Treatment Modalities for Angina with Non-Obstructive Coronary Arteries (ANOCA): A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Eligibility Criteria
2.2. Outcome Measures
2.2.1. Primary Outcome Measure
2.2.2. Secondary Outcome Measures
2.3. Search Strategies
2.3.1. Database Search
2.3.2. Study Selection and Screening
2.4. Data Extraction
- -
- Study characteristics: author, publication year, study design, enrolment period and follow-up duration.
- -
- Participants’ characteristics: total study population, age of the study population, gender, method used to diagnose ANOCA or, if prior to 2020, ‘normal coronary arteries and angina’ and endotype.
- -
- Intervention description: type of treatment modality (decision set) and comparator treatment modality (including placebo or baseline; supplementary set).
2.5. Assessment of Risk of Bias in Included Studies
2.6. Measurement of Effect and Data Synthesis
2.7. Confidence in Cumulative Evidence
3. Results
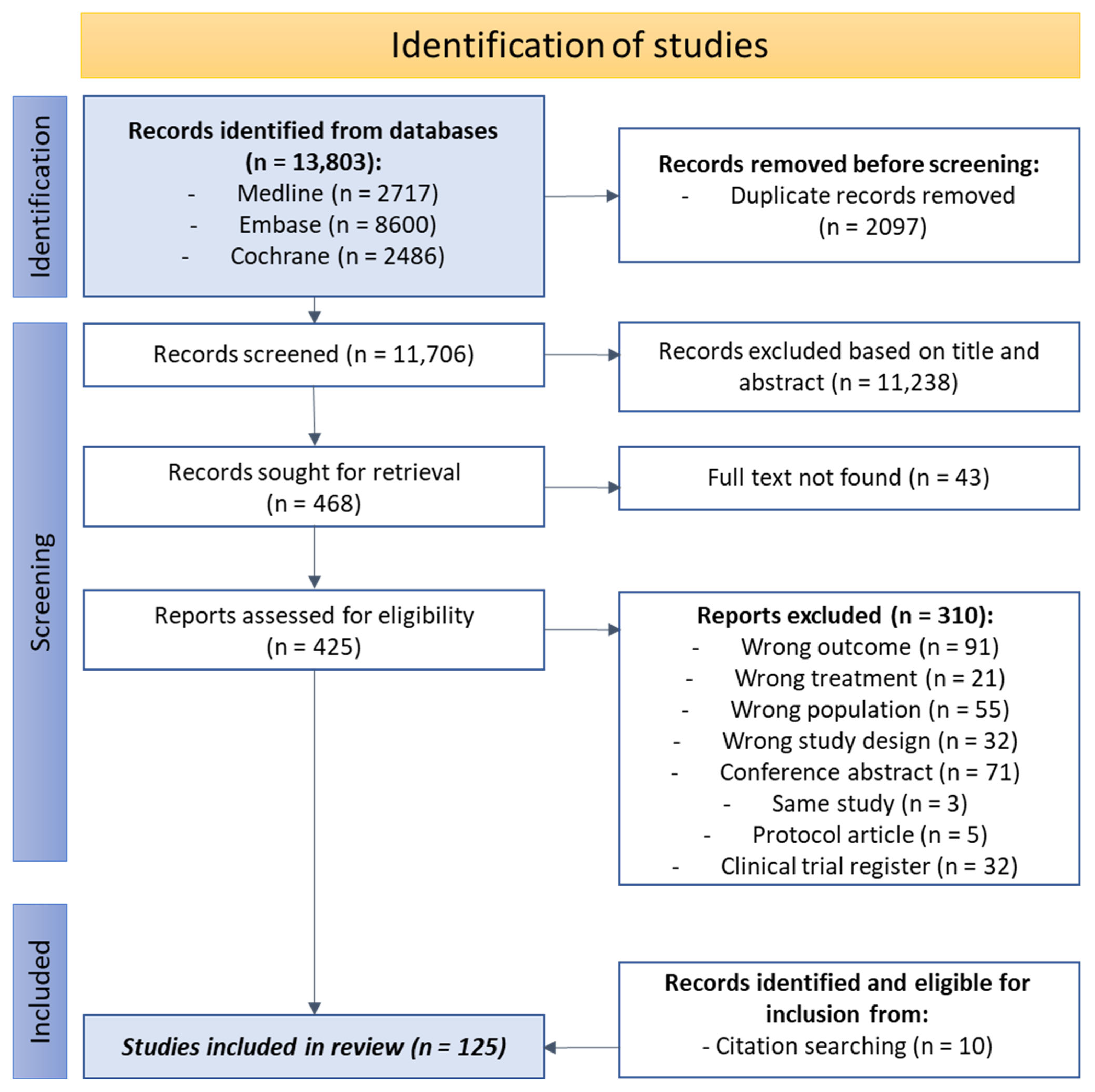
3.1. Literature Search and Study Characteristics
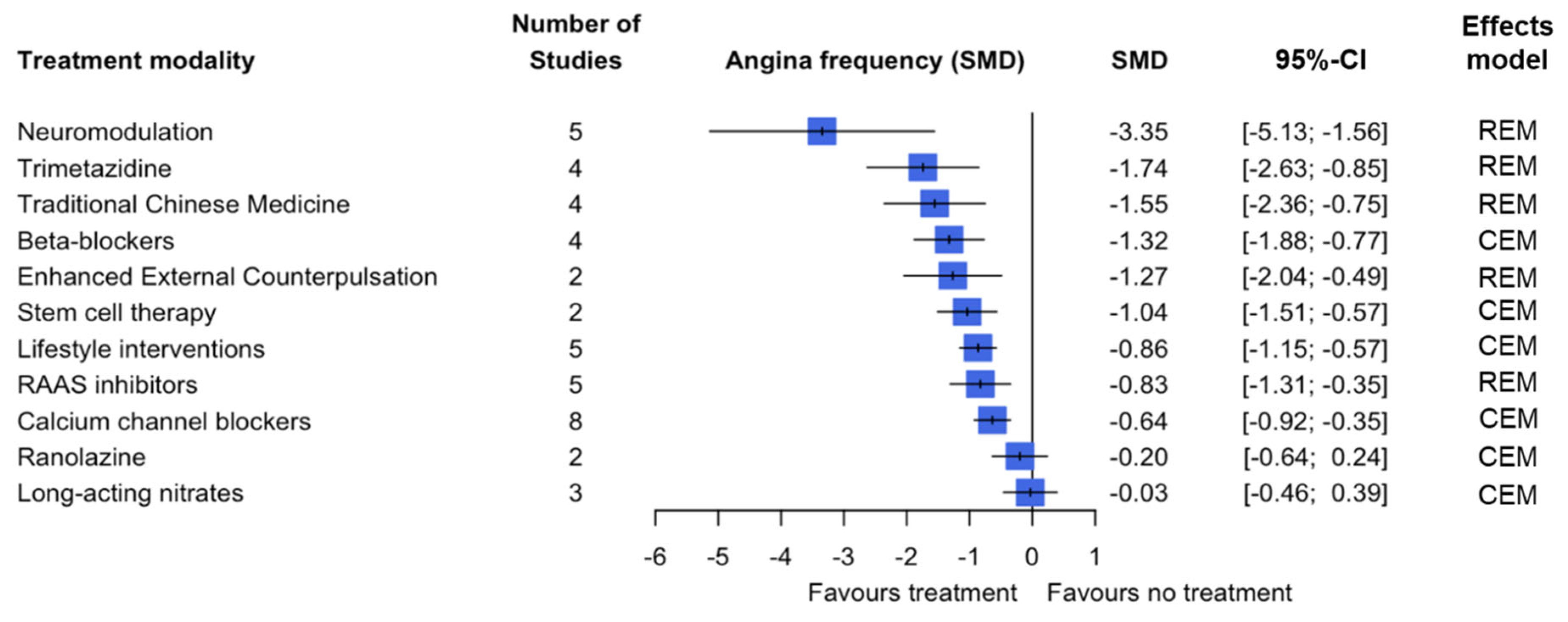
3.2. Primary Outcome
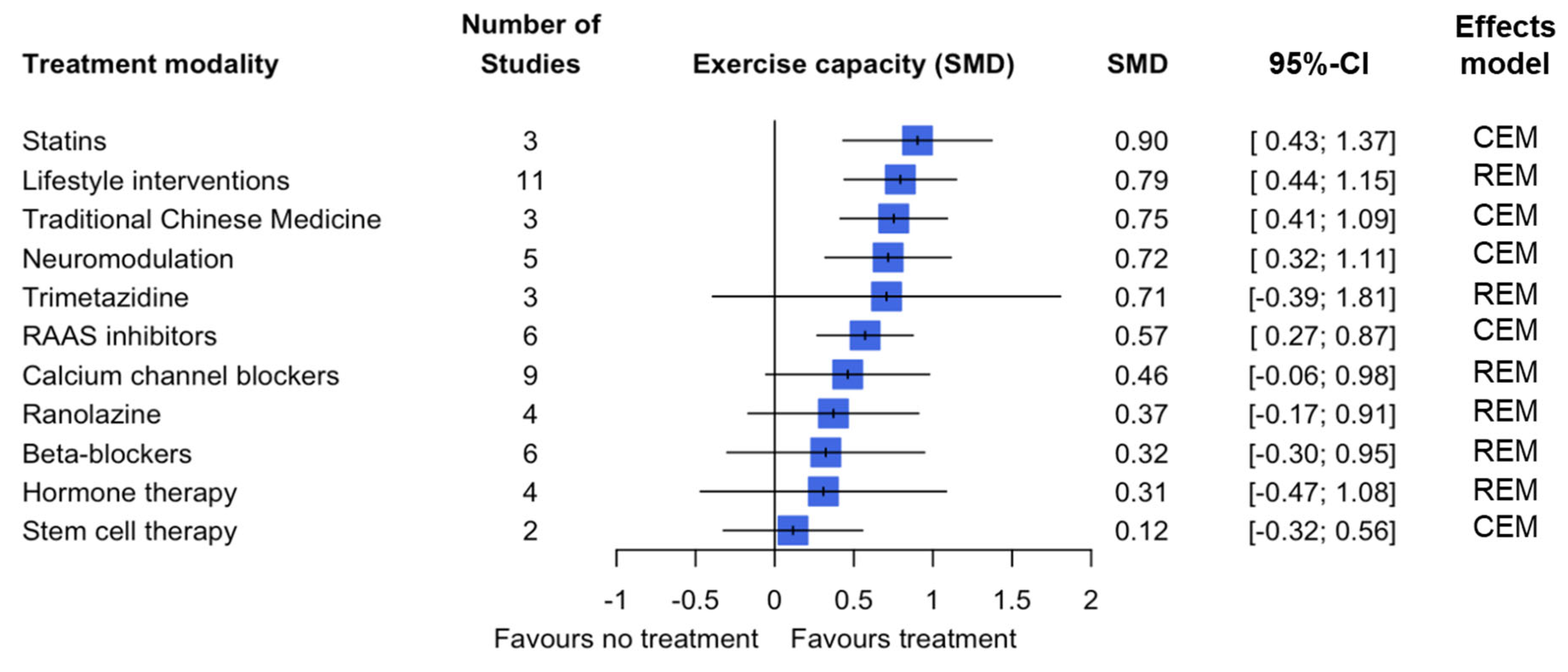
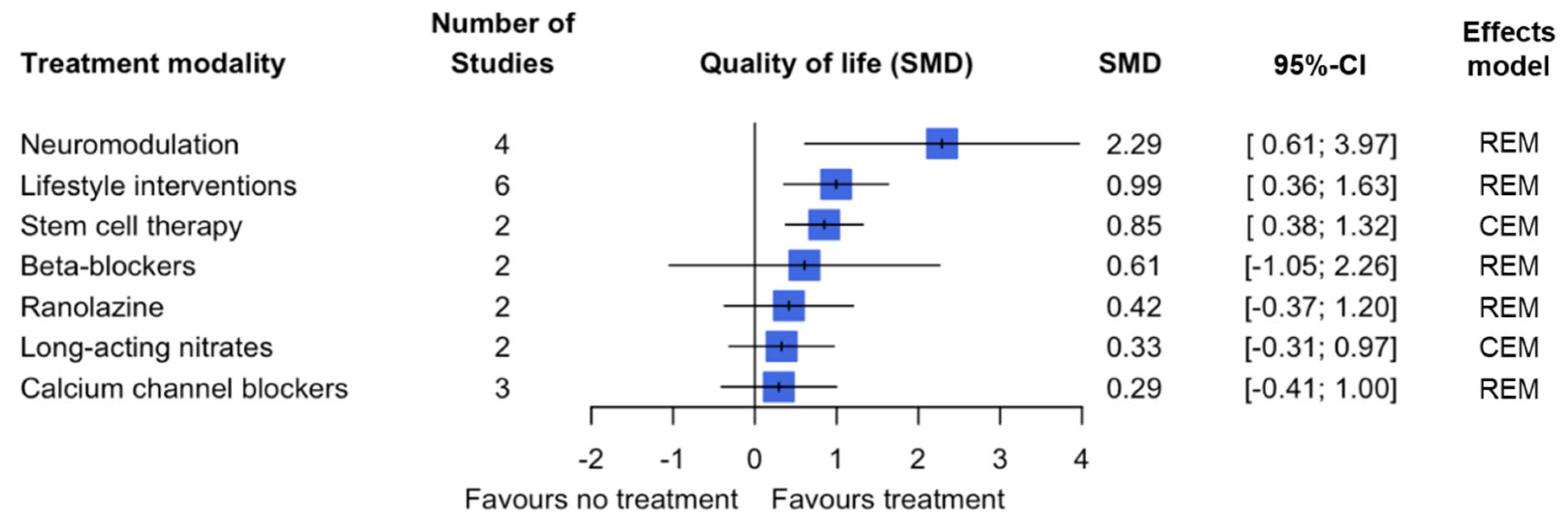
3.3. Secondary Outcomes
3.4. Publication Bias Assessment
3.5. Subgroup Analysis
3.6. Sensitivity Analysis
3.7. Confidence in Cumulative Evidence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Patel, M.R.; Peterson, E.D.; Dai, D.; Brennan, J.M.; Redberg, R.F.; Anderson, H.V.; Brindis, R.G.; Douglas, P.S. Low diagnostic yield of elective coronary angiography. N. Engl. J. Med. 2010, 362, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Jansen, T.P.J.; Konst, R.E.; de Vos, A.; Paradies, V.; Teerenstra, S.; van den Oord, S.C.H.; Dimitriu-Leen, A.; Maas, A.H.E.M.; Smits, P.C.; Damman, P.; et al. Efficacy of Diltiazem to Improve Coronary Vasomotor Dysfunction in ANOCA. J. Am. Coll. Cardiol. Imaging 2022, 15, 1473–1484. [Google Scholar] [CrossRef] [PubMed]
