Long-Term Analysis of Suicide Incidence Among Patients with Lung Cancer: A Population-Based Longitudinal Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources and Study Population
2.2. Definition of Cancer, Suicide, and Covariates
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Study Populations
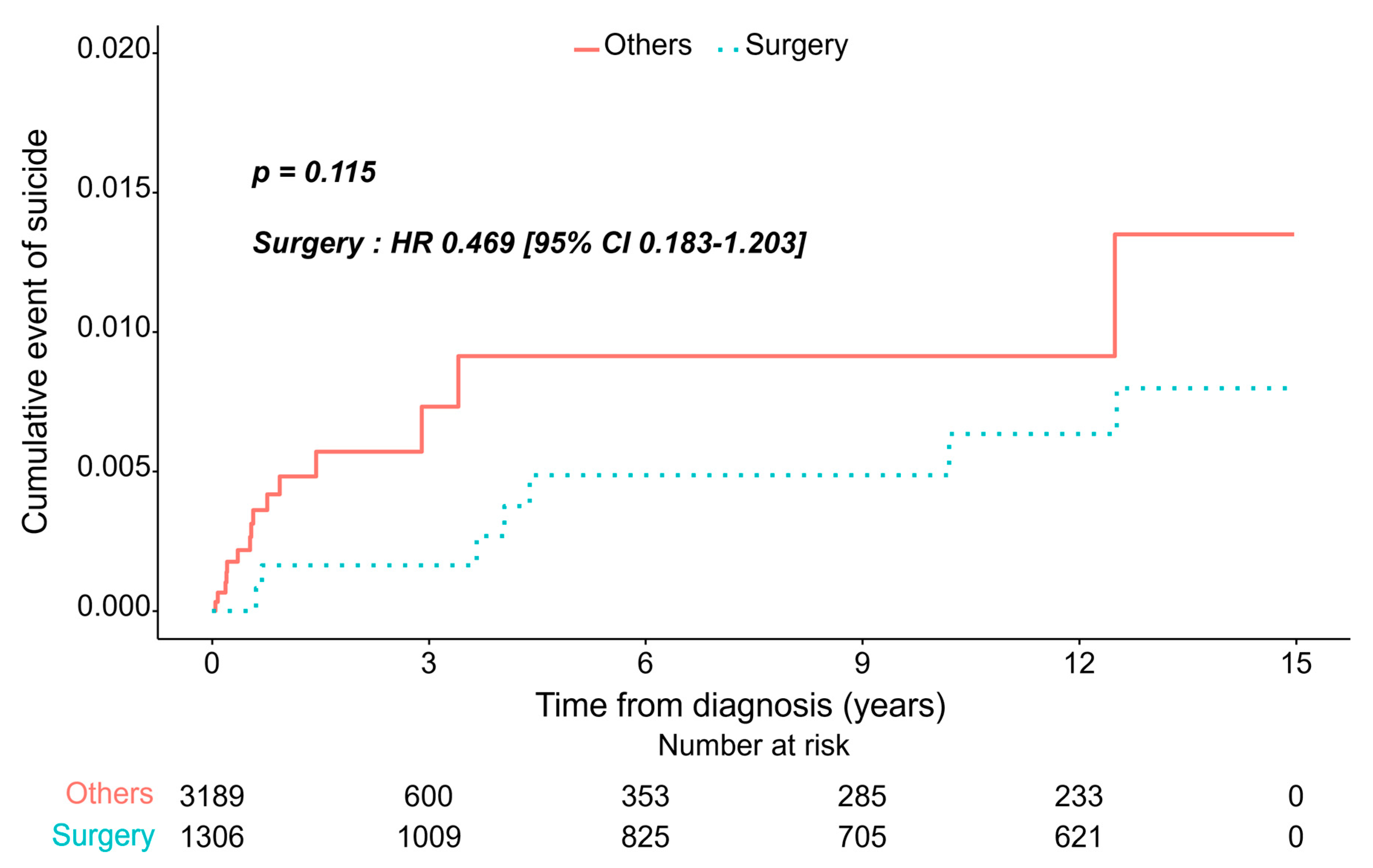
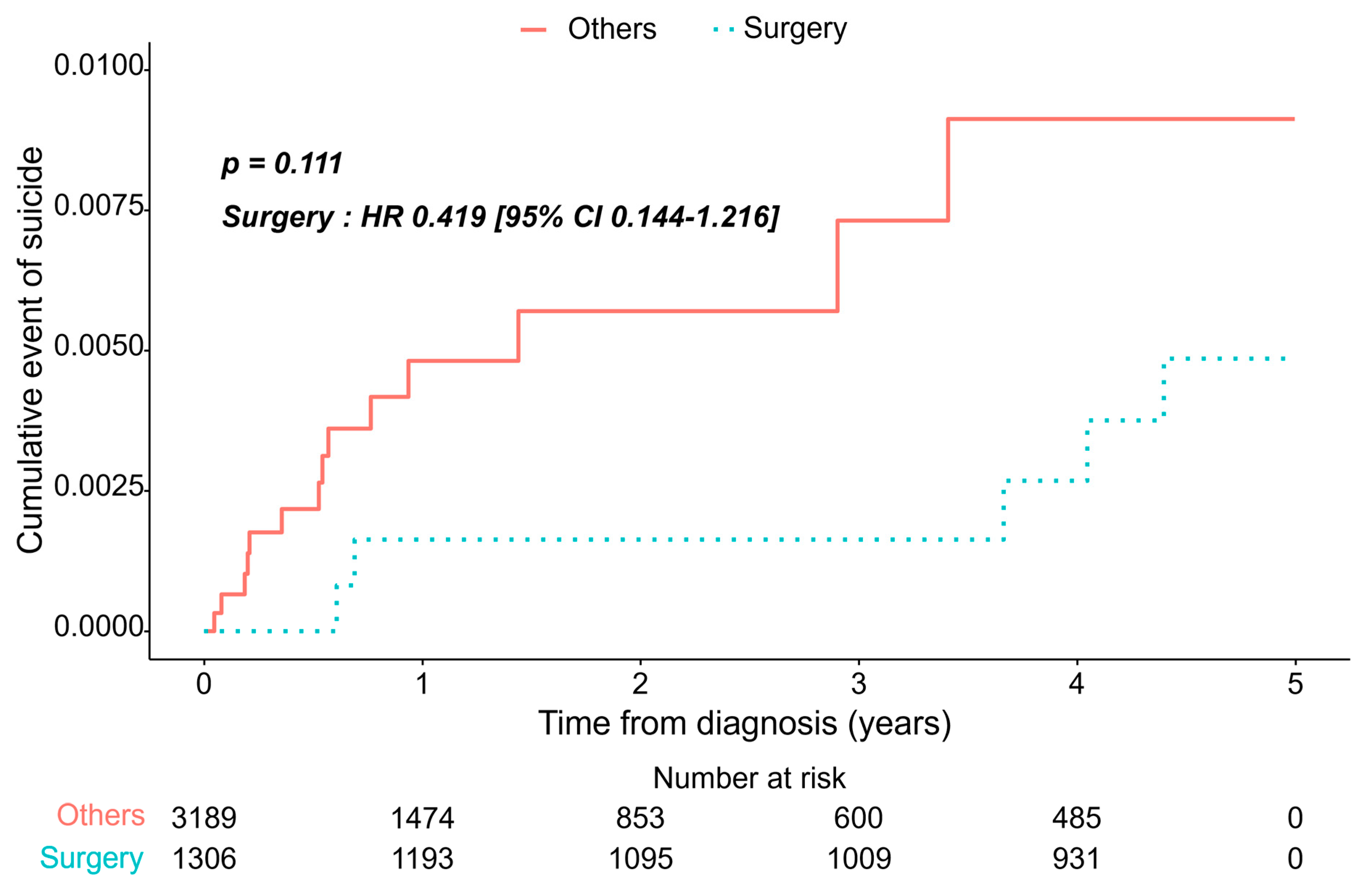
3.2. Incidence of Depression/Suicide Between Two Groups
3.3. Risk Model Prediction of Suicide
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luo, G.; Zhang, Y.; Rumgay, H.; Morgan, E.; Langselius, O.; Vignat, J.; Colombet, M.; Bray, F. Estimated Worldwide Variation and Trends in Incidence of Lung Cancer by Histological Subtype in 2022 and over Time: A Population-Based Study. Lancet Respir. Med. 2025, 13, 348–363. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Z.; Wang, J.; Liu, K.; Wu, J.; Ge, Y.; Zhu, G.; Cao, L.; Ma, X.; Li, J. Global, Regional, and National Burden of Tracheal, Bronchus, and Lung Cancer and Its Risk Factors from 1990 to 2021: Findings from the Global Burden of Disease Study 2021. eClinicalMedicine 2024, 75, 102804. [Google Scholar] [CrossRef]
- Cancer of the Lung and Bronchus—Cancer Stat Facts. Available online: https://seer.cancer.gov/statfacts/html/lungb.html (accessed on 29 April 2025).
- Jung, K.-W.; Won, Y.-J.; Kang, M.J.; Kong, H.-J.; Im, J.-S.; Seo, H.G. Prediction of Cancer Incidence and Mortality in Korea, 2022. Cancer Res. Treat. 2022, 54, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Tsuboi, M.; Herbst, R.S.; John, T.; Kato, T.; Majem, M.; Grohé, C.; Wang, J.; Goldman, J.W.; Lu, S.; Su, W.-C.; et al. Overall Survival with Osimertinib in Resected EGFR-Mutated NSCLC. N. Engl. J. Med. 2023, 389, 137–147. [Google Scholar] [CrossRef]
