Myeloma
Abstract
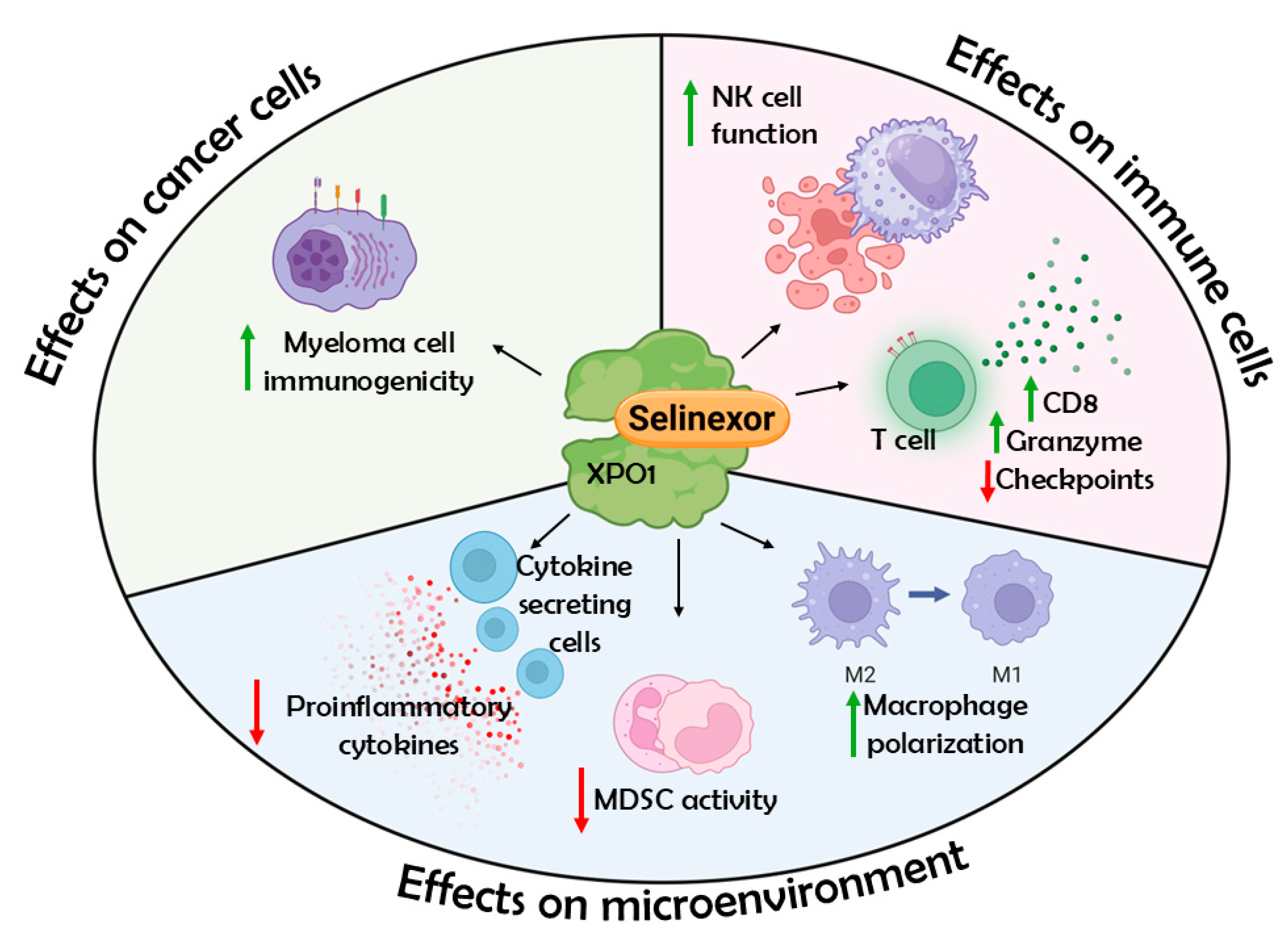
1. Introduction
2. Methods
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BCMA | B-cell maturation antigen |
| CAR-T | Chimeric antigen receptor T cell therapy |
| cilta-cel | Ciltacabtagene autoleucel |
| CNS | Central nervous system |
| EZH2 | Enhancer of Zeste homolog 2 |
| H3K27me3 | Histone H3 at Lys 27 (tri-methylation) |
| HLA-E | Human Leukocyte Antigen E |
| ide-cel | Idecabtagene vicleucel |
| IMiD | Immunomodulatory drug |
| IQR | Interquartile range |
| MDSCs | Myeloid-derived suppressor cells |
| MM | Multiple myeloma |
| NETs | Neutrophil extracellular traps |
| ORR | Overall response rate |
| OS | Overall survival |
| PBMCs | Peripheral blood mononuclear cells |
| PFS | Progression-free survival |
| RRMM | Relapsed/refractory multiple myeloma |
| XPO1 | Exportin 1 |
References
- Tanenbaum, B.; Miett, T.; Patel, S.A. The Emerging Therapeutic Landscape of Relapsed/Refractory Multiple Myeloma. Ann. Hematol. 2023, 102, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Rytlewski, J.; Madduri, D.; Fuller, J.; Campbell, T.B.; Mashadi-Hossein, A.; Thompson, E.G.; Jiang, Y.; Martin, N.; Sangurdekar, D.; Finney, O.; et al. Effects of Prior Alkylating Therapies on Preinfusion Patient Characteristics and Starting Material for CAR T Cell Product Manufacturing in Late-Line Multiple Myeloma. Blood 2020, 136, 7–8. [Google Scholar] [CrossRef]
- Firestone, R.S.; McAvoy, D.; Shekarkhand, T.; Serrano, E.; Hamadeh, I.; Wang, A.; Zhu, M.; Qin, W.G.; Patel, D.; Tan, C.R.; et al. CD8 Effector T Cells Enhance Teclistamab Response in BCMA-Exposed and -Naïve Multiple Myeloma. Blood Adv. 2023, 8, 1600–1611. [Google Scholar] [CrossRef]
- Afrough, A.; Hashmi, H.; Hansen, D.K.; Sidana, S.; Ahn, C.; Peres, L.C.; Dima, D.; Freeman, C.L.; Puglianini, O.C.; Kocoglu, M.H.; et al. Real-World Impact of Bridging Therapy on Outcomes of Ide-Cel for Myeloma in the U.S. Myeloma Immunotherapy Consortium. Blood Cancer J. 2024, 14, 63. [Google Scholar] [CrossRef]