- Kunadian, V.; Chieffo, A.; Camici, P.G.; Berry, C.; Escaned, J.; Maas, A.H.E.M.; Prescott, E.; Karam, N.; Appelman, Y.; Fraccaro, C.; et al. An EAPCI Expert Consensus Document on Ischaemia with Non-Obstructive Coronary Arteries in Collaboration with European Society of Cardiology Working Group on Coronary Pathophysiology & Microcirculation Endorsed by Coronary Vasomotor Disorders International Study Group. Eur. Heart J. 2020, 41, 3504–3520. [Google Scholar]
- Maddox, T.M.; Stanislawski, M.A.; Grunwald, G.K.; Bradley, S.M.; Ho, M.; Tsai, T.T.; Patel, M.R.; Sandhu, A.; Valle, J.; Magid, D.J.; et al. Nonobstructive Coronary Artery Disease and Risk of Myocardial Infarction. JAMA 2014, 213, 1754–1763. [Google Scholar] [CrossRef]
- Tavella, R.; Cutri, N.; Tucker, G.; Adams, R.; Spertus, J.; Beltrame, J.F. Natural history of patients with insignificant coronary artery disease. Eur. Heart J. Qual. Care Clin. Outcomes 2016, 2, 117–124. [Google Scholar] [CrossRef]
- Jespersen, L.; Hvelplund, A.; Adildstrøm, S.Z.; Pedersen, F.; Galatius, S.; Madsen, J.K.; Jørgensen, F.; Kelbæk, H.; Prescott, E. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur. Heart J. 2012, 33, 734–744. [Google Scholar] [CrossRef] [PubMed]
- Andreotti, F.; Koskinas, K.C.; Rossello, X.; Adamo, M.; Ainslie, J.; Banning, A.P.; Budaj, A.; Buechel, R.R.; Chiariello, G.A.; Chieffo, A.; et al. 2024 ESC Guidelines for the management of chronic coronary syndromes. Eur. Heart J. 2024, 45, 3415–3537. [Google Scholar]
- Ford, T.; Berry, C. How to diagnose and manage angina without obstructive coronary artery disease; Lessons from the British Heart Foundation CorMicA trial. Interv. Cardiol. Rev. 2019, 14, 76–82. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 6.4 (Updated August 2023); Cochrane: London, UK, 2023; Available online: www.training.cochrane.org/handbook (accessed on 5 December 2024).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality If Nonrandomized Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm (accessed on 5 December 2024).
