Sodium Oxybate (SMO) as Part of Agonist Opioid Treatment in Alcohol–Heroin-Addicted Patients
Abstract
1. Introduction
2. Materials and Methods
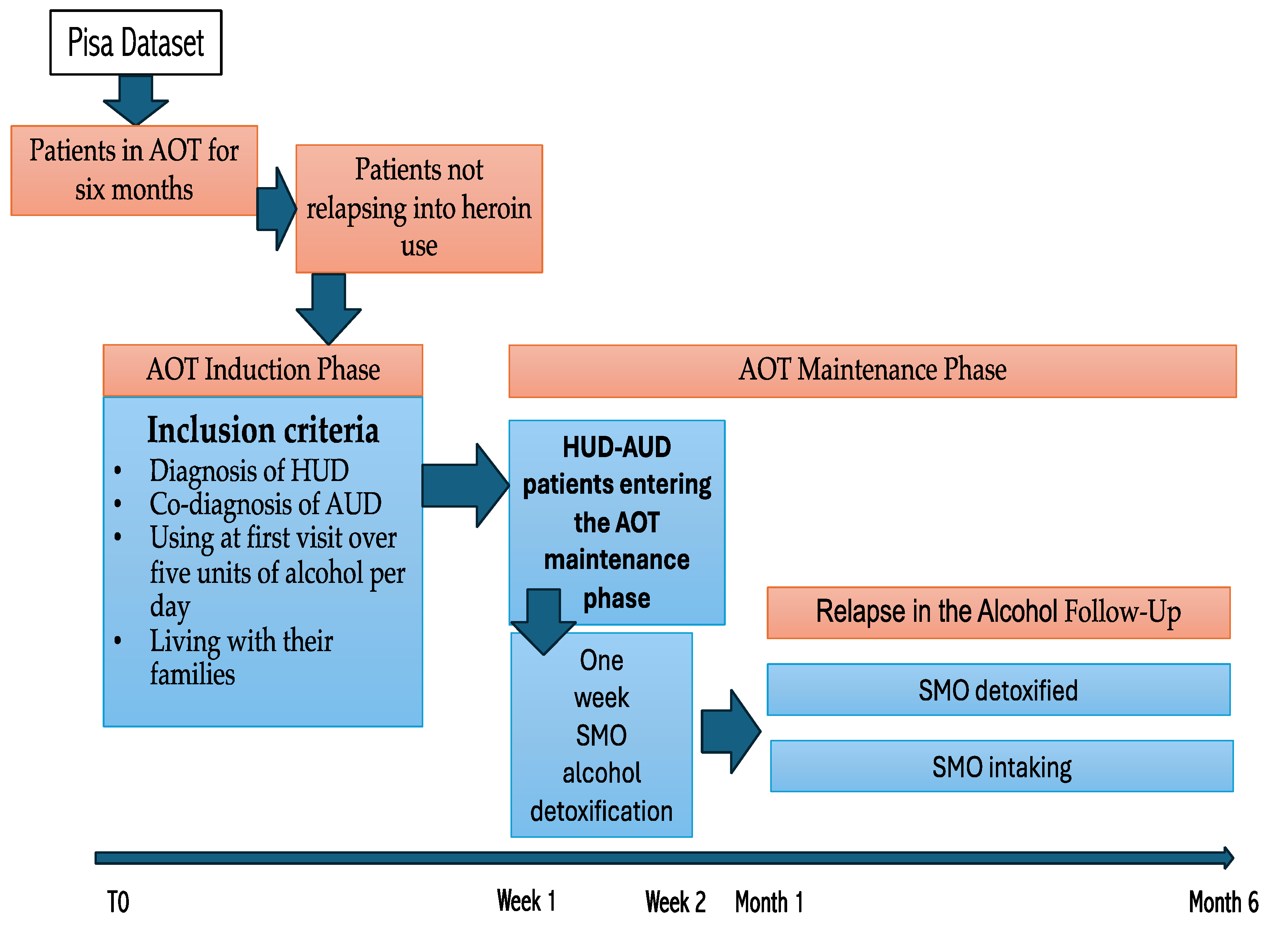
2.1. Design of the Study
2.2. Sample
2.3. Assessment
2.4. Procedure
2.5. Data Analysis
3. Results
3.1. Retention in Treatment
3.2. Predictors of Terminal Events
3.3. Clinical Global Index
4. Discussion
- Supervised administration: Self-administered doses of SMO should be monitored by a caregiver or healthcare provider to minimize risk.
