Impact of Prior Selinexor Exposure on Outcomes of Chimeric Antigen Receptor T-Cell Therapy for Relapsed/Refractory Multiple Myeloma: An Exploratory Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Clinical Assessments
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics and Treatment History
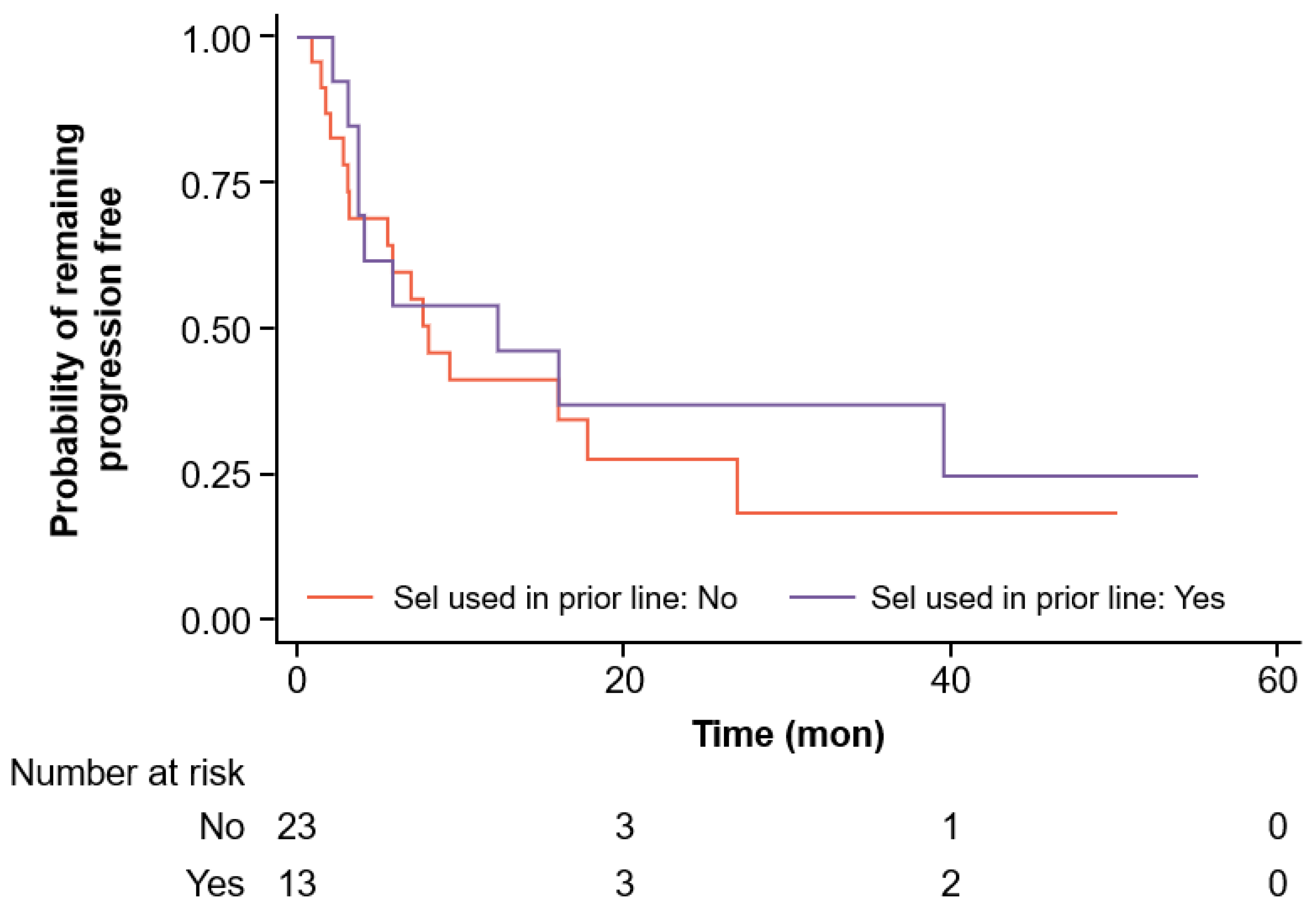
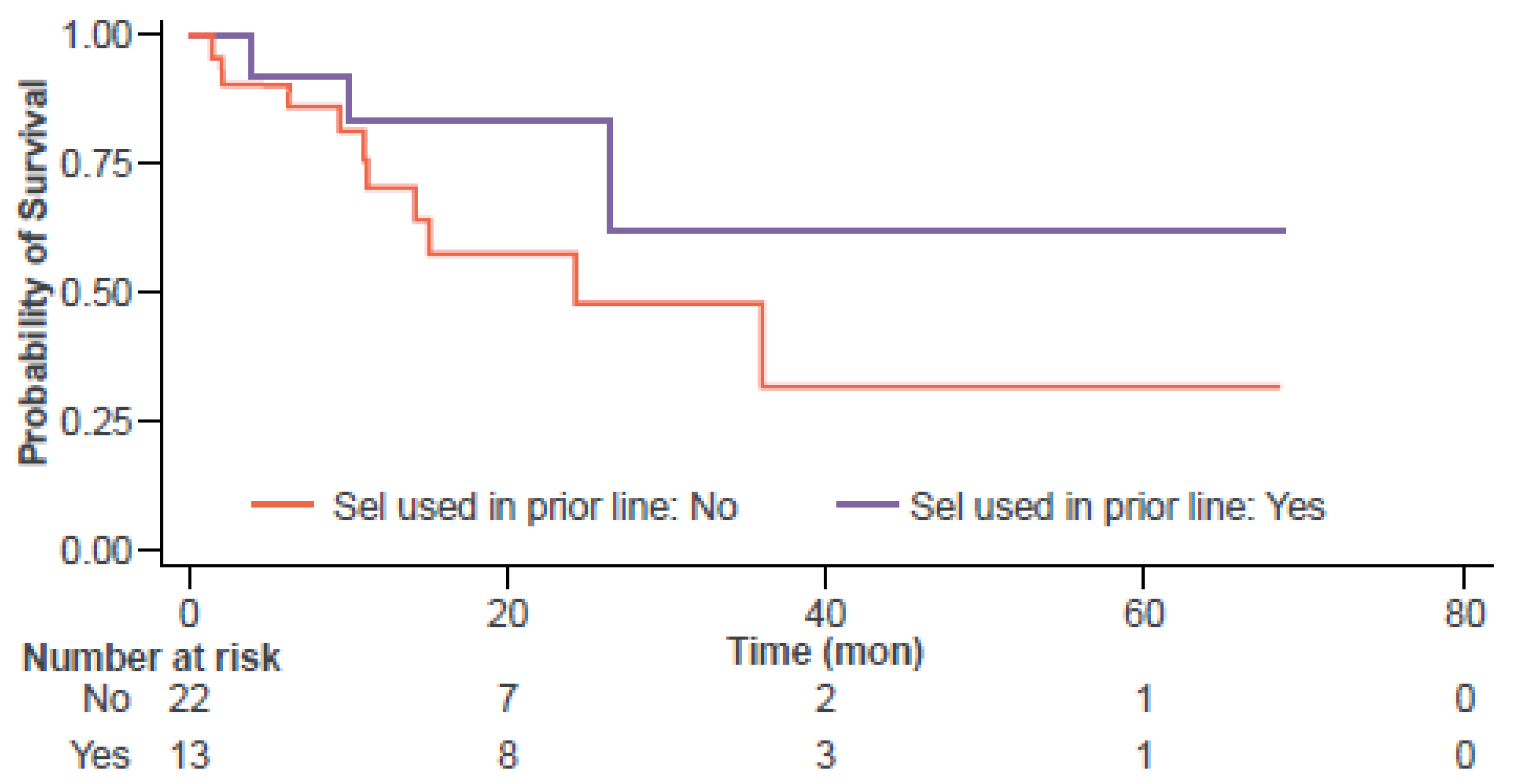
3.2. Efficacy Outcomes
3.3. Safety Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Munshi, N.C.; Anderson, L.D., Jr.; Shah, N.; Madduri, D.; Berdeja, J.; Lonial, S.; Raje, N.; Lin, Y.; Siegel, D.; Oriol, A.; et al. Idecabtagene Vicleucel in Relapsed and Refractory Multiple Myeloma. N. Engl. J. Med. 2021, 384, 705–716. [Google Scholar] [CrossRef] [PubMed]
- San-Miguel, J.; Dhakal, B.; Yong, K.; Spencer, A.; Anguille, S.; Mateos, M.V.; Fernández de Larrea, C.; Martínez-López, J.; Moreau, P.; Touzeau, C.; et al. Cilta-cel or Standard Care in Lenalidomide-Refractory Multiple Myeloma. N. Engl. J. Med. 2023, 389, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.D., Jr.; Dhakal, B.; Jain, T.; Oluwole, O.O.; Shah, G.L.; Sidana, S.; Perales, M.A.; Pasquini, M.C. Chimeric Antigen Receptor T Cell Therapy for Myeloma: Where Are We Now and What Is Needed to Move Chimeric Antigen Receptor T Cells Forward to Earlier Lines of Therapy? Expert Panel Opinion from the American Society for Transplantation and Cellular Therapy. Transpl. Cell Ther. 2024, 30, 17–37. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hashmi, H.; Hansen, D.K.; Peres, L.C.; Puglianini, O.C.; Freeman, C.; De Avila, G.; Sidana, S.; Shune, L.; Sborov, D.W.; Davis, J.; et al. Factors associated with refractoriness or early progression after idecabtagene vicleucel in patients with relapsed/ refractory multiple myeloma: US Myeloma Immunotherapy Consortium real world experience. Haematologica 2024, 109, 1514–1524. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rytlewski, J.; Fuller, J.; Mertz, D.R.; Freeman, C.; Manier, S.; Shah, N.; Campbell, T.B. P866: Correlative analysis to define patient profiles associated with manufacturing and clinical endpoints in relapsed/refractory multiple myeloma patients treated with idecabtagene vicleucel (BB2121). Hemasphere 2022, 6, 759–760. [Google Scholar] [CrossRef]
