Oesophageal Perforation Surgical Treatment: What Affects the Outcome? A Multicenter Experience
Abstract
1. Introduction
2. Methods
2.1. Study Design
- Oesophageal perforations, ischaemia, and fistula occurring after elective oesophageal surgery.
- Patients treated exclusively with endoscopic approaches.
- Oesophageal tumours causing perforations.
2.2. Diagnosis and Symptoms
2.3. Management
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| OP | Oesophageal perforation |
| CT | Computed tomography |
| RE | Rigid oesophagoscopy |
| EGDS | Oesophagogastroduodenoscopy |
| ICU | Intensive care unit |
| FB | Foreign body |
| DTV | Deep vein thrombosis |
References
- Chirica, M.; Kelly, M.D.; Siboni, S.; Aiolfi, A.; Riva, C.G.; Asti, E.; Ferrari, D.; Leppäniemi, A.; Ten Broek, R.P.G.; Brichon, P.Y.; et al. Esophageal emergencies: WSES guidelines. World J. Emerg. Surg. 2019, 14, 26. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kaman, L.; Iqbal, J.; Kundil, B.; Kochhar, R. Management of Esophageal Perforation in Adults. Gastroenterol. Res. 2010, 3, 235–244. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dziedzic, D.; Prokopowicz, J.; Orlowski, T. Open surgery versus stent placement in failed primary surgical treatment of esophageal perforation—A single institutional experience. Scand. J. Gastroenterol. 2016, 51, 1031–1036. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Hou, L.; Qin, D.; Huang, T.; Yuan, T. Current treatment and outcome of esophageal perforation: A single-center experience and a pooled analysis. Medicine 2021, 100, e25600. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sdralis, E.I.K.; Petousis, S.; Rashid, F.; Lorenzi, B.; Charalabopoulos, A. Epidemiology, diagnosis, and management of esophageal perforations: Systematic review. Dis. Esophagus. 2017, 30, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Jones, W.G., II; Ginsberg, R.J. Esophageal perforation: A continuing challenge. Ann. Thorac. Surg. 1992, 53, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Bresadola, V.; Terrosu, G.; Favero, A.; Cattin, F.; Cherchi, V.; Adani, G.L.; Marcellino, M.G.; Bresadola, F.; De Anna, D. Treatment of perforation in the healthy esophagus: Analysis of 12 cases. Langenbecks Arch. Surg. 2008, 393, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Lampridis, S.; Mitsos, S.; Hayward, M.; Lawrence, D.; Panagiotopoulos, N. The insidious presentation and challenging management of esophageal perforation following diagnostic and therapeutic interventions. J. Thorac. Dis. 2020, 12, 2724–2734. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bladergroen, M.R.; Lowe, J.E.; Postlethwait, R.W. Diagnosis and recommended management of esophageal perforation and rupture. Ann. Thorac. Surg. 1986, 42, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Søreide, J.A.; Viste, A. Esophageal perforation: Diagnostic work-up and clinical decision-making in the first 24 hours. Scand. J. Trauma Resusc. Emerg. Med. 2011, 19, 66. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maniatis, V.; Chryssikopoulos, H.; Roussakis, A.; Kalamara, C.; Kavadias, S.; Papadopoulos, A.; Andreou, J.; Stringaris, K. Perforation of the alimentary tract: Evaluation with computed tomography. Abdom. Imaging 2000, 25, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Brinster, C.J.; Singhal, S.; Lee, L.; Marshall, M.B.; Kaiser, L.R.; Kucharczuk, J.C. Evolving options in the management of esophageal perforation. Ann. Thorac. Surg. 2004, 77, 1475–1483. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, P.L.; Pinto, J.V.; Costa, L.; Marques, P.; Moura, C.P. Rigid Esophagoscopy for Foreign Body Extraction: Results and Complications in the Endoscopic Era. Cureus 2024, 16, e53040. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sung, S.W.; Park, J.J.; Kim, Y.T.; Kim, J.H. Surgery in thoracic esophageal perforation: Primary repair is feasible. Dis. Esophagus. 2002, 15, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Sepesi, B.; Raymond, D.P.; Peters, J.H. Esophageal perforation: Surgical, endoscopic and medical management strategies. Curr. Opin. Gastroenterol. 2010, 26, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Salo, J.A.; Isolauri, J.O.; Heikkilä, L.J.; Markkula, H.T.; Heikkinen, L.O.; Kivilaakso, E.O.; Mattila, S.P. Management of delayed esophageal perforation with mediastinal sepsis. Esophagectomy or primary repair? J. Thorac. Cardiovasc. Surg. 1993, 106, 1088–1091. [Google Scholar] [CrossRef] [PubMed]
- Attar, S.; Hankins, J.R.; Suter, C.M.; Coughlin, T.R.; Sequeira, A.; McLaughlin, J.S. Esophageal perforation: A therapeutic challenge. Ann. Thorac. Surg. 1990, 50, 45–49; discussion 50–51. [Google Scholar] [CrossRef] [PubMed]