- Use of lower single doses: Particularly in HUD patients, as higher doses may be more easily distinguishable and potentially reinforcing [75].
- Monitoring of subjective effects: Gradual and stable therapeutic effects are preferable, as they are associated with lower risk of misuse.
- Timing of SMO initiation: SMO should only be introduced once the patient has achieved opioid stabilization and sustained abstinence from heroin.
- Hierarchical pharmacological approach: MMT should be considered a prerequisite before initiating SMO therapy.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AUD | Alcohol Use Disorder |
| CGI | Clinical Global Impressions |
| CNS | Central Nervous System |
| DSM | Diagnostic Statistical Manual |
| GABA | Gamma-aminobutyric Acid |
| GHB | Gamma-Hydroxybutyric Acid |
| HUD | Heroin Use Disorder |
| MM | Methadone Maintenance |
| MMT | Methadone Maintenance Treatment |
| OUD | Opioid Use Disorder |
| RCT | Randomized Controlled Trial |
| SMO | Sodium Oxybate |
| SUD | Substance Use Disorder |
References
- Pacini, M.; Maremmani, A.G.I.; Ceccanti, M.; Maremmani, I. Former Heroin-Dependent Alcohol Use Disorder Patients. Prevalence, Addiction History and Clinical Features. Alcohol Alcohol. 2015, 50, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Pacini, M.; Rocca, F.D.; Maremmani, A.G.I.; Maremmani, I. Cocaine use in opioid and alcohol substance use disorders. Heroin Addict. Relat. Clin. Probl. 2023, 25, 37–46. [Google Scholar]
- Morley, K.I.; Lynskey, M.T.; Moran, P.; Borschmann, R.; Winstock, A.R. Polysubstance use, mental health and high-risk behaviours: Results from the 2012 Global Drug Survey. Drug Alcohol. Rev. 2015, 34, 427–437. [Google Scholar] [CrossRef]
- Maremmani, A.G.I.; Pacini, M.; Pani, P.P.; Ceccanti, M.; Bacciardi, S.; Akiskal, H.S.; Maremmani, I. Possible trajectories of addictions: The role of bipolar spectrum. Heroin Addict. Relat. Clin. Probl. 2016, 18, 23–32. [Google Scholar]
- Ellis, J.D.; Rabinowitz, J.A.; Ware, O.D.; Wells, J.; Dunn, K.E.; Huhn, A.S. Patterns of polysubstance use and clinical comorbidity among persons seeking substance use treatment: An observational study. J. Subst. Use Addict. Treat. 2023, 146, 208932. [Google Scholar] [CrossRef]
- Connor, J.P.; Gullo, M.J.; White, A.; Kelly, A.B. Polysubstance use: Diagnostic challenges, patterns of use and health. Curr. Opin. Psychiatry 2014, 27, 269–275. [Google Scholar] [CrossRef]
- Franklyn, A.M.; Eibl, J.K.; Gauthier, G.J.; Pellegrini, D.; Lightfoot, N.E.; Marsh, D.C. The impact of cocaine use in patients enrolled in opioid agonist therapy in Ontario, Canada. Int. J. Drug Policy 2017, 48, 1–8. [Google Scholar] [CrossRef]
- McLellan, A.T.; Lewis, D.C.; O’Brien, C.P.; Kleber, H.D. Drug dependence, a chronic medical illness: Implications for treatment, insurance, and outcomes evaluation. JAMA 2000, 284, 1689–1695. [Google Scholar] [CrossRef]
- Bonfiglio, N.S.; Mascia, M.L.; Penna, M.P. Digital Treatment Paths for Substance Use Disorders (SUDs). Int. J. Environ. Res. Public Health 2022, 19, 7322. [Google Scholar] [CrossRef]
- Coulson, C.; Ng, F.; Geertsema, M.; Dodd, S.; Berk, M. Client-reported reasons for non-engagement in drug and alcohol treatment. Drug Alcohol. Rev. 2009, 28, 372–378. [Google Scholar] [CrossRef]