- Firestone, R.S.; McAvoy, D.; Shekarkhand, T.; Serrano, E.; Hamadeh, I.; Wang, A.; Zhu, M.; Qin, W.G.; Patel, D.; Tan, C.R.; et al. CD8 effector T cells enhance teclistamab response in BCMA-exposed and -naïve multiple myeloma. Blood Adv. 2024, 8, 1600–1611. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Binder, A.F.; Walker, C.J.; Mark, T.M.; Baljevic, M. Impacting T-cell fitness in multiple myeloma: Potential roles for selinexor and XPO1 inhibitors. Front. Immunol. 2023, 14, 1275329. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Baljevic, M.; Moreau, P.; Tuchman, S.A.; Callander, N.S.; Lentzsch, S.; Van Domelen, D.R.; Bentur, O.S.; Monge, J.; Biran, N. Effectiveness of anti-B-cell maturation antigen (BCMA)-targeting therapy after selinexor treatment. J. Clin. Oncol. 2023, 41, e20034. [Google Scholar] [CrossRef]
- Kumar, S.; Paiva, B.; Anderson, K.C.; Durie, B.; Landgren, O.; Moreau, P.; Munshi, N.; Lonial, S.; Bladé, J.; Mateos, M.V.; et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016, 17, e328–e346. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Santomasso, B.D.; Locke, F.L.; Ghobadi, A.; Turtle, C.J.; Brudno, J.N.; Maus, M.V.; Park, J.H.; Mead, E.; Pavletic, S.; et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol. Blood Marrow Transplant. 2019, 25, 625–638. [Google Scholar] [CrossRef] [PubMed]
- Hansen, D.K.; Sidana, S.; Peres, L.C.; Colin Leitzinger, C.; Shune, L.; Shrewsbury, A.; Gonzalez, R.; Sborov, D.W.; Wagner, C.; Dima, D.; et al. Idecabtagene Vicleucel for Relapsed/Refractory Multiple Myeloma: Real-World Experience From the Myeloma CAR T Consortium. J. Clin. Oncol. 2023, 41, 2087–2097. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ehsan, H.; Robinson, M.; Voorhees, P.M.; Cassetta, K.; Borden, S.; Atrash, S.; Bhutani, M.; Varga, C.; Pineda-Roman, M.; Friend, R.; et al. Efficacy of Selinexor in Relapsed/Refractory Multiple Myeloma (RRMM) Patients with del17p and Other High-Risk Abnormalities (A Retrospective Single-Center Study). Life 2024, 14, 384. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Afrough, A.; Hashmi, H.; Hansen, D.K.; Sidana, S.; Ahn, C.; Peres, L.C.; Dima, D.; Freeman, C.L.; Puglianini, O.C.; Kocoglu, M.H.; et al. Real-world impact of bridging therapy on outcomes of ide-cel for myeloma in the U.S. Myeloma Immunotherapy Consortium. Blood Cancer J. 2024, 14, 63. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yee, A.J.; Huff, C.A.; Chari, A.; Vogl, D.T.; Gavriatopoulou, M.; Nooka, A.K.; Moreau, P.; Dingli, D.; Cole, C.E.; Lonial, S.; et al. Response to Therapy and the Effectiveness of Treatment with Selinexor and Dexamethasone in Patients with Penta-Exposed Triple-Class Refractory Myeloma Who Had Plasmacytomas. Blood 2019, 134 (Suppl. S1), 3140. [Google Scholar] [CrossRef]
- Grosicki, S.; Moreau, P.; Kauffman, M.G.; Dimopoulos, M.A.; Richardson, P.G.; Delimpasi, S. Multiple myeloma triplet therapies: Baseline characteristics and control groups—Authors’ reply. Lancet 2021, 397, 1621–1623. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.K.; Biran, N.; Phull, P.; Vesole, D.H.; Siegel, D.S.; Parmar, H. Sequential Administration of Selinexor and CAR-T Therapy in Relapsed/ Refractory Multiple Myeloma. Blood 2023, 142 (Suppl. S1), 6930. [Google Scholar] [CrossRef]
- Wang, D.; Fu, H.; Que, Y.; Ruan, H.; Xu, M.; Long, X.; Yu, Q.; Li, C.; Li, Z.; Cai, S.; et al. A novel two-step administration of XPO-1 inhibitor may enhance the effect of anti-BCMA CAR-T in relapsed/refractory extramedullary multiple myeloma. J. Transl. Med. 2023, 21, 812. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kang, Y.; Neff, J.; Gasparetto, C.; Wang, X.; Ellero, A.; Walker, C. Fitness and Mechanisms of Drug Resistance in Selinexor Treated Patients with Relapsed/Refractory Multiple Myeloma. In Proceedings of the 20th International Myeloma Society Annual Meeting, Athens, Greece, 27–30 September 2023. [Google Scholar]
- Daneshmandi, S.; Yan, Q.; Choi, J.E.; Katsuta, E.; MacDonald, C.R.; Goruganthu, M.; Roberts, N.; Repasky, E.A.; Singh, P.K.; Attwood, K.; et al. Exportin 1 governs the immunosuppressive functions of myeloid-derived suppressor cells in tumors through ERK1/2 nuclear export. Cell Mol. Immunol. 2024, 21, 873–891. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fisher, J.G.; Doyle, A.D.P.; Graham, L.V.; Forconi, F.; Cragg, M.S.; Walker, C.J.; Khakoo, S.I.; Blunt, M.D. Selinexor Enhances Natural Killer Cell Function Against Multiple Myeloma Cells. Blood 2023, 142 (Suppl. S1), 6599. [Google Scholar] [CrossRef]