- Gandhi, L.; Rodríguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; De Angelis, F.; Domine, M.; Clingan, P.; Hochmair, M.J.; Powell, S.F.; et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 2078–2092. [Google Scholar] [CrossRef]
- Saji, H.; Okada, M.; Tsuboi, M.; Nakajima, R.; Suzuki, K.; Aokage, K.; Aoki, T.; Okami, J.; Yoshino, I.; Ito, H.; et al. Segmentectomy versus Lobectomy in Small-Sized Peripheral Non-Small-Cell Lung Cancer (JCOG0802/WJOG4607L): A Multicentre, Open-Label, Phase 3, Randomised, Controlled, Non-Inferiority Trial. Lancet 2022, 399, 1607–1617. [Google Scholar] [CrossRef]
- Wang, T.; Molassiotis, A.; Chung, B.P.M.; Tan, J.-Y. Unmet Care Needs of Advanced Cancer Patients and Their Informal Caregivers: A Systematic Review. BMC Palliat. Care 2018, 17, 96. [Google Scholar] [CrossRef] [PubMed]
- Shakeel, S.; Tung, J.; Rahal, R.; Finley, C. Evaluation of Factors Associated With Unmet Needs in Adult Cancer Survivors in Canada. JAMA Netw. Open 2020, 3, e200506. [Google Scholar] [CrossRef]
- Hartung, T.J.; Brähler, E.; Faller, H.; Härter, M.; Hinz, A.; Johansen, C.; Keller, M.; Koch, U.; Schulz, H.; Weis, J.; et al. The Risk of Being Depressed Is Significantly Higher in Cancer Patients than in the General Population: Prevalence and Severity of Depressive Symptoms across Major Cancer Types. Eur. J. Cancer 2017, 72, 46–53. [Google Scholar] [CrossRef]
- Zaorsky, N.G.; Churilla, T.M.; Egleston, B.L.; Fisher, S.G.; Ridge, J.A.; Horwitz, E.M.; Meyer, J.E. Causes of Death among Cancer Patients. Ann. Oncol. 2017, 28, 400–407. [Google Scholar] [CrossRef]
- Pringle, B.; Colpe, L.J.; Heinssen, R.K.; Schoenbaum, M.; Sherrill, J.T.; Claassen, C.A.; Pearson, J.L. A Strategic Approach for Prioritizing Research and Action to Prevent Suicide. Psychiatr. Serv. 2013, 64, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Andersen, B.L.; Rowland, J.H.; Somerfield, M.R. Screening, Assessment, and Care of Anxiety and Depressive Symptoms in Adults with Cancer: An American Society of Clinical Oncology Guideline Adaptation. J. Oncol. Pract. 2015, 11, 133–134. [Google Scholar] [CrossRef]