- Betts, K.S.; Chan, G.; McIlwraith, F.; Dietze, P.; Whittaker, E.; Burns, L.; Alati, R. Differences in polysubstance use patterns and drug-related outcomes between people who inject drugs receiving and not receiving opioid substitution therapies. Addiction 2016, 111, 1214–1223. [Google Scholar] [CrossRef]
- Anglin, M.D.; Almog, I.J.; Fisher, D.G.; Peters, K.R. Alcohol use by heroin addicts: Evidence for an inverse relationship. A study of methadone maintenance and drug-free treatment samples. Am. J. Drug Alcohol. Abus. 1989, 15, 191–207. [Google Scholar] [CrossRef]
- Maremmani, A.G.I.; Pacini, M.; Maremmani, I. What we have learned from the Methadone Maintenance Treatment of Dual Disorder Heroin Use Disorder patients. Int. J. Environ. Res. Public Health 2019, 16, 447. [Google Scholar] [CrossRef]
- Weitzman, E.R.; Ong, M.S. Rising Prevalence of Comorbid Alcohol and Opioid Use Disorders in Adolescents and Young Adults in the United States. J. Gen. Intern. Med. 2019, 34, 1987–1989. [Google Scholar] [CrossRef] [PubMed]
- Chambers, C.D.; Taylor, W.J.; Moffett, A.D. The incidence of cocaine abuse among methadone maintenance patients. Int. J. Addict. 1972, 7, 427–441. [Google Scholar] [CrossRef] [PubMed]
- Green, J.; Jaffe, J.H.; Carlisi, J.A.; Zaks, A. Alcohol use in the opiate use cycle of the heroin addict. Int. J. Addict. 1978, 13, 1021–1033. [Google Scholar] [CrossRef]
- Novick, D.M.; Richman, B.L.; Friedman, J.M.; Friedman, J.E.; Fried, C.; Wilson, J.P.; Townley, A.; Kreek, M.J. The medical status of methadone maintenance patients in treatment for 11–18 years. Drug Alcohol Depend. 1993, 33, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Novick, D.M.; Salsitz, E.A.; Joseph, H.; Kreek, M.J. Methadone Medical Maintenance: An Early 21st-Century Perspective. J. Addict. Dis. 2015, 34, 226–237. [Google Scholar] [CrossRef]
- van den Brink, W.; Addolorato, G.; Aubin, H.J.; Benyamina, A.; Caputo, F.; Dematteis, M.; Gual, A.; Lesch, O.M.; Mann, K.; Maremmani, I.; et al. Efficacy and safety of sodium oxybate in alcohol-dependent patients with a very high drinking risk level. Addict. Biol. 2018, 23, 969–986. [Google Scholar] [CrossRef]
- Garbutt, J.C.; West, S.L.; Carey, T.S.; Lohr, K.N.; Crews, F.T. Pharmacological treatment of alcohol dependence: A review of the evidence. JAMA 1999, 281, 1318–1325. [Google Scholar] [CrossRef]
- Skala, K.; Caputo, F.; Mirijello, A.; Vassallo, G.; Antonelli, M.; Ferrulli, A.; Walter, H.; Lesch, O.; Addolorato, G. Sodium oxybate in the treatment of alcohol dependence: From the alcohol withdrawal syndrome to the alcohol relapse prevention. Expert. Opin. Pharmacother. 2014, 15, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Leone, M.A.; Vigna-Taglianti, F.; Avanzi, G.; Brambilla, R.; Faggiano, F. Gamma-hydroxybutyrate (GHB) for treatment of alcohol withdrawal and prevention of relapses. Cochrane Database Syst. Rev. 2010, CD006266. [Google Scholar] [CrossRef]
- Maitre, M. The gamma-hydroxybutyrate signalling system in brain: Organization and functional implications. Prog. Neurobiol. 1997, 51, 337–361. [Google Scholar] [CrossRef] [PubMed]
- Crunelli, V.; Emri, Z.; Leresche, N. Unravelling the brain targets of gamma-hydroxybutyric acid. Curr. Opin. Pharmacol. 2006, 6, 44–52. [Google Scholar] [CrossRef]