- Stadel, R.; Liu, R.; Landesman, Y.; Wald, D.; Vasanna, S.H.; de Lima, M.J.G. Sequential Administration of Selinexor then CD19 CAR-T Cells Exhibits Enhanced Efficacy in a Mouse Model of Human Non-Hodgkin’s Lymphoma. Blood 2022, 140 (Suppl. S1), 7413–7414. [Google Scholar] [CrossRef]
- Dima, D.; Rossi, A.; Costa, B.; Mark, T.; Ijioma, S.; Ray, D.; Dranitsaris, G.; Sadek, N.; Sheng, T.; Moshier, E.; et al. Survival Analysis of Selinexor-Exposed Relapsed/Refractory Multiple Myeloma (RRMM) Treated with Chimeric Antigen Receptor T-Cell (CAR-T) Therapy: A Real-World Exploratory Analysis. In Proceedings of the 21st International Myeloma Society Annual Meeting, Rio de Janeiro, Brazil, 25–28 September 2024. [Google Scholar]
| Parameter | n = 45 |
|---|---|
| Median age at MM diagnosis (range) | 54 (37–75) |
| Median age at the time of CAR-T therapy (range) | 64 (50–78) |
| Female | 55.6% (25) |
| Race | |
| White | 75.6% (34) |
| Black | 17.8% (8) |
| Other | 6.7% (3) |
| ISS prior to CAR-T | |
| Stage I | 31.1% (14) |
| Stage II | 48.9% (22) |
| Stage III | 11.1% (5) |
| Not Documented | 8.9% (4) |
| ECOG Performance Status | |
| 0 or 1 | 88.9% (40) |
| ≥2 | 11.1% (5) |
| Cytogenetics | |
| t(4;14) | 11.1% (5) |
| t(14;16) | 4.4% (2) |
| del(17p) | 15.6% (7) |
| 1q21 gain/amp | 55.6% (25) |
| High-risk cytogenetics abnormalities 1 | 40% (18) |
| Prior drug exposure | |
| lenalidomide | 100% (45) |
| pomalidomide | 97.8% (44) |
| bortezomib | 97.8% (44) |
| carfilzomib | 100% (45) |
| daratumumab | 100% (45) |
| Prior ASCT | 91.1% (41) |
| Second ASCT | 31.1% (14) |
| Median time between last ASCT and CAR-T infusion (IQR)—months | 52.0 (9.6–165) |
| Parameter | n = 45 |
|---|---|
| Selinexor regimen | |
| selinexor/bortezomib/dexamethasone | 28.9% (13) |
| selinexor/daratumumab/carfilzomib/dexamethasone | 4.4% (2) |
| selinexor/daratumumab/melphalan/prednisone | 2.2% (1) |
| selinexor/dexamethasone | 8.9% (4) |
| selinexor/bortezomib/isatuximab/dexamethasone | 2.2% (1) |
| selinexor/isatuximab/dexamethasone | 4.4% (2) |
| selinexor/carfilzomib | 8.9% (4) |
| selinexor/carfilzomib/dexamethasone | 20% (9) |
| selinexor/carfilzomib/venetoclax | 2.2% (1) |
| selinexor/pomalidomide/dexamethasone | 2.2% (1) |
| Not documented | 15.6% (7) |
| Median number of LOTs (IQR) | 7 (4–15) |
| Selinexor starting dose | |