- Costa, L.J.; Banerjee, R.; Mian, H.; Weisel, K.; Bal, S.; Derman, B.A.; Htut, M.M.; Nagarajan, C.; Rodriguez, C.; Richter, J.; et al. International Myeloma Working Group Immunotherapy Committee Recommendation on Sequencing Immunotherapy for Treatment of Multiple Myeloma. Leukemia 2025, 39, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.D.; Dhakal, B.; Jain, T.; Oluwole, O.O.; Shah, G.L.; Sidana, S.; Perales, M.-A.; Pasquini, M.C. Chimeric Antigen Receptor T Cell Therapy for Myeloma: Where Are We Now and What Is Needed to Move Chimeric Antigen Receptor T Cells Forward to Earlier Lines of Therapy? Expert Panel Opinion from the American Society for Transplantation and Cellular Therapy. Transplant. Cell. Ther. 2024, 30, 17–37. [Google Scholar] [CrossRef]
- Tai, Y.-T.; Landesman, Y.; Acharya, C.; Calle, Y.; Zhong, M.Y.; Cea, M.; Tannenbaum, D.; Cagnetta, A.; Reagan, M.; Munshi, A.A.; et al. CRM1 Inhibition Induces Tumor Cell Cytotoxicity and Impairs Osteoclastogenesis in Multiple Myeloma: Molecular Mechanisms and Therapeutic Implications. Leukemia 2014, 28, 155–165. [Google Scholar] [CrossRef]
- Tyler, P.M.; Servos, M.M.; de Vries, R.C.; Klebanov, B.; Kashyap, T.; Sacham, S.; Landesman, Y.; Dougan, M.; Dougan, S.K. Clinical Dosing Regimen of Selinexor Maintains Normal Immune Homeostasis and T-Cell Effector Function in Mice: Implications for Combination with Immunotherapy. Mol. Cancer Ther. 2017, 16, 428–439. [Google Scholar] [CrossRef]
- Chari, A.; Vogl, D.T.; Gavriatopoulou, M.; Nooka, A.K.; Yee, A.J.; Huff, C.A.; Moreau, P.; Dingli, D.; Cole, C.; Lonial, S.; et al. Oral Selinexor-Dexamethasone for Triple-Class Refractory Multiple Myeloma. N. Engl. J. Med. 2019, 381, 727–738. [Google Scholar] [CrossRef]
- Grosicki, S.; Simonova, M.; Spicka, I.; Pour, L.; Kriachok, I.; Gavriatopoulou, M.; Pylypenko, H.; Auner, H.W.; Leleu, X.; Doronin, V.; et al. Once-per-Week Selinexor, Bortezomib, and Dexamethasone versus Twice-per-Week Bortezomib and Dexamethasone in Patients with Multiple Myeloma (BOSTON): A Randomised, Open-Label, Phase 3 Trial. Lancet 2020, 396, 1563–1573. [Google Scholar] [CrossRef]
- White, D.; Schiller, G.J.; Madan, S.; Lentzsch, S.; Chubar, E.; Lavi, N.; Van Domelen, D.R.; Bentur, O.S.; Baljevic, M. Efficacy and Safety of Once Weekly Selinexor 40 Mg versus 60 Mg with Pomalidomide and Dexamethasone in Relapsed and/or Refractory Multiple Myeloma. Front. Oncol. 2024, 14, 1352281. [Google Scholar] [CrossRef] [PubMed]
- Gasparetto, C.; Schiller, G.J.; Tuchman, S.A.; Callander, N.S.; Baljevic, M.; Lentzsch, S.; Rossi, A.C.; Kotb, R.; White, D.; Bahlis, N.J.; et al. Once Weekly Selinexor, Carfilzomib and Dexamethasone in Carfilzomib Non-Refractory Multiple Myeloma Patients. Br. J. Cancer 2022, 126, 718–725. [Google Scholar] [CrossRef]