- Grillo, H.C.; Wilkins, E.W., Jr. Esophageal repair following late diagnosis of intrathoracic perforation. Ann. Thorac. Surg. 1975, 20, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Kotsis, L.; Agócs, L. The effectiveness of diaphragmatic pedicled grafts in esophageal injuries and wall reconstruction. Eur. J. Cardiothorac. Surg. 1998, 14, 218–220. [Google Scholar] [CrossRef] [PubMed]
- Cameron, J.L.; Kieffer, R.F.; Hendrix, T.R.; Mehigan, D.G.; Baker, R.R. Selective nonoperative management of contained intrathoracic esophageal disruptions. Ann. Thorac. Surg. 1979, 27, 404–408. [Google Scholar] [CrossRef]
- Teh, E.; Edwards, J.; Duffy, J.; Beggs, D. Boerhaave’s syndrome: A review of management and outcome. Interact Cardiovasc. Thorac. Surg. 2007, 6, 640–643. [Google Scholar] [CrossRef] [PubMed]
- Kluger, Y.; Ishay, O.B.; Sartelli, M.; Katz, A.; Ansaloni, L.; Gomez, C.A.; Biffl, W.; Catena, F.; Fraga, G.P.; Di Saverio, S.; et al. Caustic ingestion management: World society of emergency surgery preliminary survey of expert opinion. World J. Emerg. Surg. 2015, 10, 48. Erratum in World J. Emerg. Surg. 2015, 10, 56. [CrossRef]. [CrossRef] [PubMed] [PubMed Central]