| ≤60 mg | 13.3% (6) |
| 70 mg | 2.2% (1) |
| 80 mg | 60% (27) |
| 100 mg | 13.3% (6) |
| 160 mg | 11.1% (5) |
| Median duration of therapy (IQR)—months | 2.7 (0.7–11.3) |
| Median duration of therapy when used in bridging (IQR)—months | 2.6 (2–6.5) |
| Median duration of therapy when not used in bridging (IQR)—months | 2.9 (0.7–10.8) |
| Median PFS with selinexor-based therapy (IQR)—months | 2.3 (1–13.3) |
| Median time from last dose of selinexor to CAR-T infusion—months | 3.9 (0.7–22) |
| Selinexor given as part of bridging regimen before CAR-T | 24.4% (11) |
| Selinexor given as part of the LOT immediately preceding CAR-T | 44.4% (20) |
| Parameter | n = 45 |
|---|---|
| Product | |
| Idecabtagene vicleucel | 60% (27) |
| Ciltacabtagene autoleucel | 35.6% (16) |
| CC-98633/BMS-986354 | 4.4% (2) |
| Prior selinexor exposure | 100% (45) |
| Median number of LOTs (IQR) | 9 (6–15) |
| Bridging regimen administered | 68.9% (31) |
| Median duration of bridging therapy (IQR)—days | 21 (4–43) |
| Parameter | n = 45 |
|---|---|
| Hematology Parameters (mean; std dev) | |
| Platelets at baseline—K/L | 133 (84) |
| Platelets at day 30—K/L | 65.3 (52.0) |
| Platelets at day 100—K/L | 126 (74.9) |
| Hemoglobin at baseline—g/dL | 10.0 (1.9) |
| Hemoglobin at day 30—g/dL | 9.1 (1.9) |
| Hemoglobin at day 100—g/dL | 10.7 (1.9) |
| Absolute neutrophil count at baseline—K/L | 3.0 (1.9) |
| Absolute neutrophil count at day 30—K/L | 1.3 (1.06) |
| Absolute neutrophil count at day 100—K/L | 2.4 (1.2) |
| Best response to CAR-T therapy | |
| sCR | 33.3% (15) |
| CR | 20% (9) |
| VGPR | 26.7% (12) |
| PR | 8.9% (4) |
| SD | 8.9% (4) |
| PD | 2.2% (1) |
| Median time to best response (IQR)—months | 1.8 (0.8–4.5) |
| Median duration of response (IQR)—months | 8.1 (2.6–39) |
| CRS events (any grade) | 75.6% (34) |
| Grade of CRS | |
| Grade 1 | 26 |
| Grade 2 | 8 |
| Median duration of CRS (IQR)—days | 2 days (1–7) |
| ICANS events (any grade) | 17.8% (8) |
| Grade of ICANS | |
| Grade 1 | 5 |
| Grade 2 | 1 |
| Grade 3 | 2 |
| Median duration of ICANS (IQR)—days | 1 (1–4) |
| Variable 1 | Hazard Ratio 2 | (95% CI) | Impact on Risk of Progression |
|---|---|---|---|
| Selinexor used in line immediately prior to CAR-T | 0.40 | (0.14–1.09) | ↓ by 60% |
| ECOG PS: ≥1 vs. 0 | 2.30 | (0.88–6.10) | ↑ 2.3 times |