- Holland, J.C.; Bultz, B.D. National comprehensive Cancer Network (NCCN) The NCCN Guideline for Distress Management: A Case for Making Distress the Sixth Vital Sign. J. Natl. Compr. Cancer Netw. 2007, 5, 3–7. [Google Scholar] [CrossRef]
- Potter, A.L.; Haridas, C.; Neumann, K.; Kiang, M.V.; Fong, Z.V.; Riddell, C.A.; Pope, H.G.; Yang, C.-F.J. Incidence, Timing, and Factors Associated With Suicide Among Patients Undergoing Surgery for Cancer in the US. JAMA Oncol. 2023, 9, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, L.; Heinrich, M.; Baurecht, H.; Langguth, B.; Kreuzer, P.M.; Knüttel, H.; Leitzmann, M.F.; Seliger, C. Suicide Mortality Risk among Patients with Lung Cancer-A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2023, 20, 4146. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Zhang, W.-Q.; Hu, S.-Q.; Shen, W.-Q.; Chen, H.-L. Incidence and Risk Factors of Suicide in Patients with Lung Cancer: A Scoping Review. Support. Care Cancer 2022, 30, 2945–2957. [Google Scholar] [CrossRef]
- Boyes, A.W.; Girgis, A.; D’Este, C.A.; Zucca, A.C.; Lecathelinais, C.; Carey, M.L. Prevalence and Predictors of the Short-Term Trajectory of Anxiety and Depression in the First Year After a Cancer Diagnosis: A Population-Based Longitudinal Study. J. Clin. Oncol. 2013, 31, 2724–2729. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, D.R.; Forsberg, C.W.; Ganzini, L.; Au, D.H.; Gould, M.K.; Provenzale, D.; Slatore, C.G. Longitudinal Changes in Depression Symptoms and Survival Among Patients with Lung Cancer: A National Cohort Assessment. J. Clin. Oncol. 2016, 34, 3984–3991. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, S.; Peng, P.; Ma, F.; Tang, F. Prediction of Risk of Suicide Death among Lung Cancer Patients after the Cancer Diagnosis. J. Affect. Disord. 2021, 292, 448–453. [Google Scholar] [CrossRef]