- Zwischenberger, J.B.; Savage, C.; Bidani, A. Surgical aspects of esophageal disease: Perforation and caustic injury. Am. J. Respir. Crit. Care Med. 2002, 165, 1037–1040. [Google Scholar] [CrossRef] [PubMed]
- Raymond, D.P.; Watson, T.J. Esophageal Diversion. In Operative Techniques in Thoracic and Cardiovascular Surgery; Springer: Berlin/Heidelberg, Germany, 2025; Volume 13, pp. 138–146. [Google Scholar]
- Kochhar, R.; Poornachandra, K.S.; Puri, P.; Dutta, U.; Sinha, S.K.; Sethy, P.K.; Wig, J.D.; Nagi, B.; Singh, K. Comparative evaluation of nasoenteral feeding and jejunostomy feeding in acute corrosive injury: A retrospective analysis. Gastrointest Endosc. 2009, 70, 874–880. [Google Scholar] [CrossRef] [PubMed]
- Chirica, M.; Veyrie, N.; Munoz-Bongrand, N.; Zohar, S.; Halimi, B.; Celerier, M.; Cattan, P.; Sarfati, E. Late morbidity after colon interposition for corrosive esophageal injury: Risk factors, management, and outcome. A 20-years experience. Ann. Surg. 2010, 252, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Angorn, I.B.; Haffejee, A.A. Retrosternal gastric bypass for the palliative treatment of unresectable oesophageal carcinoma. A simple technique. S. Afr. Med. J. 1983, 64, 901–904. [Google Scholar] [PubMed]