- Sinha, A.; Rahman, H.; Douiri, A.; Demir, O.M.; De Silva, K.; Clapp, B.; Webb, I.; Gulati, A.; Pinho, P.; Dutta, U.; et al. ChaMP-CMD: A Phenotype-Blinded, Randomized Controlled, Cross-Over Trial. Circulation 2024, 149, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Kook, H.; Hong, S.J.; Yang, K.S.; Lee, S.; Kim, J.S.; Park, C.G. Comparison of nebivolol versus diltiazem in improving coronary artery spasm and quality of life in patients with hypertension and vasospastic angina: A prospective, randomized, double-blind pilot study. PLoS ONE 2020, 15, e0239039. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Q.; Zhao, J.; Li, X.; Sun, X.; Yang, H.; Wu, Z.; Yang, J. Effects of combination of statin and calcium channel blocker in patients with cardiac syndrome X. Coron. Artery Dis. 2014, 25, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Oikawa, Y.; Matsuno, S.; Yajima, J.; Nakamura, M.; Ono, T.; Ishiwata, S.; Fujimoto, Y.; Aizawa, T. Effects of treatment with once-daily nifedipine CR and twice-daily benidipine on prevention of symptomatic attacks in patients with coronary spastic angina pectoris–Adalat Trial vs. Coniel in Tokyo against Coronary Spastic Angina (ATTACK CSA). J. Cardiol. 2010, 55, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Özçelik, F.; Altun, A.; Özbay, G. Antianginal and Anti-Ischemic Effects of Nisoldipine and Ramipril in Patients with Syndrome X. Clin. Cardiol. 1999, 22, 361–365. [Google Scholar] [CrossRef]
- Lanza, G.A.; Colonna, G.; Pasceri, V.; Maseri, A. Atenolol Versus Amlodipine Versus Isosorbide-5-Mononitrate on Anginal Symptoms in Syndrome X. Am. J. Cardiol. 1999, 84, 854–856. [Google Scholar] [CrossRef]
- Vogt, M.; Motz, W.; Strauer, B.E. Antihypertensive Long-term Therapy with Isradipine; Improvement of coronary flow reserve in patients with arterial and microvascular angina. Arzneimittelforschung 1994, 44, 1321–1328. [Google Scholar]
- Cannon, R.O.; Brush, J.E.; Schenke, W.H.; Tracy, C.M.; Epstein, S.E. Beneficia land Detrimental Effects of Lidoflazine in Microvascular Angina. Am. J. Cardiol. 1990, 66, 37–41. [Google Scholar] [CrossRef]
- Romeo, F.; Gaspardone, A.; Ciavolella, M.; Gioffrè, P.; Reale, A. Verapamil Versus Acebutolol for Syndrome X. Am. J. Cardiol. 1988, 62, 312–313. [Google Scholar] [CrossRef]
- Prida, X.E.; Gelman, J.S.; Feldman, R.L.; Hill, J.A.; Pepine, C.J.; Scott, E. Comparison of Diltiazem and Nifedipine Alone and in Combination in Patients with Coronary Artery Spasm. J. Am. Coll. Cardiol. 1987, 9, 412–419. [Google Scholar] [CrossRef]
- Kugiyama, K.; Yasue, H.; Horio, Y.; Morikami, Y.; Fujii, H.; Koga, Y.; Kojima, A.; Takahashi, M. Effects of propranolol and nifedipine on exercise-induced attack in patients with variant angina: Assessment by exercise thallium-201 myocardial scintigraphy with quantitative rotational tomography. Circulation 1986, 74, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Gelman, J.S.; Feldman, R.L.; Scott, E.; Pepine, C.J. Nicardipine for Angina Pectoris at Rest and Coronary Arterial Spasm. Am. J. Cardiol. 1985, 56, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Cannon, R.O.; Watson, R.M.; Rosing, D.R.; Epstein, S.E. Efficacy of Calcium Channel Blocker Therapy for Angina Pectoris Resulting from Small-Vessel Coronary Artery Disease and Abnormal Vasodilator Reserve. Am. J. Cardiol. 1985, 56, 242–246. [Google Scholar] [CrossRef]
- Pitcher, D.; Sowton, E. Double-blind trial of nifedipine in angina patients with normal coronary arteries. Pharmatherapeutica 1981, 3, 6–13. [Google Scholar]
- Freedman, B.; Dunn, R.F.; Richmond, D.R.; Kelly, D.T. Coronary Artery Spasm During Exercise: Treatment with Verapamil. Circulation 1981, 64, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Sugisawa, J.; Matsumoto, Y.; Takeuchi, M.; Suda, A.; Tsuchiya, S.; Ohyama, K.; Nishimiya, K.; Akizuki, M.; Sato, K.; Ohura, S.; et al. Beneficial effects of exercise training on physical performance in patients with vasospastic angina. Int. J. Cardiol. 2021, 328, 14–21. [Google Scholar] [CrossRef]
- Bove, K.B.; Nilsson, M.; Pedersen, L.R.; Mikkelsen, N.; Suhrs, H.E.; Astrup, A.; Prescott, E. Comprehensive treatment of microvascular angina in overweight women—A randomized controlled pilot trial. PLoS ONE 2020, 15, e0240722. [Google Scholar] [CrossRef]
- Rahmani, R.; Niyazi, S.; Sobh-Rakhshankhah, A.; Guazzi, M.; Mazaheri, R.; Hashemi, N.; Khoddami-Vishteh, H.R. Effects of a Cardiac Rehabilitation Program Versus Usual Care on Cardiopulmonary Function in Patients with Cardiac Syndrome X. J. Cardiopulm. Rehabil. Prev. 2020, 40, 41–47. [Google Scholar] [CrossRef]
- Szot, W.; Zajac, J.; Kubinyi, A.; Kostkiewicz, M. The effects of cardiac rehabilitation on overall physical capacity and myocardial perfusion in women with microvascular angina. Kardiol. Pol. 2016, 74, 431–438. [Google Scholar] [CrossRef]
- De Carvalho, E.E.V.; Santi, G.L.; Crescêncio, J.C.; de Oliveira, L.F.L.; Reis, D.C.C.; Figueiredo, A.B.; Pintya, A.O.; Lima-Filho, M.O.; Gallo-Júnior, L.; Marin-Neto, J.A.; et al. Pilot study testing the effect of physical training over the myocardial perfusion and quality of life in patients with primary microvascular angina. J. Nucl. Cardiol. 2015, 22, 130–137. [Google Scholar] [CrossRef]
- Feizi, A.; Ghaderi, C.; Dehghani, M.R.; Khalkhali, H.R.; Sheikhi, S. Effect of phase III cardiac rehabilitation and relaxation on the quality of life in patients with cardiac syndrome X. Iran. J. Nurs. Midwifery Res. 2012, 17, 547–552. [Google Scholar]
- Asbury, E.A.; Kanji, N.; Ernst, E.; Barbir, M.; Collins, P. Autogenic training to manage symptomology in women with chest pain and normal coronary arteries. Menopause 2009, 16, 60–65. [Google Scholar] [CrossRef]
- Asbury, E.A.; Slattery, C.; Grant, A.; Evans, L.; Barbir, M.; Collins, P. Cardiac rehabilitation for the treatment of women with chest pain and normal coronary arteries. Menopause 2008, 15, 454–460. [Google Scholar] [CrossRef]
- Tyni-Lenne, R.; Stryjan, S.; Eriksson, B.; Berglund, M.; Sylven, C. Beneficial therapeutic effects of physical training and relaxation therapy in women with coronary syndrome X. Physiother. Res. Int. 2002, 7, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, C.; Brown, S.; Kaski, J.C. Effects of Transcendental Meditation on Symptoms and Electrocardiographic Changes in Patients with Cardiac Syndrome X. Am. J. Cardiol. 2000, 85, 653–655. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, B.E.; Tyni-Lenne, R.; Svedenhag, J.; Hallin, R.; Jensen-Urstad, K.; Jensen-Urstad, M.; Bergman, K.; Sylvén, C. Physical Training in Syndrome X: Physical Training Counteracts Deconditioning and Pain in Syndrome X. J. Am. Coll. Cardiol. 2000, 36, 1619–1625. [Google Scholar] [CrossRef] [PubMed]
- Michelsen, M.M.; Rask, A.B.; Suhrs, E.; Raft, K.F.; Høst, N.; Prescott, E. Effect of ACE-inhibition on coronary microvascular function and symptoms in normotensive women with microvascular angina: A randomized placebo-controlled trial. PLoS ONE 2018, 13, e0196962. [Google Scholar] [CrossRef]
- Bavry, A.A.; Handberg, E.M.; Huo, T.; Lerman, A.; Quyyumi, A.A.; Shufelt, C.; Sharaf, B.; Merz, C.N.B.; Cooper-DeHoff, R.; Sopko, G.; et al. Aldosterone inhibition and coronary endothelial function in women without obstructive coronary artery disease: An ancillary study of the National Heart, Lung, and Blood Institute-sponsored Women’s Ischemia Syndrome Evaluation. Am. Heart J. 2014, 167, 826–832. [Google Scholar] [CrossRef]
- Pauly, D.F.; Johnson, B.D.; Anderson, R.D.; Handberg, E.M.; Smith, K.M.; Cooper-DeHoff, R.M.; Sopko, G.; Sharaf, B.M.; Kelsey, S.F.; Merz, C.N.B.; et al. In women with symptoms of cardiac ischemia, nonobstructive coronary arteries, and microvascular dysfunction, angiotensin-converting enzyme inhibition is associated with improved microvascular function: A double-blind randomized study from the National Heart, Lung and Blood Institute Women’s Ischemia Syndrome Evaluation (WISE). Am. Heart J. 2011, 162, 678–684. [Google Scholar]