- Rahouma, M.; Kamel, M.; Abouarab, A.; Eldessouki, I.; Nasar, A.; Harrison, S.; Lee, B.; Shostak, E.; Morris, J.; Stiles, B.; et al. Lung Cancer Patients Have the Highest Malignancy-Associated Suicide Rate in USA: A Population-Based Analysis. ecancermedicalscience 2018, 12, 859. [Google Scholar] [CrossRef]
- Zaorsky, N.G.; Zhang, Y.; Tuanquin, L.; Bluethmann, S.M.; Park, H.S.; Chinchilli, V.M. Suicide among Cancer Patients. Nat. Commun. 2019, 10, 207. [Google Scholar] [CrossRef]
- Li, B.; Evans, D.; Faris, P.; Dean, S.; Quan, H. Risk Adjustment Performance of Charlson and Elixhauser Comorbidities in ICD-9 and ICD-10 Administrative Databases. BMC Health Serv. Res. 2008, 8, 12. [Google Scholar] [CrossRef]
- Laor, A.; Tal, S.; Guller, V.; Zbar, A.P.; Mavor, E. The Charlson Comorbidity Index (CCI) as a Mortality Predictor after Surgery in Elderly Patients. Am. Surg. 2016, 82, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.; Bernardini, J.; Piraino, B. Charlson Comorbidity Index as a Predictor of Outcomes in Incident Peritoneal Dialysis Patients. Am. J. Kidney Dis. 2001, 37, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Frenkel, W.J.; Jongerius, E.J.; Mandjes-van Uitert, M.J.; van Munster, B.C.; de Rooij, S.E. Validation of the Charlson Comorbidity Index in Acutely Hospitalized Elderly Adults: A Prospective Cohort Study. J. Am. Geriatr. Soc. 2014, 62, 342–346. [Google Scholar] [CrossRef] [PubMed]
- Lung Cancer Survival Rates|5-Year Survival Rates for Lung Cancer. Available online: https://www.cancer.org/cancer/types/lung-cancer/detection-diagnosis-staging/survival-rates.html (accessed on 30 April 2025).