- Keating, G.M. Sodium oxybate: A review of its use in alcohol withdrawal syndrome and in the maintenance of abstinence in alcohol dependence. Clin. Drug Investig. 2014, 34, 63–80. [Google Scholar] [CrossRef]
- Guiraud, J.; Spanagel, R.; van den Brink, W. Substitution therapy for patients with alcohol dependence: Mechanisms of action and efficacy. Int. Rev. Neurobiol. 2024, 175, 187–239. [Google Scholar] [CrossRef] [PubMed]
- Guiraud, J.; Addolorato, G.; Antonelli, M.; Aubin, H.J.; de Bejczy, A.; Benyamina, A.; Cacciaglia, R.; Caputo, F.; Dematteis, M.; Ferrulli, A.; et al. Sodium oxybate for the maintenance of abstinence in alcohol-dependent patients: An international, multicenter, randomized, double-blind, placebo-controlled trial. J. Psychopharmacol. 2022, 36, 1136–1145. [Google Scholar] [CrossRef]
- Hechler, V.; Gobaille, S.; Bourguignon, J.J.; Maitre, M. Extracellular events induced by gamma-hydroxybutyrate in striatum: A microdialysis study. J. Neurochem. 1991, 56, 938–944. [Google Scholar] [CrossRef]
- Caputo, F.; Vignoli, T.; Tarli, C.; Domenicali, M.; Zoli, G.; Bernardi, M.; Addolorato, G. A Brief Up-Date of the Use of Sodium Oxybate for the Treatment of Alcohol Use Disorder. Int. J. Environ. Res. Public Health 2016, 13, 290. [Google Scholar] [CrossRef]
- Gobaille, S.; Hechler, V.; Andriamampandry, C.; Kemmel, V.; Maitre, M. gamma-Hydroxybutyrate modulates synthesis and extracellular concentration of gamma-aminobutyric acid in discrete rat brain regions in vivo. J. Pharmacol. Exp. Ther. 1999, 290, 303–309. [Google Scholar] [CrossRef]
- Chick, J.; Nutt, D.J. Substitution therapy for alcoholism: Time for a reappraisal? J. Psychopharmacol. 2012, 26, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Gallimberti, L.; Cibin, M.; Pagnin, P.; Sabbion, R.; Pani, P.P.; Pirastu, R.; Ferrara, S.D.; Gessa, G.L. Gamma-hydroxybutyric acid for treatment of opiate withdrawal syndrome. Neuropsychopharmacology 1993, 9, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Gallimberti, L.; Schifano, F.; Forza, G.; Miconi, L.; Ferrara, S.D. Clinical efficacy of gamma-hydroxybutyric acid in treatment of opiate withdrawal. Eur. Arch. Psychiatry Clin. Nuerosci 1994, 244, 113–114. [Google Scholar] [CrossRef] [PubMed]
- Wixon, H.N.; Hunt, W.A. Effect of acute and chronic ethanol treatment on gamma-aminobutyric acid levels and on aminooxyacetic acid-induced GABA accumulation. Subst. Alcohol. Actions Misuse 1980, 1, 481–491. [Google Scholar] [PubMed]
- Dole, V.P.; Nyswander, M. A Medical Treatment for Diacetylmorphine (Heroin) Addiction: A Clinical Trial with Methadone Hydrochloride. JAMA 1965, 193, 646–650. [Google Scholar] [CrossRef]
- Dole, V.P.; Nyswander, M.E. Methadone maintenance treatment. A ten-year perspective. JAMA 1976, 235, 2117–2119. [Google Scholar] [CrossRef]
- Bizzarri, J.V.; Casetti, V.; Sanna, L.; Maremmani, A.G.; Rovai, L.; Bacciardi, S.; Piacentino, D.; Conca, A.; Maremmani, I. The newer Opioid Agonist Treatment with lower substitutive opiate doses is associated with better toxicology outcome than the older Harm Reduction Treatment. Ann. Gen. Psychiatry 2016, 15, 34. [Google Scholar] [CrossRef]
- Maremmani, A.G.I.; Quaranta, G.; Bacciardi, S.; Rovai, L.; Rugani, F.; Pacini, M.; Nisita, C.; Maremmani, I. Alcohol use disorder and past heroin addiction. A successfully treated ‘masked heroinism’ patient. Heroin Addict. Relat. Clin. Probl. 2014, 16, 37–42. [Google Scholar]