- Pizzi, C.; Manfrini, O.; Fontana, F.; Bugiardini, R. Angiotensin-Converting Enzyme Inhibitors and 3-Hydroxy-3-Methylglutaryl Coenzyme A Reductase in Cardiac Syndrome X; Role of Superoxide Dismutase Activity. Circulation 2004, 109, 53–58. [Google Scholar] [CrossRef]
- Chen, J.W.; Hsu, N.W.; Wu, T.C.; Lin, S.J.; Chang, M.S. Long-term Angiotensin-Converting Enzyme Inhibition Reduces Plasma Asymmetric Dimethylarginine and Improves Endothelial Nitric Oxide Bioavailability and Coronary Microvascular Function in Patients with Syndrome X. Am. J. Cardiol. 2002, 90, 974–982. [Google Scholar] [CrossRef] [PubMed]
- Kanadaşi, M.; Demir, M.; Demirtaş, M.; Akpinar, O.; Alhan, C.C. Effects of Lisinopril, Atenolol, and Isosorbide 5-Mononitrate on Angina Pectoris and QT Dispersion in Patients with Syndrome X: An Open-Label, Randomized, Crossover Study. Curr. Ther. Res. 2002, 63, 273–283. [Google Scholar] [CrossRef]
- Nalbantgil, I.; Önder, R.; Altintig, A.; Nalbantgil, S.; Kiliçcioglu, B.; Boydak, B.; Yilmaz, H. Therapeutic Benefits of Cilazapril in Patients with Syndrome X. Cardiology 1998, 89, 130–133. [Google Scholar] [CrossRef]
- Motz, W.; Strauer, B.E. Improvement of Coronary Flow Reserve After Long-term Therapy with Enalapril. Hypertension 1996, 27, 1031–1038. [Google Scholar] [CrossRef]
- Kaski, J.C.; Rosano, G.; Gavrielides, S.; Chen, L. Effects of Angiotensin-Converting Enzyme Inhibition on Exercise-Induced Angina and ST Segment Depression in Patients with Microvascular Angina. J. Am. Coll. Cardiol. 1994, 23, 652–657. [Google Scholar] [CrossRef]
- Erdamar, H.; Sen, N.; Tavil, Y.; Yazici, H.U.; Turfan, M.; Poyraz, F.; Topal, S.; Okuyan, H.; Cemri, M.; Cengel, A. The effect of nebivolol treatment on oxidative stress and antioxidant status in patients with cardiac syndrome-X. Coron. Art. Dis. 2009, 20, 238–244. [Google Scholar] [CrossRef]
- Şen, N.; Tavil, Y.; Erdamar Yazici, H.U.; Cakir, E.; Akgül, E.Ö.; Bilgi, C.; Erbil, M.K.; Poyraz, F.; Okyay, K.; Turfan, M.; et al. Nebivolol therapy improves endothelial function and increases exercise tolerance in patients with cardiac syndrome X. Anadolu Kardiyol. Derg. 2009, 9, 371–379. [Google Scholar] [PubMed]
- Suzuki, J.; Watanabe, K.; Tsuruoka, T.; Sueda, S.; Funada, J.; Kitakaze, M.; Sekiya, M. Beneficial effects of betaxolol, a selective antagonist of beta-1 adrenoreceptors, on exercise-induced myocardial ischemia in patients with coronary vasospasm. Int. J. Cardiol. 2003, 91, 227–232. [Google Scholar] [CrossRef]
- Leonardo, F.; Fragasso, G.; rossetti, E.; Dabrowski, P.; Pagnotta, P.; Rosano, G.M.C.; Chierchia, S.L. Comparison of trimetazidine with atenolol in patients with syndrome X: Effects on diastolic function and exercise tolerance. Cardiologia 1999, 44, 1065–1069. [Google Scholar]
- Shimizu, H.; Lee, J.D.; Ogawa, K.B.; Shimizu, K.; Yamamoto, M.; Hara, A.; Nakamura, T. Efficacy of denopamine, a β1 adrenoceptor agonist, in preventing coronary artery spasm. Jpn. Circ. J. 1993, 57, 175–182. [Google Scholar] [CrossRef]
- Lim, Y.; Kim, M.C.; Ahn, Y.; Cho, K.H.; Sim, D.S.; Hong, Y.J.; Kim, J.H.; Jeong, M.H.; Baek, S.H.; Her, S.H.; et al. Prognostic Impact of Chronic Vasodilator Therapy in Patients with Vasospastic Angina. J. Am. Heart Assoc. 2022, 11, e023776. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Park, T.K.; Cho, S.W.; Oh, M.S.; Lee, D.H.; Seong, C.S.; Gwag, H.B.; Lim, A.Y.; Yang, J.H.; Song, Y.B.; et al. Impact of different nitrate therapies on long-term clinical outcomes of patients with vasospastic angina: A propensity score-matched analysis. Int. J. Cardiol. 2018, 252, 1–5. [Google Scholar] [CrossRef]
- Takahashi, J.; Nihei, T.; Takagi, Y.; Miyata, S.; Odaka, Y.; Tsunoda, R.; Seki, A.; Sumiyoshi, T.; Matsui, M.; Goto, T.; et al. Prognostic impact of chronic nitrate therapy in patients with vasospastic angina: Multicentre registry study of the Japanese coronary spasm association. Eur. Heart J. 2015, 36, 228–237. [Google Scholar] [CrossRef]
- Wu, M.; Villano, A.; Russo, G.; Di Franco, A.; Stazi, A.; Lauria, C.; Sestito, A.; Lanza, G.A.; Crea, F. Poor Tolerance and Limited Effects of Isosorbide-5-Mononitrate in Microvascular Angina. Cardiology 2015, 130, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Kosugi, M.; Nakagomi, A.; Shibui, T.; Kato, K.; Kusama, Y.; Atarashi, H.; Mizuno, K. Effect of Long-Term Nitrate Treatment on Cardiac Events in Patients with Vasospastic Angina. Circ. J. 2011, 75, 2196–2205. [Google Scholar] [CrossRef]
- Lee, Y.; hung, M.; Chen, T.; Mao, C.; Yeh, C.; Kounis, N.G.; Chen, I.Y.; Hu, P.; Hung, M. Effects of statins in patients with coronary artery spasm: A nationwide population-based study. Clin. Transl. Sci. 2024, 17, e70087. [Google Scholar] [CrossRef]
- Mori, H.; Takahashi, J.; Sato, K.; Miyata, S.; Takagi, Y.; Tsunoda, R.; Sumiyoshi, T.; Matsui, M.; Tanabe, Y.; Sueda, S.; et al. Impact of statins in patients with vasospastic angina: A multicenter registry study of the Japanese Coronary Spasm Association. J. Cardiol. 2022, 80, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Seo, W.W.; Jo, S.H.; Kim, S.E.; Han, S.H.; Lee, K.Y.; Her, S.H.; Lee, M.H.; Cho, S.S.; Baek, S.H. Clinical impact of statin therapy on vasospastic angina: Data from a Korea nation-wide cohort study. Heart Vessel. 2020, 35, 1051–1059. [Google Scholar] [CrossRef]
- Ishii, M.; Kaikita, K.; Sato, K.; Yamanaga, K.; Miyazaki, T.; Akasaka, T.; Tabata, N.; Arima, Y.; Sueta, D.; Sakamoto, K.; et al. Impact of Statin Therapy on Clinical Outcome in Patients with Coronary Spasm. J. Am. Heart Assoc. 2016, 5, e003426. [Google Scholar] [CrossRef]
- Oh, M.S.; Yang, J.H.; Lee, D.H.; Park, T.K.; Song, Y.B.; Hahn, J.Y.; Choi, J.H.; Lee, S.H.; Gwon, H.C.; Choi, S.H. Impact of statin therapy on long-term clinical outcomes of vasospastic angina without significant stenosis: A propensity-score matched analysis. Int. J. Cardiol. 2016, 223, 791–796. [Google Scholar] [CrossRef]
- Kayikcioglu, M.; Payzin, S.; Yavuzgil, O.; Kultursay, H.; Can, L.H.; Soydan, I. Benefits of statin treatment in cardiac syndrome-X. Eur. Heart J. 2003, 24, 1999–2005. [Google Scholar] [CrossRef] [PubMed]
- De Vries, J.; DeJongste, M.J.L.; Durenkamp, A.; Zijlstra, F.; Staal, M.J. The sustained benefits of long-term neurostimulation in patients with refractory chest pain and normal coronary arteries. Eur. J. Pain. 2007, 11, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Sgueglia, G.A.; Sestito, A.; Spinelli, A.; Cioni, B.; Infusino, F.; Papacci, F.; Bellocci, F.; Meglio, M.; Crea, F.; Lanza, G.A. Long-Term Follow-up of Cardiac Syndrome X Patients Treated by Spinal Cord Stimulation. Heart 2007, 93, 591–597. [Google Scholar] [CrossRef]
- Jessurun, G.A.J.; Hautvast, R.W.M.; Tio, R.A.; DeJongste, M.J.L. Electrical neuromodulation improves myocardial perfusion and ameliorates refractory angina pectoris in patients with syndrome X: Fad or future? Eur. J. Pain. 2003, 7, 507–512. [Google Scholar] [CrossRef]
- Lanza, G.A.; Sestito, A.; Sgueglia, G.A.; Infusino, F.; Papacci, F.; Visocchi, M.; Ierardi, C.; meglio, M.; Belloccci, F.; Crea, F. Effect of spinal cord stimulation on spontaneous and stress-induced angina and ‘ischemia-like’ ST-segment depression in patients with cardiac syndrome X. Eur. Heart J. 2005, 26, 983–989. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lanza, G.A.; Sestito, A.; Sandric, S.; Cioni, B.; Tamburrini, G.; Barollo, A.; Crea, F.; De Seta, F.; Meglio, M.; Bellocci, F.; et al. Spinal cord stimulation in patients with refractory anginal pain and normal coronary arteries. Ital. Heart J. 2001, 2, 25–30. [Google Scholar]
- Eliasson, T.; Albertsson, P.; Hardhammar, P.; Emanuelsson, H.; Augustinsson, L.E.; Mannheimer, C. Spinal cord stimulation in angina pectoris with normal coronary arteriograms. Coron. Art. Dis. 1993, 4, 819–827. [Google Scholar]
- Birkeland, K.; Khandwalla, R.M.; Kedan, I.; Shufelt, C.L.; Mehta, P.K.; Minissian, M.B.; Wei, J.; Handberg, E.M.; Thomson, L.E.J.; Berman, D.S.; et al. Daily Activity Measured with Wearable Technology as a Novel Measurement of Treatment Effect in Patients with Coronary Microvascular Dysfunction: Substudy of a Randomized Controlled Crossover Trial. JMIR Res. Protoc. 2017, 6, e255. [Google Scholar] [CrossRef]