- Gasparetto, C.; Lentzsch, S.; Schiller, G.; Callander, N.; Tuchman, S.; Chen, C.; White, D.; Kotb, R.; Sutherland, H.; Sebag, M.; et al. Selinexor, Daratumumab, and Dexamethasone in Patients with Relapsed or Refractory Multiple Myeloma. eJHaem 2021, 2, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Schiller, G.J.; Lipe, B.C.; Bahlis, N.J.; Tuchman, S.A.; Bensinger, W.I.; Sutherland, H.J.; Lentzsch, S.; Baljevic, M.; White, D.; Kotb, R.; et al. Selinexor-Based Triplet Regimens in Patients with Multiple Myeloma Previously Treated with Anti-CD38 Monoclonal Antibodies. Clin. Lymphoma Myeloma Leuk. 2023, 23, e286–e296.e4. [Google Scholar] [CrossRef]
- Baljevic, M.; Gasparetto, C.; Schiller, G.J.; Tuchman, S.A.; Callander, N.S.; Lentzsch, S.; Monge, J.; Kotb, R.; Bahlis, N.J.; White, D.; et al. Selinexor-Based Regimens in Patients with Multiple Myeloma after Prior Anti-B-Cell Maturation Antigen Treatment. eJHaem 2022, 3, 1270–1276. [Google Scholar] [CrossRef]
- Kang, Y.; Neff, J.; Gasparetto, C.; Wang, X.; Ellero, A.; Walker, C. P-396 Investigation of T-Cell Fitness and Mechanisms of Drug Resistance in Selinexor Treated Patients with Relapsed/Refractory Multiple Myeloma. Clin. Lymphoma Myeloma Leuk. 2023, 23, S259. [Google Scholar] [CrossRef]
- Blanquart, E.; Ekren, R.; Rigaud, B.; Joubert, M.-V.; Baylot, V.; Daunes, H.; Cuisinier, M.; Villard, M.; Carrié, N.; Mazzotti, C.; et al. NK Cells with Adhesion Defects and Reduced Cytotoxic Functions Are Associated with a Poor Prognosis in Multiple Myeloma. Blood 2024, 144, 1271–1283. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.G.; Walker, C.J.; Doyle, A.D.; Johnson, P.W.; Forconi, F.; Cragg, M.S.; Landesman, Y.; Khakoo, S.I.; Blunt, M.D. Selinexor Enhances NK Cell Activation Against Malignant B Cells via Downregulation of HLA-E. Front. Oncol. 2021, 11, 785635. [Google Scholar] [CrossRef]
- van Montfoort, N.; Borst, L.; Korrer, M.J.; Sluijter, M.; Marijt, K.A.; Santegoets, S.J.; van Ham, V.J.; Ehsan, I.; Charoentong, P.; André, P.; et al. NKG2A Blockade Potentiates CD8 T Cell Immunity Induced by Cancer Vaccines. Cell 2018, 175, 1744–1755.e15. [Google Scholar] [CrossRef]