- Pacini, M.; Maremmani, A.G.I.; Rovai, L.; Rugani, F.; Maremmani, I. Treating heroin addicts. Blocking dosages and stimulation-stabilization of opioidergic system. Heroin Addict. Relat. Clin. Probl. 2010, 12, 41–48. [Google Scholar]
- Hser, Y.I.; Anglin, M.D.; Powers, K. Longitudinal patterns of alcohol use by narcotics addicts. Recent Dev. Alcohol. 1990, 8, 145–171. [Google Scholar]
- Laqueille, X.; Launay, C.; Dervaux, A.; Kanit, M. Abuse of alcohol and benzodiazepine during substitution therapy in heroin addicts: A review of the literature. Encephale 2009, 35, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Rounsaville, B.J.; Weissman, M.M.; Kleber, H.D. The significance of alcoholism in treated opiate addicts. J. Nerv. Ment. Dis. 1982, 170, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Guiraud, J.; van den Brink, W. Sodium oxybate: A comprehensive review of efficacy and safety in the treatment of alcohol withdrawal syndrome and alcohol dependence. Int. Rev. Neurobiol. 2024, 178, 213–281. [Google Scholar] [CrossRef] [PubMed]
- Biso, L.; Spini, A.; Petragnano, F.; Maggio, R.; Scarselli, M.; Carli, M. Long-term Efficacy and Safety of Sodium Oxybate in Treating Alcohol Use Disorder: A Systematic Review and Meta-Analysis. Curr. Neuropharmacol. 2024, 23, 579–593. [Google Scholar] [CrossRef]
- Addolorato, G.; Lesch, O.M.; Maremmani, I.; Walter, H.; Nava, F.; Raffaillac, Q.; Caputo, F. Post-marketing and clinical safety experience with sodium oxybate for the treatment of alcohol withdrawal syndrome and maintenance of abstinence in alcohol-dependent subjects. Expert. Opin. Drug Saf. 2020, 19, 159–166. [Google Scholar] [CrossRef]
- American Psychiatric Association. DSM-IV-TR. Diagnostic and Statistical Manual of Mental Disorders; American Psychiatric Association: Washington, DC, USA, 2000. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, DSM-5, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Guy, W. Clinical Global Impressions. In ECDEU Assessment Manual for Psychopharmacology. Clinical Global Impressions; U.S. Department of Health, Education, and Welfare: Rockville, MD, USA, 1976; pp. 218–222. [Google Scholar]
- Lettieri, J.T.; Fung, H.L. Dose-dependent pharmacokinetics and hypnotic effects of sodium gamma-hydroxybutyrate in the rat. J. Pharmacol. Exp. Ther. 1979, 208, 7–11. [Google Scholar] [CrossRef]
- Maremmani, I.; Balestri, C.; Lamanna, F.; Tagliamonte, A. Efficacy of Split Doses of GHB Used as Anticraving in the Treatment of Alcohol Dependence. Preliminary Results. Alcoholism 1998, 34, 73–80. [Google Scholar]
- Addolorato, G.; Cibin, M.; Caputo, F.; Capristo, E.; Gessa, G.L.; Stefanini, G.F.; Gasbarrini, G. Gamma-hydroxybutyric acid in the treatment of alcoholism: Dosage fractioning utility in non-responder alcoholic patients. Drug Alcohol. Depend. 1998, 53, 7–10. [Google Scholar] [CrossRef]
- Caputo, F.; Vignoli, T.; Maremmani, I.; Bernardi, M.; Zoli, G. Gamma hydroxybutyric acid (GHB) for the treatment of alcohol dependence: A review. Int. J. Environ. Res. Public Health 2009, 6, 1917–1929. [Google Scholar] [CrossRef]
- Dole, V.P.; Nyswander, M.E.; Kreek, M.J. Narcotic blockade. Arch. Intern. Medicine 1966, 118, 304–309. [Google Scholar] [CrossRef]