- Ahmed, B.; Mondragon, J.; Sheldon, M.; Clegg, S. Impact of ranolazine on coronary microvascular dysfunction (MICRO) study. Cardiovasc. Revasc Med. 2017, 18, 431–435. [Google Scholar] [CrossRef]
- Safdar, B.; DÓnofrio, G.; Dziura, J.; Russell, R.R.; Johnson, C.; Sinusas, A.J. Ranolazine and Microvascular Angina by PET in the Emergency Department: Results from a Pilot Randomized Controlled Trial. Clin. Ther. 2017, 39, 55–63. [Google Scholar] [CrossRef]
- Merz, C.N.B.; Handberg, E.M.; Shufelt, C.L.; Mehta, P.K.; Minissian, M.B.; Wei, J.; Thomson, L.E.J.; Berman, D.S.; Shaw, L.J.; Petersen, J.W.; et al. A randomized, placebo-controlled trial of late Na current inhibition (ranolazine) in coronary microvascular dysfunction (CMD): Impact on angina and myocardial perfusion reserve. Eur. Heart J. 2016, 37, 1504–1513. [Google Scholar] [CrossRef] [PubMed]
- Villano, A.; Di Franco, A.; Nerla, R.; Sestito, A.; Tarzia, P.; Lamendola, P.; Di Monaco, A.; Sarullo, F.M.; Lanza, G.A.; Crea, F. Effects of Ivabradine and Ranolazine in patients with Microvascular Angina Pectoris. Am. J. Cardiol. 2013, 112, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Boldueva, S.A.; Leonova, I.A.; Zakharova, O.V. Efficacy of Trimetazidine and Sulodexide in Patients with Microvascular Angina. Ration. Pharmacother. Cardiol. 2020, 16, 363–369. [Google Scholar] [CrossRef]
- Galin, P.Y.; Gubanova, T.G. Clinical effectiveness of the modified release trimetazidine in microvascular angina. Russ. J. Cardiol. 2016, 12, 84–89. [Google Scholar] [CrossRef][Green Version]
- Rogacka, D.; Guzik, P.; Wykretowicz, A.; Rzezniczak, J.; Dziarmaga, M.; Wysocki, H. Effects of trimetazidine on clinical symptoms and tolerance of exercise of patients with syndrome X: A preliminary study. Coron. Art. Dis. 2000, 11, 171–177. [Google Scholar] [CrossRef]
- Nalbantgil, S.; Altintig, A.; Yilmaz, H.; Nalbantgil, I.; Önder, R. The Effect of Trimetazidine in the Treatment of Microvascular Angina. Int. J. Angiol. 1999, 8, 40–43. [Google Scholar] [CrossRef]
- Noroozi, S.; Karimi, M.; Farahani, A.V.; Omidi, N.; Zargaran, A.; Soleymani, S.; Alaeddini, F.; Rezaeizadeh, H. Efficacy of Chamomile-Lemon Balm Syrup in Patients with Conventional Drug-Resistant Cardiac Syndrome X: A Single-Arm Clinical Trial. Cresent J. Med. Biol. Sci. 2023, 10, 73–80. [Google Scholar] [CrossRef]
- Ma, X.; Yang, D.; Shen, W.; Liang, W.; Lin, Q. Therapeutic effect of Xuezhitong capsule on microvascular angina. Trop. J. Pharm. Res. 2021, 20, 1991–1997. [Google Scholar] [CrossRef]
- Cao, G.; Wang, W.; Ni, Y.; Wu, H. Clinical trial of Wenxin granules combined with aspirin for the treatment of cardiac syndrome X. Asian J. Surg. 2021, 44, 1486–1487. [Google Scholar] [CrossRef]
- Li, J.J.; Wang, Y.; Nie, S.P.; Li, Q.; Li, Y.S.; Huang, Y.; Hui, R.T. Xuezhikang, an extract of cholestin, decreases plasma inflammatory markers and endothelin-1, improve exercise-induced ischemia and subjective feelings in patients with cardiac syndrome X. Int. J. Cardiol. 2007, 122, 82–84. [Google Scholar] [CrossRef]
- Mao, J.Y.; Ge, Y.B.; Wang, H.H.; Wang, Q.; Zhang, Y.; Yu, D.L.; Zhang, Y.; Huang, Q.; Zhao, Z.Q.; Zhao, G.F.; et al. Summary of 32 Patients with Cardiac Syndrome X Treated by TCM Therapy of Regulating Qi Relieving Chest Stuffiness and Promoting Blood Circulation. Chin. J. Integr. Med. 2007, 13, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Merz, C.N.B.; Olson, M.B.; McClure, C.; Yang, Y.C.; Symons, J.; Sopko, G.; Kelsey, S.F.; Handberg, E.; Johnson, B.D.; Cooper-DeHoff, R.M.; et al. A randomized controlled trial of low-dose hormone therapy on myocardial ischemia in postmenopausal women with no obstructive coronary artery disease: Results from the National Institutes of Health/National Heart, Lung, and Blood Institute-sponsored Women’s Ischemia Syndrome Evaluation (WISE). Am. Heart J. 2010, 159, 987.e1–987.e7. [Google Scholar] [PubMed]
- Adamson, D.L.; Webb, C.M.; Collins, P. Esterified estrogens combined with methyltestosterone improve emotional well-being in postmenopausal women with chest pain and normal coronary angiograms. Menopause 2001, 4, 233–238. [Google Scholar] [CrossRef]
- Rosano, G.M.C.; Peters, N.S.; Lefroy, D.; Lindsay, D.C.; Sarrel, P.M.; Collins, P.; Poole-Wilson, P.A. 17-Beta-Estradiol Therapy Lessens Angina in Postmenopausal Women with Syndrome X. J. Am. Coll. Cardiol. 1996, 28, 1500–1505. [Google Scholar]
- Albertsson, P.A.; Emanuelsson, H.; Milsom, I. Beneficial effect of treatment with transdermal estradiol-17-β on exercise-induced angina and ST segment depression in syndrome X. Int. J. Cardiol. 1996, 54, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Henry, T.D.; Merz, C.N.B.; Wei, J.; Corban, M.T.; Quesada, O.; Joung, S.; Kotynski, C.L.; Wang, J.; Lewis, M.; Schumacher, A.M.; et al. Autologous CD34+ Stem Cell Therapy Increases Coronary Flow Reserve and Reduces Angina in Patients with Coronary Microvascular Dysfunction. Circ. Cardiovasc. Interv. 2022, 15, e010802. [Google Scholar] [CrossRef]
- Corban, M.T.; Toya, T.; Albers, D.; Sebaali, F.; Lewis, B.R.; Bois, J.; Gulati, R.; Prasad, A.; Best, P.J.M.; Bell, M.R.; et al. IMRPOvE-CED Trial: Intracoronary Autologous CD34+ Cell Therapy for Treatment of Coronary Endothelial Dysfunction in Patients with Angina and Nonobstructive Coronary Arteries. Circ. Res. 2022, 130, 326–338. [Google Scholar] [CrossRef]
- Ashokprabhu, N.D.; Fox, J.; Henry, T.D.; Schmidt, C.W.; Tierney, D.; Alvarez, Y.R.; Thompson, L.; Hamstra, M.; Shah, S.A.; Quesada, O. Enhanced External Counterpulsation for the Treatment of Angina with Nonobstructive Coronary Artery Disease. Am. J. Cardiol. 2024, 211, 89–93. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, X.; Ren, L. A Randomized Controlled Study of Enhanced External Counterpulsation with Cardiac Rehabilitation in Patients with Nonobstructive Coronary Artery Disease and Coronary Microvascular Dysfunction. Int. Heart J. 2024, 65, 380–385. [Google Scholar] [CrossRef]
- Kronhaus, K.D.; Lawson, W.E. Enhanced external counterpulsation is an effective treatment for Syndrome X. Int. J. Cardiol. 2009, 135, 256–257. [Google Scholar] [CrossRef]
- Marinescu, M.A.; Löffler, A.I.; Ouellette, M.; Smith, L.; Kramer, C.M.; Bourque, J.M. Coronary Microvascular Dysfunction, Microvascular Angina, and Treatment Strategies. JACC Cardiovasc. Imaging 2015, 8, 210–220. [Google Scholar] [CrossRef]
- Vermeltfoort, I.A.C.; Raijmakers, P.G.H.M.; Riphagen, I.I.; Odekerken, D.A.M.; Kuijper, A.F.M.; Zwijnenburg, A.; Teule, G.J.J. Definitions and incidence of cardiac syndrome X: Review and analysis of clinical data. Clin. Res. Cardiol. 2010, 99, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Kaski, J.C. Pathophysiology and Management of Patients with Chest Pain and Normal Coronary Arteriograms (Cardiac Syndrome X). Circulation 2004, 109, 568–572. [Google Scholar] [CrossRef] [PubMed]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef] [PubMed]
- Thadani, U. Challenges with Nitrate Therapy and Nitrate Tolerance: Prevalance, Prevention, and Clinical Relevance. Am. J. Cardiovasc. Drugs 2014, 14, 287–301. [Google Scholar] [CrossRef]
- Ford, T.J.; Stanley, B.; Good, R.; Rocchiccioli, P.; McEntegart, M.; Watkins, S.; Eteiba, H.; Shaukat, A.; Lindsay, M.; Robertson, K.; et al. Stratified Medical Therapy Using Invasive Coronary Function Testing in Angina: The CorMicA Trial. JACC 2018, 72, 2841–2855. [Google Scholar] [CrossRef]
- Ford, T.J.; Stanley, B.; Sidik, N.; Good, R.; Rocchiccioli, P.; McEntegart, M.; Watkins, S.; Eteiba, H.; Shaukat, A.; Lindsay, M.; et al. 1-Year Outcomes of Angina Management Guided by Invasive Coronary Function Testing (CorMicA). J. Am. Coll. Cardiol. Intv. 2020, 13, 33–45. [Google Scholar] [CrossRef]
Angina with Non-Obstructive Coronary Arteries (ANOCA), according to endotype.