- Tilanus, H.W.; Bossuyt, P.; Schattenkerk, M.E.; Obertop, H. Treatment of oesophageal perforation: A multivariate analysis. Br. J. Surg. 1991, 78, 582–585. [Google Scholar] [CrossRef] [PubMed]
- Abbas, G.; Schuchert, M.J.; Pettiford, B.L.; Pennathur, A.; Landreneau, J.; Landreneau, J.; Luketich, J.D.; Landreneau, R.J. Contemporaneous management of esophageal perforation. Surgery 2009, 146, 749–755; discussion 755–756. [Google Scholar] [CrossRef] [PubMed]
- Eroglu, A.; Turkyilmaz, A.; Aydin, Y.; Yekeler, E.; Karaoglanoglu, N. Current management of esophageal perforation: 20 years experience. Dis. Esophagus. 2009, 22, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Port, J.L.; Kent, M.S.; Korst, R.J.; Bacchetta, M.; Altorki, N.K. Thoracic esophageal perforations: A decade of experience. Ann. Thorac. Surg. 2003, 75, 1071–1074. [Google Scholar] [CrossRef] [PubMed]
- Finley, R.J.; Pearson, F.G.; Weisel, R.D.; Todd, T.R.; Ilves, R.; Cooper, J. The management of nonmalignant intrathoracic esophageal perforations. Ann. Thorac. Surg. 1980, 30, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Orringer, M.B. Transhiatal esophagectomy without thoracotomy for carcinoma of the esophagus. Adv. Surg. 1986, 19, 1–49. [Google Scholar] [CrossRef] [PubMed]