- Nassiri, F.; Price, B.; Shehab, A.; Au, K.; Cusimano, M.D.; Jenkinson, M.D.; Jungk, C.; Mansouri, A.; Santarius, T.; Suppiah, S.; et al. Life after Surgical Resection of a Meningioma: A Prospective Cross-Sectional Study Evaluating Health-Related Quality of Life. Neuro Oncol. 2019, 21, i32–i43. [Google Scholar] [CrossRef]
- Rausei, S.; Mangano, A.; Galli, F.; Rovera, F.; Boni, L.; Dionigi, G.; Dionigi, R. Quality of Life after Gastrectomy for Cancer Evaluated via the EORTC QLQ-C30 and QLQ-STO22 Questionnaires: Surgical Considerations from the Analysis of 103 Patients. Int. J. Surg. 2013, 11 (Suppl. S1), S104–S109. [Google Scholar] [CrossRef]
- Rhoten, B.A.; Murphy, B.; Ridner, S.H. Body Image in Patients with Head and Neck Cancer: A Review of the Literature. Oral. Oncol. 2013, 49, 753–760. [Google Scholar] [CrossRef]
- Avery, K.N.L.; Blazeby, J.M.; Chalmers, K.A.; Batchelor, T.J.P.; Casali, G.; Internullo, E.; Krishnadas, R.; Evans, C.; West, D. Impact on Health-Related Quality of Life of Video-Assisted Thoracoscopic Surgery for Lung Cancer. Ann. Surg. Oncol. 2020, 27, 1259–1271. [Google Scholar] [CrossRef]
- Jeantieu, M.; Gaillat, F.; Antonini, F.; Azoulay, E.; Martin, C.; Thomas, P.; Leone, M. Postoperative Pain and Subsequent PTSD-Related Symptoms in Patients Undergoing Lung Resection for Suspected Cancer. J. Thorac. Oncol. 2014, 9, 362–369. [Google Scholar] [CrossRef]
- Merlo, A.; Carlson, R.; Espey, J.; Williams, B.M.; Balakrishnan, P.; Chen, S.; Dawson, L.; Johnson, D.; Brickey, J.; Pompili, C.; et al. Postoperative Symptom Burden in Patients Undergoing Lung Cancer Surgery. J. Pain Symptom Manag. 2022, 64, 254–267. [Google Scholar] [CrossRef] [PubMed]
- Rauma, V.; Sintonen, H.; Räsänen, J.V.; Salo, J.A.; Ilonen, I.K. Long-Term Lung Cancer Survivors Have Permanently Decreased Quality of Life after Surgery. Clin. Lung Cancer 2015, 16, 40–45. [Google Scholar] [CrossRef]