- Daneshmandi, S.; Yan, Q.; Choi, J.E.; Katsuta, E.; MacDonald, C.R.; Goruganthu, M.; Roberts, N.; Repasky, E.A.; Singh, P.K.; Attwood, K.; et al. Exportin 1 Governs the Immunosuppressive Functions of Myeloid-Derived Suppressor Cells in Tumors through ERK1/2 Nuclear Export. Cell. Mol. Immunol. 2024, 21, 873–891. [Google Scholar] [CrossRef]
- Jiménez, I.; Carabia, J.; Bobillo, S.; Palacio, C.; Abrisqueta, P.; Pagès, C.; Nieto, J.C.; Castellví, J.; Martínez-Ricarte, F.; Escoda, L.; et al. Repolarization of Tumor Infiltrating Macrophages and Increased Survival in Mouse Primary CNS Lymphomas after XPO1 and BTK Inhibition. J. Neurooncol 2020, 149, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Tantravahi, S.; Patel, A.; Yap, J.; Walker, C.; Ellero, A.; Rets, A.; Prchal, J.; Deininger, M. MPN-652 Long-Term Response to Selinexor in Patients with Myelofibrosis and Refractory or Intolerant to JAK Inhibitors: Follow-Up Results of a Single-Center, Phase II, Investigator-Initiated Trial (IIT). Clin. Lymphoma Myeloma Leuk. 2024, 24, S441. [Google Scholar] [CrossRef]
- Kashyap, T.; Murray, J.; Walker, C.J.; Chang, H.; Tamir, S.; Hou, B.; Shacham, S.; Kauffman, M.G.; Tripp, R.A.; Landesman, Y. Selinexor, a Novel Selective Inhibitor of Nuclear Export, Reduces SARS-CoV-2 Infection and Protects the Respiratory System in Vivo. Antivir. Res. 2021, 192, 105115. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, T.; Argueta, C.; Aboukameel, A.; Unger, T.J.; Klebanov, B.; Mohammad, R.M.; Muqbil, I.; Azmi, A.S.; Drolen, C.; Senapedis, W.; et al. Selinexor, a Selective Inhibitor of Nuclear Export (SINE) Compound, Acts through NF-κB Deactivation and Combines with Proteasome Inhibitors to Synergistically Induce Tumor Cell Death. Oncotarget 2016, 7, 78883–78895. [Google Scholar] [CrossRef]
- Baron, S.; Rashal, T.; Vaisman, D.; Elhasid, R.; Shukrun, R. Selinexor, a Selective Inhibitor of Nuclear Export, Inhibits Human Neutrophil Extracellular Trap Formation in Vitro. Front. Pharmacol. 2022, 13, 1030991. [Google Scholar] [CrossRef]
- Masucci, M.T.; Minopoli, M.; Del Vecchio, S.; Carriero, M.V. The Emerging Role of Neutrophil Extracellular Traps (NETs) in Tumor Progression and Metastasis. Front. Immunol. 2020, 11, 1749. [Google Scholar] [CrossRef]
- Wang, D.; Fu, H.; Que, Y.; Ruan, H.; Xu, M.; Long, X.; Yu, Q.; Li, C.; Li, Z.; Cai, S.; et al. A Novel Two-Step Administration of XPO-1 Inhibitor May Enhance the Effect of Anti-BCMA CAR-T in Relapsed/Refractory Extramedullary Multiple Myeloma. J. Transl. Med. 2023, 21, 812. [Google Scholar] [CrossRef]