- Woods, J.S.; Joseph, H. From Narcotic to Normalizer: The Misperception of Methadone Treatment and the Persistence of Prejudice and Bias. Subst. Use Misuse 2018, 53, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Pacini, M.; Maremmani, A.G.I.; Maremmani, I. The Hystory of Methadone Treatment. In The Principles and Practice of Methadone Treatment; Maremmani, I., Ed.; Pacini Editore Medicina: Pisa, Italy, 2009; pp. 75–80. [Google Scholar]
- Parrino, M.W.; Maremmani, A.G.I.; Samuels, P.N.; Maremmani, I. Challenges and Opportunities for the Use of Medications to Treat Opioid Addiction in the United States and Other Nations of the World. J. Addict. Dis. 2015, 34, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Mautone, S.; Maremmani, A.G.I.; Gazzarrini, D.; Maremmani, I. Evaluating Methadone Maintenance Treatment. The Sant’Arsenio Methadone Clinic, in the Vallo di Diano region (Italy), as case study. Heroin Addict. Relat. Clin. Probl. 2016, 18, 5–14. [Google Scholar]
- Caputo, F.; Addolorato, G.; Domenicali, M.; Mosti, A.; Viaggi, M.; Trevisani, F.; Gasbarrini, G.; Bernardi, M.; Stefanin, G.F. Short-term methadone administration reduces alcohol consumption in non-alcoholic heroin addicts. Alcohol Alcohol. 2002, 37, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Caputo, F.; Addolorato, G.; Stoppo, M.; Francini, S.; Vignoli, T.; Lorenzini, F.; Del Re, A.; Comaschi, C.; Andreone, P.; Trevisani, F.; et al. Comparing and combining gamma-hydroxybutyric acid (GHB) and naltrexone in maintaining abstinence from alcohol: An open randomised comparative study. Eur. Neuropsychopharmacol. 2007, 17, 781–789. [Google Scholar] [CrossRef]
- Caputo, F.; Addolorato, G.; Lorenzini, F.; Domenicali, M.; Greco, G.; del Re, A.; Gasbarrini, G.; Stefanini, G.F.; Bernardi, M. Gamma-hydroxybutyric acid versus naltrexone in maintaining alcohol abstinence: An open randomized comparative study. Drug Alcohol. Depend. 2003, 70, 85–91. [Google Scholar] [CrossRef]
- Maremmani, I.; Lamanna, F.; Tagliamonte, A. Long-term therapy using GHB (sodium gamma hydroxybutyrate) for treatment-resistant chronic alcoholics. J. Psychoact. Drugs 2001, 33, 135–142. [Google Scholar] [CrossRef]
- Guiraud, J.; Addolorato, G.; Aubin, H.J.; Batel, P.; de Bejczy, A.; Caputo, F.; Goudriaan, A.E.; Gual, A.; Lesch, O.; Maremmani, I.; et al. Treating alcohol dependence with an abuse and misuse deterrent formulation of sodium oxybate: Results of a randomised, double-blind, placebo-controlled study. Eur. Neuropsychopharmacol. 2021, 52, 18–30. [Google Scholar] [CrossRef]
- Tambour, S.; Quertemont, E. Preclinical and clinical pharmacology of alcohol dependence. Fundam. Clin. Pharmacol. 2007, 21, 9–28. [Google Scholar] [CrossRef]
- Johnson, B.A.; Swift, R.M.; Addolorato, G.; Ciraulo, D.A.; Myrick, H. Safety and efficacy of GABAergic medications for treating alcoholism. Alcohol. Clin. Exp. Res. 2005, 29, 248–254. [Google Scholar] [CrossRef]
- Mannucci, C.; Pichini, S.; Spagnolo, E.V.; Calapai, F.; Gangemi, S.; Navarra, M.; Calapai, G. Sodium Oxybate Therapy for Alcohol Withdrawal Syndrome and Keeping of Alcohol Abstinence. Curr. Drug Metab. 2018, 19, 1056–1064. [Google Scholar] [CrossRef] [PubMed]
- Busardo, F.P.; Kyriakou, C.; Napoletano, S.; Marinelli, E.; Zaami, S. Clinical applications of sodium oxybate (GHB): From narcolepsy to alcohol withdrawal syndrome. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 4654–4663. [Google Scholar] [PubMed]