|
‘normal coronary arteries and angina’: alternative terms used:
|
| Treatment modalities and outcomes: all treatments, including pharmacological and non-pharmacological, used in patients with ANOCA or ‘normal coronary arteries and angina’ with the aim of symptom reduction and/or improvement in quality of life and/or changes in myocardial blood flow. |
| First Author | Year | Study Type | Follow-up Duration | N | Mean Age Intervention | Mean Age Control | Male (%) | Diagnosis ANOCA |
|---|---|---|---|---|---|---|---|---|
| Calcium channel blockers | 538 | |||||||
| Sinha [13] | 2024 | Prospective, randomised | 12 weeks | 87 | 62 ± 8 | 60 ± 7 | 37 | MVA—typical angina, preserved left ventricular ejection fraction (>50%) and normal CAG (FFR > 0.8) |
| Jansen [2] | 2022 | Prospective, randomised | 6 weeks | 85 | 57.7 ± 8.8 | 58.0 ± 9.3 | 34 | VSA/MVA—typical angina and CFT; CFR ≤ 2.0 and/or IMR > 25 and/or abnormal response to Ach |
| Kook [14] | 2020 | Prospective, randomised | 12 weeks | 48 | 59.5 ± 11.8 | 62.8 ± 7.2 | 66.7 | VSA—typical angina and CAG with abnormal response to Ach |
| Zhang [15] | 2014 | Prospective, randomised | 90 days | 66 | 54 ± 7 | 53 ± 8 | 45.5 | Cardiac syndrome X—typical angina, positive exercise test and normal CAG |
| Oikawa [16] | 2010 | Prospective, randomised | 8 weeks | 28 | 64.6 ± 10.8 | 61.2 ± 14 | 78.6 | VSA—typical angina and CAG with abnormal response to Ach |
| Özçelik [17] | 1999 | Prospective, randomised (cross-over) | 12 weeks | 18 | 46 ± 10 | N/A | 38.9 | MVA—typical angina, positive exercise test, negative IV ergonovine test and normal CAG |
| Lanza [18] | 1999 | Prospective, randomised (cross-over) | 16 weeks | 10 | 57 ± 6 | N/A | 40 | Cardiac syndrome X—typical angina, positive exercise test and normal CAG |
| Vogt [19] | 1994 | Prospective, unrandomised | 52 weeks | 15 | 61 ± 7 | N/A | 66.7 | Cardiac syndrome X—typical angina, positive exercise test or MPS with ischaemia and normal CAG |
| Cannon [20] | 1990 | Prospective, randomised (cross-over) | 4 weeks | 22 | 52 [30–65] | N/A | 45.5 | MVA—typical angina, normal CAG and reduced CFR |
| Romeo [21] | 1988 | Prospective, randomised (cross-over) | 9 weeks | 30 | 50 ± 9 | N/A | 10 | MVA—typical angina, positive exercise test, negative IV ergonovine test and normal CAG |
| Prida [22] | 1987 | Prospective, randomised (cross-over) | 16 weeks | 15 | 58.3 ± 10.5 | N/A | 80 | VSA—typical angina and CAG with spontaneous or Ach-induced spasm |
| Kugiyama [23] | 1986 | Prospective, randomised (cross-over) | 3 weeks | 20 | 54.2 ± 7.8 | N/A | 80 | VSA—typical angina, CAG with proven coronary spasm and normal coronary arteries |
| Gelman [24] | 1985 | Prospective, randomised (cross-over) | 4 weeks | 17 | 57.2 ± 6.15 | N/A | 94.1 | VSA—typical angina and CAG with spontaneous or Ach-induced spasm |
| Cannon [25] | 1985 | Prospective, randomised (cross-over) | 4 weeks | 26 | 53 [38–64] | N/A | 42.3 | Cardiac syndrome X—typical angina, normal CAG and abnormal vasodilator reserve |
| Pitcher [26] | 1981 | Prospective, randomised (cross-over) | 4 weeks | 33 | 49 [31–58] | N/A | 27.3 | Cardiac syndrome X—typical angina, positive exercise test and normal CAG |
| Freedman [27] | 1981 | Prospective, unrandomised | 4 days | 6 | 56 [49–64] | N/A | 83.3 | Cardiac syndrome X—typical angina, positive exercise test and normal CAG |
| Lifestyle interventions | 389 | |||||||
| Sugisawa [28] | 2021 | Prospective, randomised | 12 weeks | 20 | 58.1 ± 2.3 | 61.8 ± 3.2 | 25 | VSA—typical angina and CAG with abnormal response to Ach |
| Bove [29] | 2020 | Prospective, randomised | 24 weeks | 56 | 64.3 ± 7.6 | 63.0 ± 8.0 | 0 | MVA—typical angina and CFT; CFR ≤ 2.5 |
| Rahmani [30] | 2020 | Prospective, randomised | 4 weeks | 30 | 53 ± 9 | 54 ± 7 | 20 | Cardiac syndrome X—typical angina, positive exercise test and/or MPS with ischaemia and normal CAG |
| Szot [31] | 2016 | Prospective, unrandomised | 12 weeks | 55 | 57.3 ± 5.4 | N/A | 0 | Cardiac syndrome X—typical angina, MPS with ischaemia and normal CAG |
| de Carvalho [32] | 2015 | Prospective, unrandomised | 16 weeks | 12 | 53.8 ± 9.7 | N/A | 58.3 | Cardiac syndrome X—typical angina, MPS with ischaemia and normal CAG |
| Feizi [33] | 2012 | Prospective, randomised | 8 weeks | 40 | 50.5 ± 7.1 | 52.4 ± 6.3 | 0 | Cardiac syndrome X—typical angina, MPS with ischaemia and normal CAG |
| Asbury [34] | 2009 | Prospective, randomised | 16 weeks | 53 | 58.1 ± 7.2 | 56.1 ± 8.6 | 0 | Cardiac syndrome X—typical angina, positive exercise test and normal CAG |
| Asbury [35] | 2008 | Prospective, randomised | 8 weeks | 64 | 58.1 ± 9.4 | 56.4 ± 7.8 | 0 | Cardiac syndrome X—typical angina, positive exercise test and normal CAG |
| Tyni-Lenne [36] | 2002 | Prospective, randomised | 8 weeks | 24 | 57 ± 7 | 55 ± 8 | 0 | Cardiac syndrome X—typical angina, positive exercise test and normal CAG |
| Cunningham [37] | 2000 | Prospective, unrandomised | 12 weeks | 9 | 56 [48–66] | N/A | 0 | Cardiac syndrome X—typical angina, positive exercise test and normal CAG |
| Eriksson [38] | 2000 | Prospective, randomised | 16 weeks | 26 | 57 ± 7 | 53 ± 10 | 0 | Cardiac syndrome X—typical angina, positive exercise test and normal CAG |
| RAAS inhibitors | 339 | |||||||
| Michelsen [39] | 2018 | Prospective, randomised | 24 weeks | 63 | 58.6 ± 11.6 | 57.3 ± 12.5 | 0 | MVA—typical angina, CAG with no epicardial stenosis > 50% and CFVR < 2.2 (adenosine stress echocardiography) |
| Bavry [40] | 2014 | Prospective, randomised | 16 weeks | 51 | 54 ± 10 | 54 ± 11 | 0 | MVA—typical angina, CAG with no epicardial stenosis > 50% and endothelial dysfunction (<5% diameter increase Ach) |
| Pauly [41] | 2011 | Prospective, randomised | 16 weeks | 61 | 56 ± 8 | 51 ± 10 | 0 | Cardiac syndrome X—typical angina, CAG with no epicardial stenosis > 50% and CFR < 3.0 |
| Pizzi [42] | 2004 | Prospective, randomised | 24 weeks | 45 | 59.6 ± 8.7 | 57.6 ± 9.6 | 11.1 | Cardiac syndrome X—typical angina, positive exercise test, normal CAG and no coronary spasm during ergonovine IV |
| Chen [43] | 2002 | Prospective, randomised | 8 weeks | 20 | 66.3 ± 3.5 | 67.7 ± 2.9 | 75 | Cardiac syndrome X—typical angina, positive exercise test, normal CAG and no evidence of coronary spasm |
| Kanadaşi [44] | 2002 | Prospective, randomised (cross-over) | 16 weeks | 21 | 49.5 ± 10.4 | N/A | 14.3 | Cardiac syndrome X—typical angina, positive exercise test and normal CAG |
| Özçelik [17] | 1999 | See previous * | ||||||
| Nalbantgil [45] | 1998 | Prospective, randomised (cross-over) | 10 weeks | 35 | 43.9 ± 6.4 | N/A | 22.9 | MVA—typical angina, positive exercise test and normal CAG |