- Sudalagunta, P.R.; Renatino-Canevarolo, R.; Meads, M.B.; Noyes, D.; Achille, A.; Silva, M.; Zhao, X.; Alugubelli, R.R.; Lastorino, D.; Hampton, O.; et al. Selinexor Disrupts Epigenetic Programing and Modulates Immunogenicity in Multiple Myeloma. Blood 2023, 142, 3301. [Google Scholar] [CrossRef]
- Costa, B.A.; Dima, D.; Mark, T.; Sadek, N.L.; Ijioma, S.; Ray, D.; Goel, U.; Dranitsaris, G.; Sheng, T.; Moshier, E.; et al. Impact of Prior Selinexor Exposure on Outcomes of Chimeric Antigen Receptor T-Cell Therapy for Relapsed/Refractory Multiple Myeloma: An Exploratory Analysis. J. Clin. Med. 2025, 14, 1316. [Google Scholar] [CrossRef]
- Gill, S.K.; Biran, N.; Phull, P.; Vesole, D.H.; Siegel, D.S.; Parmar, H. Sequential Administration of Selinexor and CAR-T Therapy in Relapsed/ Refractory Multiple Myeloma. Blood 2023, 142, 6930. [Google Scholar] [CrossRef]
- Binder, A.F.; Walker, C.J.; Mark, T.M.; Baljevic, M. Impacting T-Cell Fitness in Multiple Myeloma: Potential Roles for Selinexor and XPO1 Inhibitors. Front. Immunol. 2023, 14, 1275329. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khouri, J.; Sborov, D.; Rossi, A.; Martin, T.; Kashyap, T.; Mark, T.; Baljevic, M. Focusing on Selinexor for Holding and Bridging Prior to CAR-T in Relapsed/Refractory Multiple Myeloma. J. Clin. Med. 2025, 14, 4071. https://doi.org/10.3390/jcm14124071
Khouri J, Sborov D, Rossi A, Martin T, Kashyap T, Mark T, Baljevic M. Focusing on Selinexor for Holding and Bridging Prior to CAR-T in Relapsed/Refractory Multiple Myeloma. Journal of Clinical Medicine. 2025; 14(12):4071. https://doi.org/10.3390/jcm14124071
Chicago/Turabian StyleKhouri, Jack, Douglas Sborov, Adriana Rossi, Thomas Martin, Trinayan Kashyap, Tomer Mark, and Muhamed Baljevic. 2025. "Focusing on Selinexor for Holding and Bridging Prior to CAR-T in Relapsed/Refractory Multiple Myeloma" Journal of Clinical Medicine 14, no. 12: 4071. https://doi.org/10.3390/jcm14124071
APA StyleKhouri, J., Sborov, D., Rossi, A., Martin, T., Kashyap, T., Mark, T., & Baljevic, M. (2025). Focusing on Selinexor for Holding and Bridging Prior to CAR-T in Relapsed/Refractory Multiple Myeloma. Journal of Clinical Medicine, 14(12), 4071. https://doi.org/10.3390/jcm14124071