| EMD present prior to CAR-T therapy | 3.54 | (1.26–9.92) | ↑ 3.5 times |
| Male gender | 0.46 | (0.19–1.12) | ↓ by 54% |
| Time from the last dose of selinexor to CAR-T (mon) | 0.94 | (0.89–1.00) | ↓ by 6% for each additional month |
| Variable 1 | Hazard Ratio 2 | (95% CI) | Impact on Risk of Death |
|---|---|---|---|
| Selinexor used in line immediately prior to CAR-T | 0.08 | (0.02–0.46) | ↓ by 92% |
| Age ≥ 60 years | 3.83 | (0.94–15.5) | ↑ 3.8 times |
| Albumin level (g/dL) prior to CAR-T | 0.16 | (0.04–0.62) | ↓ by 84% risk with higher levels |
| Time from the last dose of selinexor to CAR-T (mon) | 0.86 | (0.74–0.98) | ↓ by 14% for each additional month |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, B.A.; Dima, D.; Mark, T.; Sadek, N.L.; Ijioma, S.; Ray, D.; Goel, U.; Dranitsaris, G.; Sheng, T.; Moshier, E.; et al. Impact of Prior Selinexor Exposure on Outcomes of Chimeric Antigen Receptor T-Cell Therapy for Relapsed/Refractory Multiple Myeloma: An Exploratory Analysis. J. Clin. Med. 2025, 14, 1316. https://doi.org/10.3390/jcm14041316
Costa BA, Dima D, Mark T, Sadek NL, Ijioma S, Ray D, Goel U, Dranitsaris G, Sheng T, Moshier E, et al. Impact of Prior Selinexor Exposure on Outcomes of Chimeric Antigen Receptor T-Cell Therapy for Relapsed/Refractory Multiple Myeloma: An Exploratory Analysis. Journal of Clinical Medicine. 2025; 14(4):1316. https://doi.org/10.3390/jcm14041316
Chicago/Turabian StyleCosta, Bruno Almeida, Danai Dima, Tomer Mark, Norah Layla Sadek, Stephen Ijioma, David Ray, Utkarsh Goel, George Dranitsaris, Tianxiang Sheng, Erin Moshier, and et al. 2025. "Impact of Prior Selinexor Exposure on Outcomes of Chimeric Antigen Receptor T-Cell Therapy for Relapsed/Refractory Multiple Myeloma: An Exploratory Analysis" Journal of Clinical Medicine 14, no. 4: 1316. https://doi.org/10.3390/jcm14041316
APA StyleCosta, B. A., Dima, D., Mark, T., Sadek, N. L., Ijioma, S., Ray, D., Goel, U., Dranitsaris, G., Sheng, T., Moshier, E., Mouhieddine, T. H., Khouri, J., & Rossi, A. (2025). Impact of Prior Selinexor Exposure on Outcomes of Chimeric Antigen Receptor T-Cell Therapy for Relapsed/Refractory Multiple Myeloma: An Exploratory Analysis. Journal of Clinical Medicine, 14(4), 1316. https://doi.org/10.3390/jcm14041316