- Kissane, D.W.; Grabsch, B.; Love, A.; Clarke, D.M.; Bloch, S.; Smith, G.C. Psychiatric Disorder in Women with Early Stage and Advanced Breast Cancer: A Comparative Analysis. Aust. N. Z. J. Psychiatry 2004, 38, 320–326. [Google Scholar] [CrossRef]
- Shi, Y.; Cai, J.; Wu, Z.; Jiang, L.; Xiong, G.; Gan, X.; Wang, X. Effects of a Nurse-Led Positive Psychology Intervention on Sexual Function, Depression and Subjective Well-Being in Postoperative Patients with Early-Stage Cervical Cancer: A Randomized Controlled Trial. Int. J. Nurs. Stud. 2020, 111, 103768. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.M.; Colebaugh, C.A.; Flowers, K.M.; Edwards, R.R.; Partridge, A.H.; Dominici, L.S.; Schreiber, K.L. Early Postoperative Psychological Distress as a Mediator of Subsequent Persistent Postsurgical Pain Outcomes among Younger Breast Cancer Patients. Breast Cancer Res. Treat. 2022, 196, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Vodermaier, A.; Linden, W.; MacKenzie, R.; Greig, D.; Marshall, C. Disease Stage Predicts Post-Diagnosis Anxiety and Depression Only in Some Types of Cancer. Br. J. Cancer 2011, 105, 1814–1817. [Google Scholar] [CrossRef] [PubMed]
- Song, I.-A.; Park, H.Y.; Oh, T.K. Effect of Preoperative Psychiatric Morbidity on Postoperative Outcomes of Lung Cancer Surgery: A Nationwide Cohort Study in South Korea. J. Psychosom. Res. 2022, 161, 111002. [Google Scholar] [CrossRef] [PubMed]
- Barberan-Garcia, A.; Ubré, M.; Roca, J.; Lacy, A.M.; Burgos, F.; Risco, R.; Momblán, D.; Balust, J.; Blanco, I.; Martínez-Pallí, G. Personalised Prehabilitation in High-Risk Patients Undergoing Elective Major Abdominal Surgery: A Randomized Blinded Controlled Trial. Ann. Surg. 2018, 267, 50. [Google Scholar] [CrossRef]
- Cho, A.-R.; Najafi, T.; Ramanakumar, A.V.; Ferri, L.; Spicer, J.; Najmeh, S.; Cools-Lartigue, J.; Sirois, C.; Soh, S.; Kim, D.J.; et al. The Effect of Multimodal Prehabilitation on Postoperative Outcomes in Lung Cancer Surgery. J. Thorac. Cardiovasc. Surg. 2025, 169, 1631–1644.e2. [Google Scholar] [CrossRef]