- Marinelli, E.; Beck, R.; Malvasi, A.; Lo Faro, A.F.; Zaami, S. Gamma-hydroxybutyrate abuse: Pharmacology and poisoning and withdrawal management. Arh. Hig. Rada Toksikol. 2020, 71, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Heier, E.C.; Eyer, F.; Rabe, C.; Geith, S.; Dargan, P.I.; Wood, D.M.; Heyerdahl, F.; Dines, A.M.; Giraudon, I.; Erik Hovda, K.; et al. Clinical effect of ethanol co-use in patients with acute drug toxicity involving the use of central nervous system depressant recreational drugs. Eur. J. Emerg. Med. 2022, 29, 291–300. [Google Scholar] [CrossRef]
- Addolorato, G.; Leggio, L.; Ferrulli, A.; Caputo, F.; Gasbarrini, A. The therapeutic potential of gamma-hydroxybutyric acid for alcohol dependence: Balancing the risks and benefits. A focus on clinical data. Expert. Opin. Investig. Drugs 2009, 18, 675–686. [Google Scholar] [CrossRef]
- Nemeth, Z.; Kun, B.; Demetrovics, Z. The involvement of gamma-hydroxybutyrate in reported sexual assaults: A systematic review. J. Psychopharmacol. 2010, 24, 1281–1287. [Google Scholar] [CrossRef]
- Addolorato, G.; Leggio, L.; Abenavoli, L.; Gasbarrini, G.; Caputo, F.; Vignoli, T.; Lorenzini, F.; Bernardi, M. Gamma hydroxybutyrric acid (GHB) withdrawal does not occur at therapeutic dosage. Drug Alcohol. Depend. 2005, 77, 209. [Google Scholar] [CrossRef]
- Caputo, F. Gamma-hydroxybutyrate (GHB) for the treatment of alcohol dependence: A call for further understanding. Alcohol Alcohol. 2011, 46, 3. [Google Scholar] [CrossRef]
- Caputo, F.; Francini, S.; Stoppo, M.; Lorenzini, F.; Vignoli, T.; Del Re, A.; Comaschi, C.; Leggio, L.; Addolorato, G.; Zoli, G.; et al. Incidence of craving for and abuse of gamma-hydroxybutyric acid (GHB) in different populations of treated alcoholics: An open comparative study. J. Psychopharmacol. 2009, 23, 883–890. [Google Scholar] [CrossRef]
- Kalivas, P.W.; Volkow, N.D. The neural basis of addiction: A pathology of motivation and choice. Am. J. Psychiatry 2005, 162, 1403–1413. [Google Scholar] [CrossRef]
- Rosen, M.I.; Pearsall, H.R.; Woods, S.W.; Kosten, T.R. Effects of gamma-hydroxybutyric acid (GHB) in opioid-dependent patients. J. Subst. Abus. Treat. 1997, 14, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.M.; Pierce, R.C. Cocaine-induced alterations in dopamine receptor signaling: Implications for reinforcement and reinstatement. Pharmacol. Ther. 2005, 106, 389–403. [Google Scholar] [CrossRef] [PubMed]
- Maremmani, I.; Pacini, M. Use of sodium gamma-hydroxybutyrate (GHB) in alcoholic heroin addicts and polydrug-abusers. Heroin Addict. Relat. Clin. Probl. 2007, 9, 55–76. [Google Scholar]
- Caputo, F. It is time for a responsible administration of gamma hydroxybutyrate and methadone. Heroin Addict. Relat. Clin. Probl. 2010, 12, 49–52. [Google Scholar]
- Dominguez, A.; Soca Gallego, L.; Patel, P.; Parmar, M. Sodium Oxybate. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]

| MM/SMO-Maintained | MM/SMO-Detoxified | p | ||
|---|---|---|---|---|
| Med (Q1–Q3) | Med (Q1–Q3) | z * | ||
| Age | 37 (28–51) | 42 (33–51) | 0.63 | 0.528 |
| N (%) | N (%) | χ2 | ||
| Sex, female | 13 (61.9) | 9 (42.9) | 1.52 | 0.217 |
| Education, <8 years | 13 (61.9) | 15 (71.4) | 0.43 | 0.513 |
| Marital status, with partner | 14 (66.7) | 13 (61.9) | 0.10 | 0.747 |
| Employment, blue collar | 14 (66.7) | 13 (61.9) | 0.15 | 0.929 |