| Motz [46] | 1996 | Prospective, unrandomised | 12 weeks | 15 | 58 ± 6 | N/A | 66.7 | Cardiac syndrome X—typical angina, positive exercise test and normal CAG |
| Kaski [47] | 1994 | Prospective, randomised (cross-over) | 4 weeks | 10 | 53 ± 6 | N/A | 30 | Cardiac syndrome X—typical angina, positive exercise test, abnormal coronary flow reserve and normal CAG |
| Beta-blockers | 219 | |||||||
| Kook [14] | 2020 | See previous * | ||||||
| Erdamar [48] | 2009 | Prospective, randomised | 12 weeks | 30 | 47.6 ± 7.2 | 49.1 ± 7.3 | 43.3 | Cardiac syndrome X—typical angina, positive exercise test, normal CAG and absence of coronary spasm |
| Sen [49] | 2009 | Prospective, randomised | 12 weeks | 34 | 47.2 ± 7.3 | 49.5 ± 7.3 | 70.6 | Cardiac syndrome X—typical angina, positive exercise test, normal CAG and absence of coronary spasm |
| Suzuki [50] | 2003 | Prospective, unrandomised | 12 weeks | 12 | 56.3 ± 8.2 | N/A | 58.3 | VSA—typical angina and CAG with abnormal response to Ach |
| Kanadaşi [44] | 2002 | See previous * | ||||||
| Lanza [18] | 1999 | See previous * | ||||||
| Leonardo [51] | 1999 | Prospective, randomised (cross-over) | 8 weeks | 16 | 62 ± 7 | N/A | 18.8 | Cardiac syndrome X—typical angina, positive exercise test and normal CAG |
| Shimizu [52] | 1993 | Prospective, randomised (cross-over) | 1 week | 10 | 57.5 ± 6.7 | N/A | 100 | VSA—typical angina, CAG with no epicardial stenosis > 50% and spontaneous or Ach-induced coronary spasm |
| Romeo [21] | 1988 | See previous * | ||||||
| Kugiyama [23] | 1986 | See previous * | ||||||
| Long-acting nitrates | 2792 | |||||||
| Lim [53] | 2022 | Prospective, unrandomised | 24 months | 568 | 54.9 ± 11.3 | 55.6 ± 11.5 | 55.5 | VSA—typical angina, normal CAG and positive ergonovine provocation test |
| Kim [54] | 2018 | Prospective, unrandomised | 54.7 months | 1127 | 56.7 ± 9.3 | 56.6 ± 9.8 | 85.5 | VSA—typical angina, normal CAG and positive ergonovine provocation test |
| Takahashi [55] | 2015 | Prospective, unrandomised | 32 months | 826 | 66 [58–73] | 66 [59,60,61,62,63,64,65,66,67,68,69,70,71,72,73] | 74.8 | VSA—typical angina, CAG with no epicardial stenosis > 50% and positive ergonovine/Ach provocation test |
| Wu [56] | 2015 | Prospective, randomised (cross-over) | 4 weeks | 9 | 59 ± 9 | N/A | 22.2 | MVA—typical angina, normal CAG, positive exercise test and CFR < 2.0 (Doppler LAD) |
| Kosugi [57] | 2011 | Prospective, unrandomised | 70.5 months | 231 | 61.0 ± 10.6 | 59.2 ± 9.9 | 66.7 | VSA—typical angina, normal CAG and positive Ach provocation test |
| Kanadaşi [44] | 2002 | See previous * | ||||||
| Lanza [18] | 1999 | See previous * | ||||||
| Statins | 7479 | |||||||
| Lee [58] | 2024 | Prospective, unrandomised | 4.8 years | 4432 | 57.8 ± 11.6 | 58.5 ± 13.1 | 45.6 | VSA—typical angina, normal CAG and positive ergonovine/Ach provocation test |
| Mori [59] | 2022 | Prospective, unrandomised | 726 days | 422 | 65.5 ± 9.5 | 64.6 ± 10.3 | 74.4 | VSA—typical angina, CAG with no epicardial stenosis > 50% and positive ergonovine/Ach provocation test |
| Seo [60] | 2020 | Prospective, unrandomised | 104 weeks | 1658 | 55.9 ± 10.9 | 53.5 ± 11.5 | 60.6 | VSA—typical angina, normal CAG and positive ergonovine provocation test |
| Ishii [61] | 2016 | Prospective, unrandomised | 60 months | 256 | 64.6 ± 9.9 | 64.8 ± 9.7 | 43.8 | VSA—typical angina, normal CAG and positive Ach provocation test |
| Oh [62] | 2016 | Prospective, unrandomised | 4.5 years | 562 | 55.8 ± 9.2 | 55.7 ± 9.2 | 85.2 | VSA—typical angina, normal CAG and positive ergonovine provocation test |
| Zhang [15] | 2014 | See previous * | ||||||
| Pizzi [42] | 2004 | See previous * | ||||||
| Kayikciolgu [63] | 2003 | Prospective, randomised | 12 weeks | 38 | 45 ± 7 | 47 ± 4 | 42.1 | Cardiac syndrome X—typical angina, positive exercise test and normal CAG |
| Neuromodulation | 77 | |||||||
| de Vries [64] | 2007 | Prospective, unrandomised | 5.1 years | 12 | 56.7 ± 8.2 | N/A | 37.5 | Cardiac syndrome X—typical angina and normal CAG |
| Sgueglia [65] | 2007 | Prospective, unrandomised | 36 months | 28 | 60.9 ± 8.5 | 60.9 ± 8.8 | 28.6 | Cardiac syndrome X—typical angina, positive exercise test OR perfusion defect MPS and normal CAG |
| Jessurun [66] | 2003 | Prospective, unrandomised | 4 weeks | 8 | 55 ± 7 | N/A | 37.5 | Cardiac syndrome X—typical angina, normal CAG and heterogeneous myocardial perfusion MPS |
| Lanza [67] | 2005 | Prospective, randomised (cross-over) | 7 weeks | 10 | 58.6 ± 5.7 | N/A | 30 | Cardiac syndrome X—typical angina, positive exercise test OR perfusion defect MPS and normal CAG |
| Lanza [68] | 2001 | Prospective, unrandomised | 1 month | 7 | 59.3 ± 11 | N/A | 57.1 | Cardiac syndrome X—typical angina, positive exercise test and normal CAG |
| Eliasson [69] | 1993 | Prospective, unrandomised | 1 week | 12 | 61 ± 6 | N/A | 33.3 | Cardiac syndrome X—typical angina, positive exercise test and normal CAG |
| Ranolazine | 246 | |||||||
| Sinha [13] | 2024 | See previous * | ||||||
| Birkeland [70] | 2017 | Prospective, randomised (cross-over) | 2 weeks | 30 | 54 ± 10.6 | N/A | 3.3 | MVA—typical angina, CAG with no epicardial stenosis > 50% and CFR < 2.5 OR MPRI < 2.0 |
| Ahmed [71] | 2017 | Prospective, unrandomised | 4 weeks | 7 | 57.6 ± 11.3 | N/A | 57.1 | MVA—typical angina, CAG with no epicardial stenosis > 50%, positive exercise test OR perfusion defect MPS OR stress echo with RWMA and IMR > 20 |
| Safdar [72] | 2017 | Prospective, randomised | 4 weeks | 31 | 50 ± 5 | 50 ± 7 | 29 | MVA—typical angina, normal CAG and CFR < 2.5 |
| Merz [73] | 2016 | Prospective, randomised (cross-over) | 2 weeks | 132 | 55.2 ± 9.8 | N/A | 4 | MVA—typical angina, CAG with no epicardial stenosis > 50%, CFR < 2.5 OR no dilation with Ach OR MPRI < 2.0 |
| Villano [74] | 2013 | Prospective, randomised | 4 weeks | 46 | 57 ± 11 | 60 ± 9 | 19.6 | MVA—typical angina, positive exercise test, normal CAG, CFR < 2.5 (Doppler LAD) and no vasospastic angina |
| Trimetazidine | 195 | |||||||
| Boldueva [75] | 2020 | Prospective, randomised | 3 months | 60 | 58.4 ± 6.5 | 57.3 ± 6.4 | 43.3 | MVA—typical angina, positive exercise test, normal CAG and ischaemia using PET |
| Galin [76] | 2016 | Prospective, unrandomsed | 6 months | 50 | 55.2 ± 3.8 | N/A | 32 | Cardiac syndrome X—typical angina, CAG with no epicardial stenosis > 50% and positive exercise test |
| Rogacka [77] | 2000 | Prospective, unrandomised | 6 months | 34 | 46 [32–60] | N/A | 41.2 | Cardiac syndrome X—typical angina, positive exercise test and normal CAG |