- Molenaar, C.J.L.; Minnella, E.M.; Coca-Martinez, M.; ten Cate, D.W.G.; Regis, M.; Awasthi, R.; Martínez-Palli, G.; López-Baamonde, M.; Sebio-Garcia, R.; Feo, C.V.; et al. Effect of Multimodal Prehabilitation on Reducing Postoperative Complications and Enhancing Functional Capacity Following Colorectal Cancer Surgery: The PREHAB Randomized Clinical Trial. JAMA Surg. 2023, 158, 572–581. [Google Scholar] [CrossRef]
- Sebio-Garcia, R.; Celada-Castro, C.; Arguis, M.J.; Sisó, M.; Torné, A.; Tena, B.; Díaz-Feijoo, B.; Martinez-Palli, G. Multimodal Prehabilitation Improves Functional Capacity in Patients with Advanced Ovarian Cancer Undergoing Cytoreductive Surgery. Int. J. Gynecol. Cancer 2024, ijgc–2024–005686. [Google Scholar] [CrossRef] [PubMed]
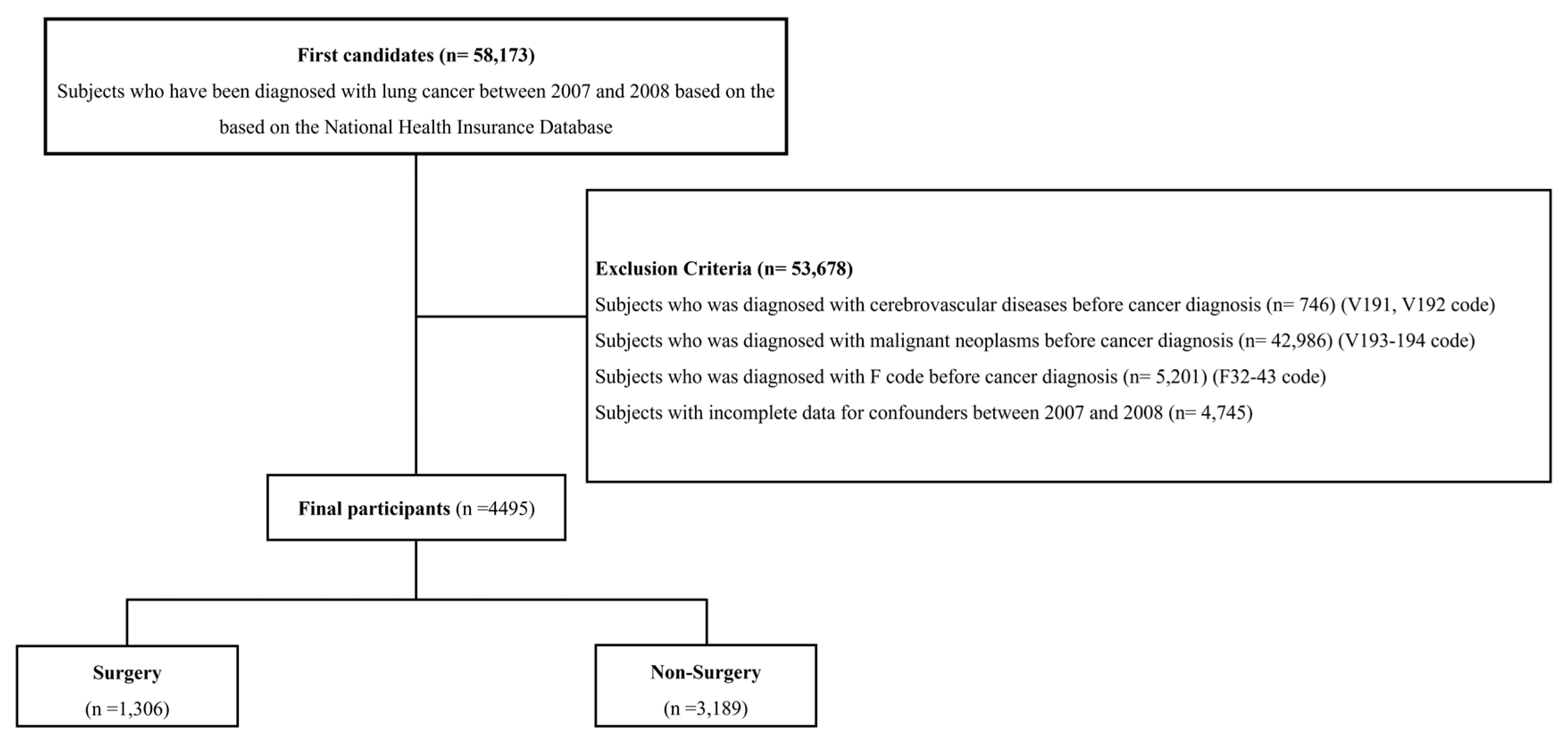
| Surgical Resection | Others | p-Value | |
|---|---|---|---|
| Clinical Variables | (n = 1306) | (n = 3189) | |
| Age, years | 63.4 (±9.1) | 68.0 (±10.1) | <0.001 |
| <50 | 103 (7.9%) | 177 (5.6%) | |
| 50–59 | 305 (23.3%) | 455 (14.3%) | |
| 60–69 | 548 (42.0%) | 942 (29.5%) | |
| ≥70 | 350 (26.8%) | 1615 (50.6%) | |
| BMI, kg/m2 | 23.5 (±3.0) | 22.9 (±3.1) | <0.001 |
| Systolic Blood pressure, mmHg | 126 (±16) | 129 (±17) | <0.001 |
| Fasting blood sugar, mg/dL | 101.4 (±28.1) | 103.7 (±35.6) | 0.034 |
| Total cholesterol, mg/dL | 192.5 (±35.8) | 190.3 (±39.2) | 0.126 |
| SGPT, U/L | 24.0 (±16.8) | 23.8 (±19.3) | 0.538 |
| Charlson comorbidity index | <0.001 | ||
| 2 | 350 (26.8%) | 562 (17.6%) | |
| 3 or more | 956 (73.2%) | 2627 (82.4%) | |
| Life-style related factors | |||
| Alcohol consumption | <0.001 | ||
| Rare | 97 (7.4%) | 90 (2.8%) | |
| Moderate | 992 (76.0%) | 2506 (78.6%) | |
| Heavy | 217 (16.6%) | 593 (18.6%) | |
| Smoking history | <0.001 | ||
| Never smoker | 692 (53.0%) | 1523 (47.8%) | |
| Former smoker | 218 (16.7%) | 455 (14.3%) | |
| Current smoker | 396 (30.3%) | 1211 (38.0%) | |
| Physical activity | <0.001 | ||
| Low | 24 (1.8%) | 30 (0.9%) | |