| Income, adequate | 18 (85.7) | 19 (90.5) | 0.23 | 0.634 |
| Living situation, not alone | 17 (81.0) | 15 (71.4) | 0.52 | 0.469 |
| Illness Severity | Efficacy Index | Therapeutic Effect |
|---|---|---|
| 0. Not assessed | 0. Not assessed | 1. Marked/No side effect |
| 1. Normal, not at all ill | 1. Very much improved | 2. Marked/Do not interfere with patient functioning |
| 2. Borderline mentally ill | 2. Much improved | 3. Marked/Interferes with patient functioning |
| 3. Mildly ill | 3. Minimally improved | 4. Marked/Outweighs therapeutic effect |
| 4. Moderately ill | 4. No change | 5. Moderate/No side effect |
| 5. Markedly ill | 5. Minimally worse | 6. Moderate/Do not interfere with patient functioning |
| 6. Severely ill | 6. Much worse | 7. Moderate/Interferes with patient functioning |
| 8. Moderate/Outweighs therapeutic effect | ||
| 9. Minimal/No side effect | ||
| 10. Minimal/Do not interfere with patient functioning | ||
| 11. Minimal/Interferes with patient functioning | ||
| 12. Minimal/Outweighs therapeutic effect | ||
| 13. Unchanged or worse/No side effect | ||
| 14. Unchanged or worse/Do not interfere with patient functioning | ||
| 15. Unchanged or worse/Interferes with patient functioning | ||
| 16. Unchanged or worse/Outweighs therapeutic effect |
| N | B | Exp(B) | 95% CI | p | |
|---|---|---|---|---|---|
| Male sex (N = 20) Female sex (N = 22) | 22 20 | −0.41 | 1.00 0.96 | 0.02–1.94 | 0.935 |
| Age | 42 | −0.02 | 0.97 | 0.93–1.01 | 0.204 |
| Baseline severity of illness | 42 | 0.44 | 1.56 | 0.72–2.40 | 0.296 |
| MM-SMO detoxified MM-SMO maintained | 21 21 | −1.89 | 1.00 0.15 | 0.03–0.27 | 0.001 |
| MM/SMO- Detoxified | MM/SMO- Maintained | z * | p | |
|---|---|---|---|---|
| Med (Q1–Q3) | Med (Q1–Q3) | |||
| Endpoint CGI illness severity | 5.00 (4.00–6.00) | 4.00 (3.00–4.50) | −2.42 | 0.015 |
| Endpoint CGI efficacy index | 4.00 (3.00–4.00) | 1.00 (1.00–2.00) | −4.58 | 0.000 |
| Endpoint CGI therapeutic effect | 13.00 (9.00–14.00) | 2.00 (1.00–5.50) | −4.40 | 0.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maremmani, A.G.I.; Della Rocca, F.; Pacini, M.; Bacciardi, S.; Cimino, S.; Cerniglia, L.; Miccoli, M.; Maremmani, I. Sodium Oxybate (SMO) as Part of Agonist Opioid Treatment in Alcohol–Heroin-Addicted Patients. J. Clin. Med. 2025, 14, 4016. https://doi.org/10.3390/jcm14124016
Maremmani AGI, Della Rocca F, Pacini M, Bacciardi S, Cimino S, Cerniglia L, Miccoli M, Maremmani I. Sodium Oxybate (SMO) as Part of Agonist Opioid Treatment in Alcohol–Heroin-Addicted Patients. Journal of Clinical Medicine. 2025; 14(12):4016. https://doi.org/10.3390/jcm14124016
Chicago/Turabian StyleMaremmani, Angelo G. I., Filippo Della Rocca, Matteo Pacini, Silvia Bacciardi, Silvia Cimino, Luca Cerniglia, Mario Miccoli, and Icro Maremmani. 2025. "Sodium Oxybate (SMO) as Part of Agonist Opioid Treatment in Alcohol–Heroin-Addicted Patients" Journal of Clinical Medicine 14, no. 12: 4016. https://doi.org/10.3390/jcm14124016
APA StyleMaremmani, A. G. I., Della Rocca, F., Pacini, M., Bacciardi, S., Cimino, S., Cerniglia, L., Miccoli, M., & Maremmani, I. (2025). Sodium Oxybate (SMO) as Part of Agonist Opioid Treatment in Alcohol–Heroin-Addicted Patients. Journal of Clinical Medicine, 14(12), 4016. https://doi.org/10.3390/jcm14124016