| Leonardo [51] | 1999 | See previous * | ||||||
| Nalbantgil [78] | 1999 | Prospective, randomised (cross-over) | 10 weeks | 35 | 43.9 ± 6.4 | N/A | 22.9 | Cardiac syndrome X—typical angina, positive exercise test and normal CAG |
| Traditional Chinese medicine | 356 | |||||||
| Noroozi [79] | 2023 | Prospective, unrandomised | 90 days | 28 | 50.6 ± 6 | N/A | 42.9 | Cardiac syndrome X—typical angina, positive exercise test and normal CAG |
| Ma [80] | 2021 | Prospective, randomised | 12 weeks | 171 | 60.2 ± 6.2 | 59.1 ± 6.2 | Not repor. | Cardiac syndrome X—typical angina, positive exercise test, normal CAG and negative ergonovine test |
| Cao [81] | 2021 | Prospective, randomised | Not repor. | 70 | 60.6 ± 10 | 61.9 ± 9.3 | 56.9 | Cardiac syndrome X—typical angina, positive exercise test and normal CAG |
| Li [82] | 2007 | Prospective, randomised | 3 months | 36 | Not repor. | Not repor. | Not repor. | Cardiac syndrome X—typical angina, positive exercise test and normal CAG |
| Mao [83] | 2007 | Prospective, unrandomised | 14 days | 51 | 51.2 ± 6.2 | 50.8 ± 6.5 | 21.6 | Cardiac syndrome X—typical angina, positive exercise test and normal CAG |
| Hormone therapy | 110 | |||||||
| Merz [84] | 2010 | Prospective, randomised | 12 weeks | 37 | 56 ± 9 | 59 ± 7 | 0 | Cardiac syndrome X—typical angina, CAG with no epicardial stenosis > 50% and positive exercise test OR perfusion defect MPS OR CFR < 2.25 |
| Adamson [85] | 2001 | Prospective, randomised (cross-over) | 16 weeks | 32 | 58 ± 2 | N/A | 0 | Cardiac syndrome X—typical angina, positive exercise test and normal CAG |
| Rosano [86] | 1996 | Prospective, randomised (cross-over) | 18 weeks | 26 | 56.8 [47–65] | N/A | 0 | Cardiac syndrome X—typical angina, positive exercise test and normal CAG |
| Albertsson [87] | 1996 | Prospective, randomised (cross-over) | 1 week | 15 | 58 ± 6 | N/A | 0 | Cardiac syndrome X—typical angina, positive exercise test and normal CAG |
| Autologous CD34+ stem cell therapy | 40 | |||||||
| Henry [88] | 2022 | Prospective, unrandomised | 6 months | 20 | 54.3 ± 12.7 | N/A | 15 | MVA—typical angina, CAG with no epicardial stenosis > 40% and CFR ≤ 2.5 |
| Corban [89] | 2022 | Prospective, unrandomised | 6 months | 20 | 51.0 ± 12.1 | N/A | 25 | MVA—typical angina, CAG with no epicardial stenosis > 40% and CFR ≤ 2.5 |
| Enhanced External Counterpulsation | 181 | |||||||
| Ashokprabhu [90] | 2024 | Retrospective, unrandomised | 7 weeks | 101 | 60.6 ± 11.3 | N/A | 37.6 | ANOCA—CCS class III or IV and absence of obstructive coronary arteries (CAG or CCTA stenosis < 50%) |
| Zhang [91] | 2024 | Prospective, randomised | 1 year | 80 | 50.5 ± 16.8 | 51.2 ± 14.6 | 67.5 | MVA—typical angina, MPR < 2.0 (CMR) and absence of obstructive coronary arteries (CAG or CCTA stenosis < 50%) |
| Kronhaus [92] | 2009 | Prospective, unrandomised | 12 months | 30 | 64.9 ± 10.7 | N/A | 27 | Cardiac syndrome X—typical angina, no obstructive coronary arteries (<50%) and pharmacological or exercise-induced ischaemia. |
| Angina Pectoris Frequency | Exercise Capacity | Quality of Life | CCS Class | Coronary Blood Flow | Survival | |
|---|---|---|---|---|---|---|
| Calcium channel blockers | n = 188 | n = 223 | n = 99 | n = 88 | n = 110 | |
| Lifestyle interventions | n = 153 | n = 232 | n = 194 | n = 66 | ||
| RAAS inhibitors | n = 153 | n = 116 | n = 149 | |||
| Beta-blockers | n = 55 | n = 110 | n = 25 | |||
| EECP | n = 181 | n = 131 | ||||
| Long-acting nitrates | n = 55 | n = 19 | n = 4375 | |||
| Neuromodulation | n = 58 | n = 69 | n = 57 | |||
| Ranolazine | n = 80 | n = 109 | n = 147 | n = 47 | ||
| Trimetazidine | n = 129 | n = 85 | ||||
| Statins | n = 86 | n = 7222 | ||||
| Traditional Chinese medicine | n = 271 | n = 104 | ||||
| Hormone therapy | n = 94 | |||||
| Stem cell therapy | n = 40 | n = 40 | n = 40 | n = 40 |
| RoB 2 | |||||||
| Study | D1 | D2 | D3 | D4 | D5 | Overall | |
| Jansen et al. [2] | |||||||
| Bove et al. [29] | |||||||
| Sugisawa et al. [28] | |||||||
| Asbury et al. [34] | |||||||
| Michelsen et al. [35] | |||||||
| Cao et al. [82] | |||||||
| Oikawa et al. [16] | |||||||
| Chen et al. [43] | |||||||
| Villano et al. [74] | |||||||
| Ma et al. [80] | |||||||
| Asbury et al. [35] | |||||||
| Boldueva et al. [75] | |||||||
| Zhang et al. [91] | |||||||
| RoB 2—cross-over | |||||||
| Study | D1 | S | D2 | D3 | D4 | D5 | Overall |
| Lanza et al. [18] | |||||||
| Leonardo et al. [51] | |||||||
| Prida et al. [22] | |||||||
| Wu et al. [56] | |||||||
| Gelman et al. [24] | |||||||
| Lanza et al. [67] | |||||||
| Shimizu et al. [52] | |||||||
| Merz et al. [73] | |||||||
| Kanadaşi et al. [44] | |||||||
| Özçelik et al. [17] | |||||||
| Cannon et al. [25] | |||||||
| Nalbantgil et al. [78] | |||||||
| NOS | |||||||
| Study | Selection | Comparability | Outcomes | Overall | |||
| Sgueglia et al. [65] | 3 | 1 | 3 | Good | |||
| Jessurun et al. [66] | 2 | 1 | 2 | Fair | |||
| Mao et al. [83] | 3 | 1 | 2 | Good | |||
| Lanza et al. [68] | 2 | 1 | 3 | Good | |||
| Cunningham et al. [37] | 2 | 1 | 3 | Good | |||
| De Vries et al. [64] | 2 | 1 | 2 | Fair | |||
| Henry et al. [88] | 2 | 1 | 3 | Good | |||
| Noroozi et al. [79] | 2 | 1 | 2 | Fair | |||
| Galin et al. [76] | 2 | 1 | 2 | Fair | |||
| Corban et al. [89] | 3 | 1 | 3 | Good | |||
| Ashokprabhu et al. [90] | 3 | 1 | 3 | Good | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vervaat, F.E.; de Vos, A.; Schenk, J.; Tonino, P.A.L.; Wijnbergen, I.F. Treatment Modalities for Angina with Non-Obstructive Coronary Arteries (ANOCA): A Systematic Review and Meta-Analysis. J. Clin. Med. 2025, 14, 4069. https://doi.org/10.3390/jcm14124069
Vervaat FE, de Vos A, Schenk J, Tonino PAL, Wijnbergen IF. Treatment Modalities for Angina with Non-Obstructive Coronary Arteries (ANOCA): A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2025; 14(12):4069. https://doi.org/10.3390/jcm14124069
Chicago/Turabian StyleVervaat, Fabienne E., Annemiek de Vos, Jimmy Schenk, Pim A. L. Tonino, and Inge F. Wijnbergen. 2025. "Treatment Modalities for Angina with Non-Obstructive Coronary Arteries (ANOCA): A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 14, no. 12: 4069. https://doi.org/10.3390/jcm14124069
APA StyleVervaat, F. E., de Vos, A., Schenk, J., Tonino, P. A. L., & Wijnbergen, I. F. (2025). Treatment Modalities for Angina with Non-Obstructive Coronary Arteries (ANOCA): A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 14(12), 4069. https://doi.org/10.3390/jcm14124069