| Moderate | 1057 (80.9%) | 2784 (87.3%) | |
| High | 225 (17.2%) | 375 (11.8%) | |
| Income level | <0.001 | ||
| Low (0–6) | 246 (18.8%) | 676 (21.2%) | |
| Middle (7–13) | 360 (27.6%) | 1020 (32.0%) | |
| High (13–20) | 700 (53.6%) | 1493 (46.8%) | |
| Suicide | 0.035 | ||
| No | 1303 (99.8%) | 3164 (99.2%) | |
| Yes | 3 (0.2%) | 25 (0.8%) |
| Surgery | Others | p-Value | |
|---|---|---|---|
| (n = 1306) | (n = 3189) | ||
| Model1: adjusted for age, sex | |||
| Hazard ratio (95% CI) | 0.469 (0.183–1.203) | 1 (Ref.) | 0.115 |
| Model 2: adjusted for BMI, SBP, Total cholesterol, Fasting blood glucose, SGOT, in addition to variables in Model 1 | |||
| Hazard ratio (95% CI) | 0.481 (0.184–1.256) | 1 (Ref.) | 0.135 |
| Model 3: CCI, alcohol consumption, smoking history, physical activity, income levels, and depression history, in addition to variables in Model 2 | |||
| Hazard ratio (95% CI) | 0.489 (0.185–1.291) | 1 (Ref.) | 0.222 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, E.; Woo, W.; Lee, S.; Kang, H.-T. Long-Term Analysis of Suicide Incidence Among Patients with Lung Cancer: A Population-Based Longitudinal Study. J. Clin. Med. 2025, 14, 4070. https://doi.org/10.3390/jcm14124070
Kim E, Woo W, Lee S, Kang H-T. Long-Term Analysis of Suicide Incidence Among Patients with Lung Cancer: A Population-Based Longitudinal Study. Journal of Clinical Medicine. 2025; 14(12):4070. https://doi.org/10.3390/jcm14124070
Chicago/Turabian StyleKim, Eunjoo, Wongi Woo, Sungsoo Lee, and Hee-Taik Kang. 2025. "Long-Term Analysis of Suicide Incidence Among Patients with Lung Cancer: A Population-Based Longitudinal Study" Journal of Clinical Medicine 14, no. 12: 4070. https://doi.org/10.3390/jcm14124070
APA StyleKim, E., Woo, W., Lee, S., & Kang, H.-T. (2025). Long-Term Analysis of Suicide Incidence Among Patients with Lung Cancer: A Population-Based Longitudinal Study. Journal of Clinical Medicine, 14(12), 4070. https://doi.org/10.3390/jcm14124070