- Javed, A.; Pal, S.; Dash, N.R.; Sahni, P.; Chattopadhyay, T.K. Outcome following surgical management of corrosive strictures of the esophagus. Ann. Surg. 2011, 254, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Chirica, M.; Bonavina, L.; Kelly, M.D.; Sarfati, E.; Cattan, P. Caustic ingestion. Lancet 2017, 389, 2041–2052. [Google Scholar] [CrossRef] [PubMed]
- Hermansson, M.; Johansson, J.; Gudbjartsson, T.; Hambreus, G.; Jönsson, P.; Lillo-Gil, R.; Smedh, U.; Zilling, T. Esophageal perforation in South of Sweden: Results of surgical treatment in 125 consecutive patients. BMC Surg. 2010, 10, 31. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shaker, H.; Elsayed, H.; Whittle, I.; Hussein, S.; Shackcloth, M. The influence of the ‘golden 24-h rule’ on the prognosis of oesophageal perforation in the modern era. Eur. J. Cardiothorac. Surg. 2010, 38, 216–222. [Google Scholar] [CrossRef] [PubMed]
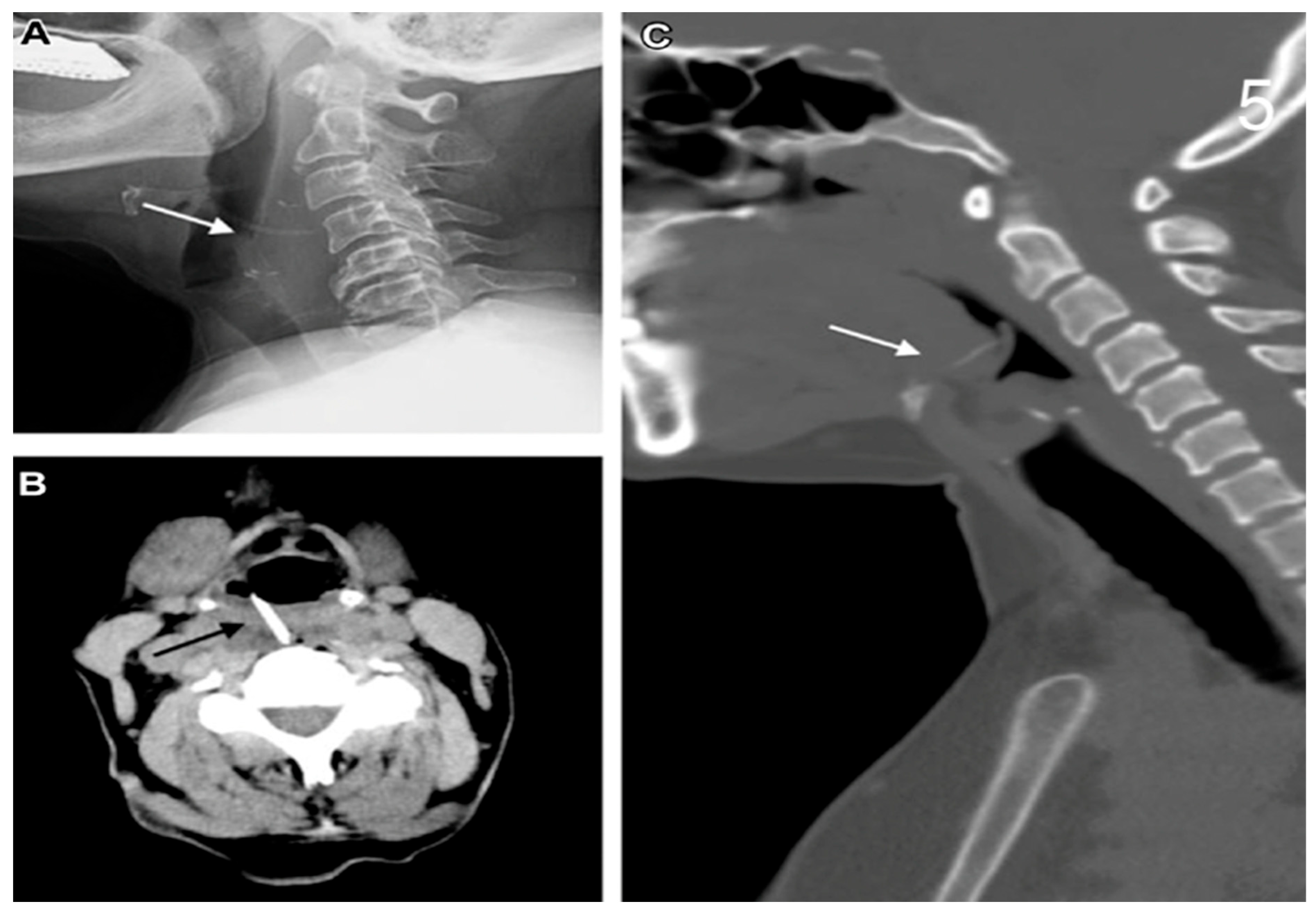
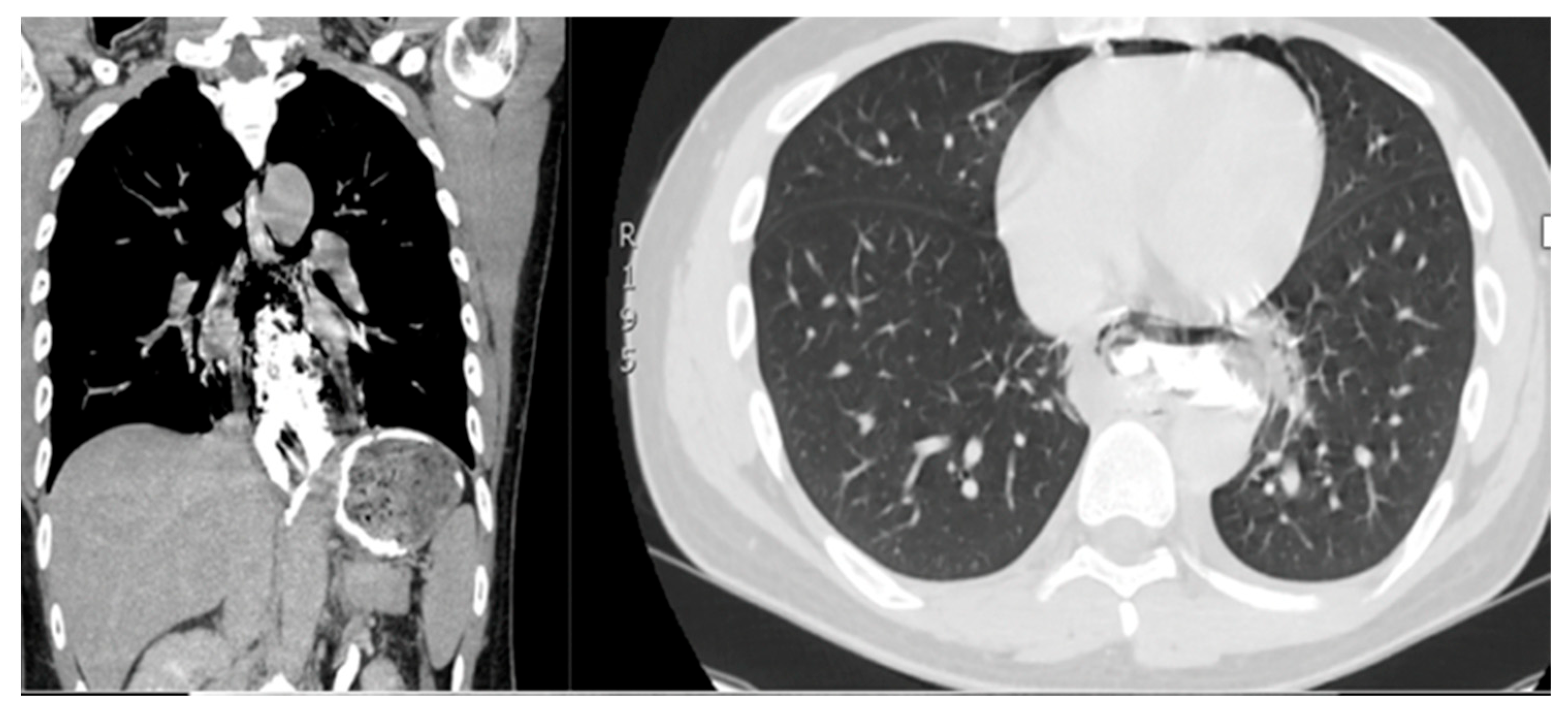
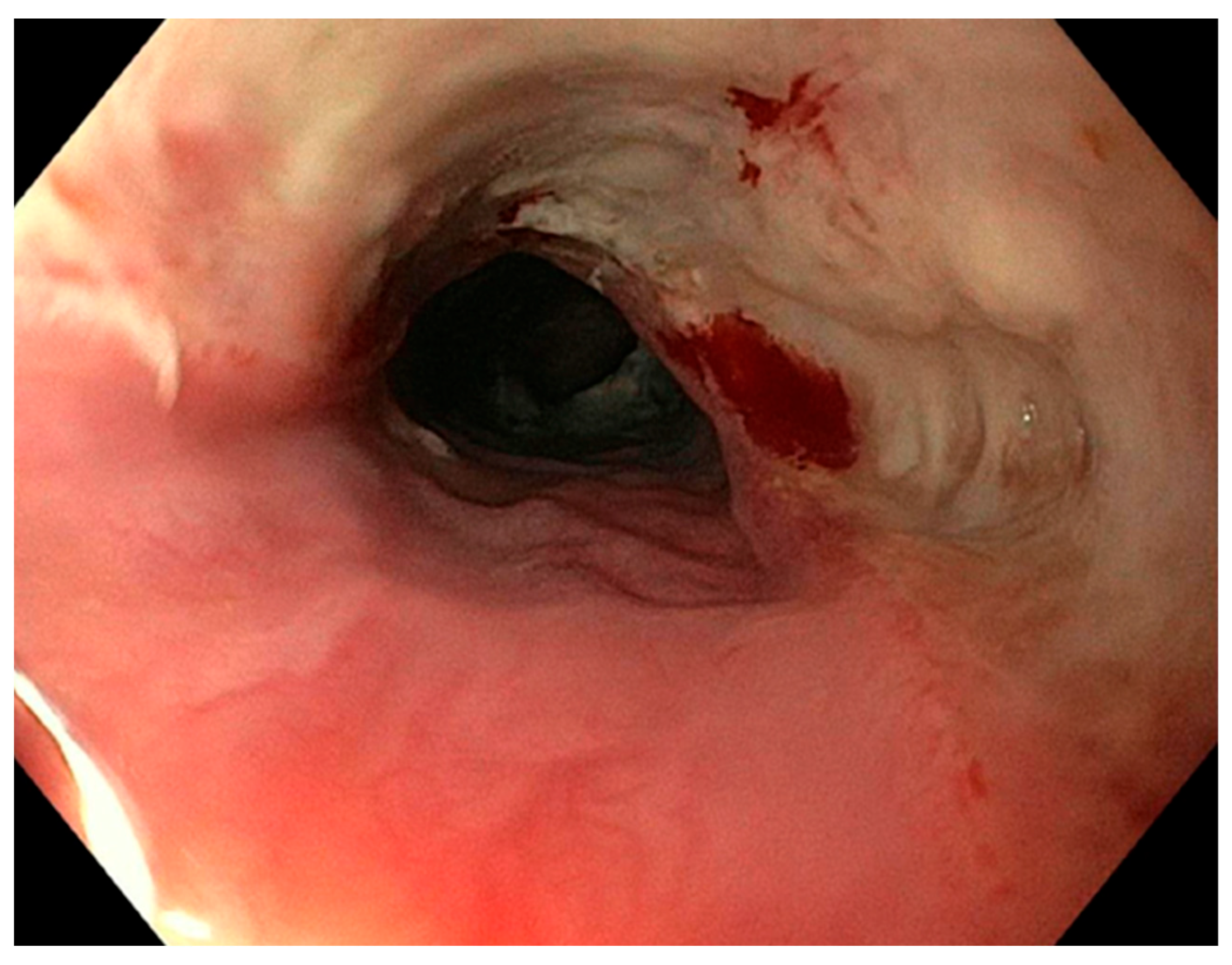

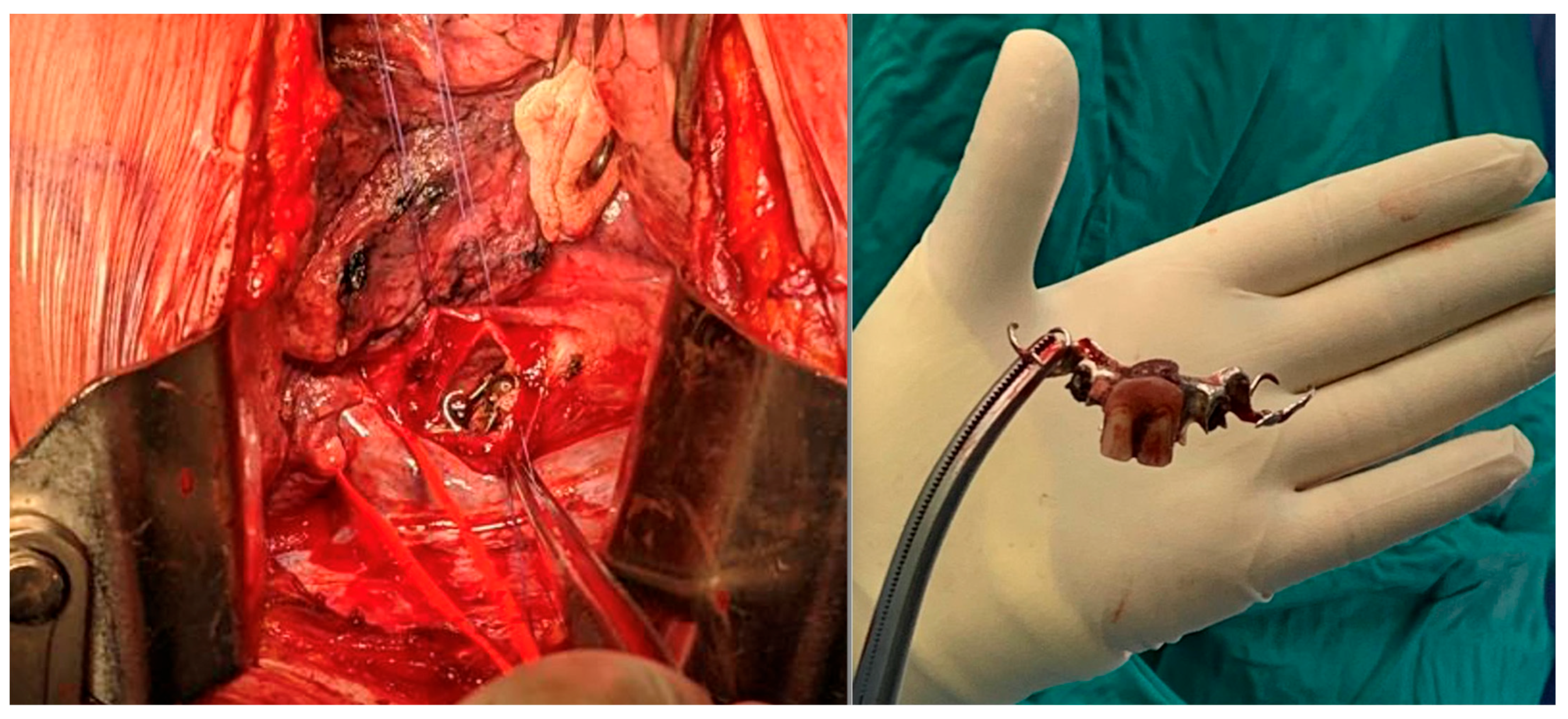
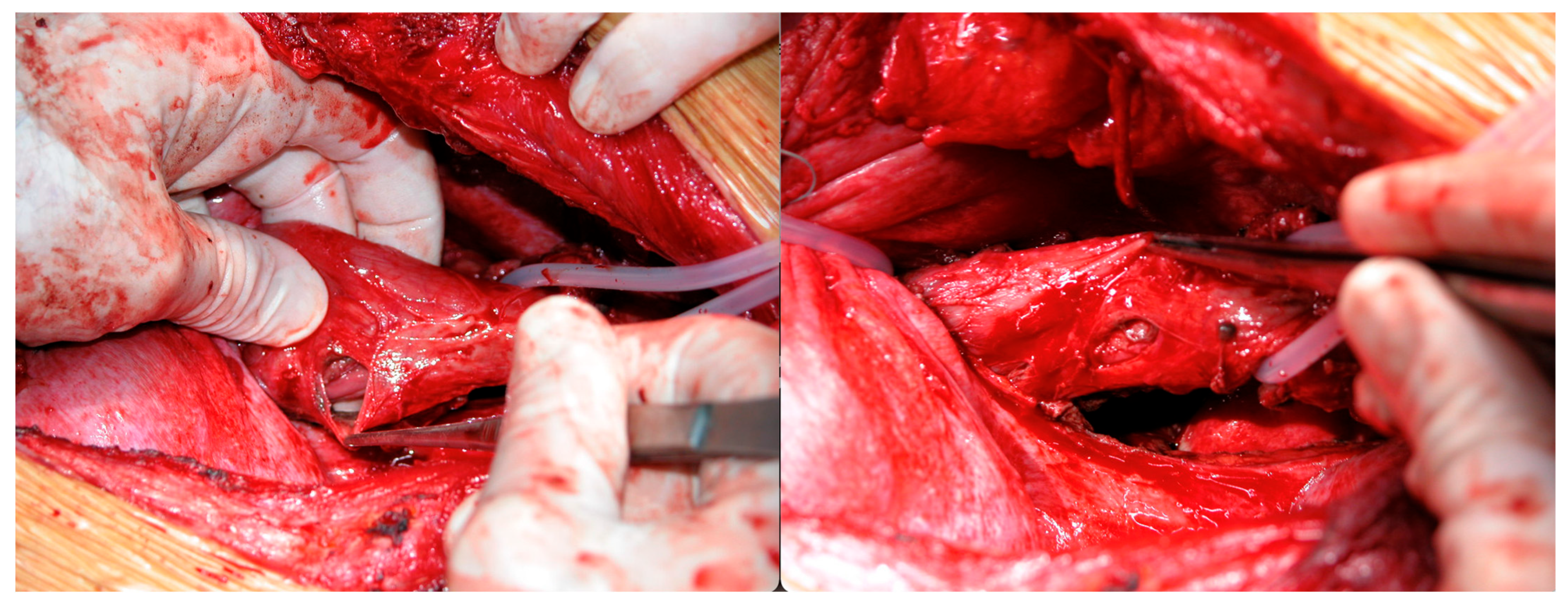
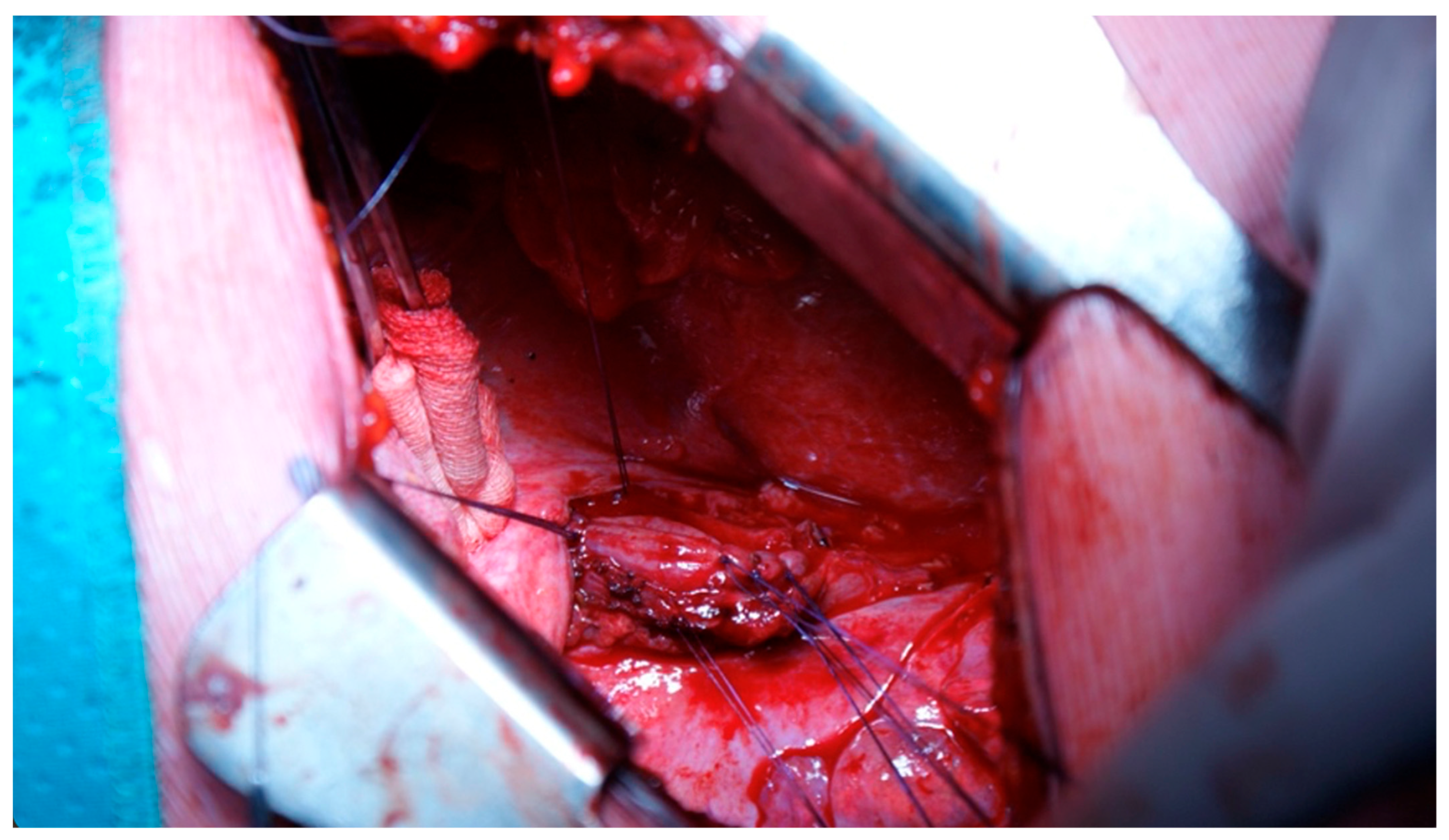

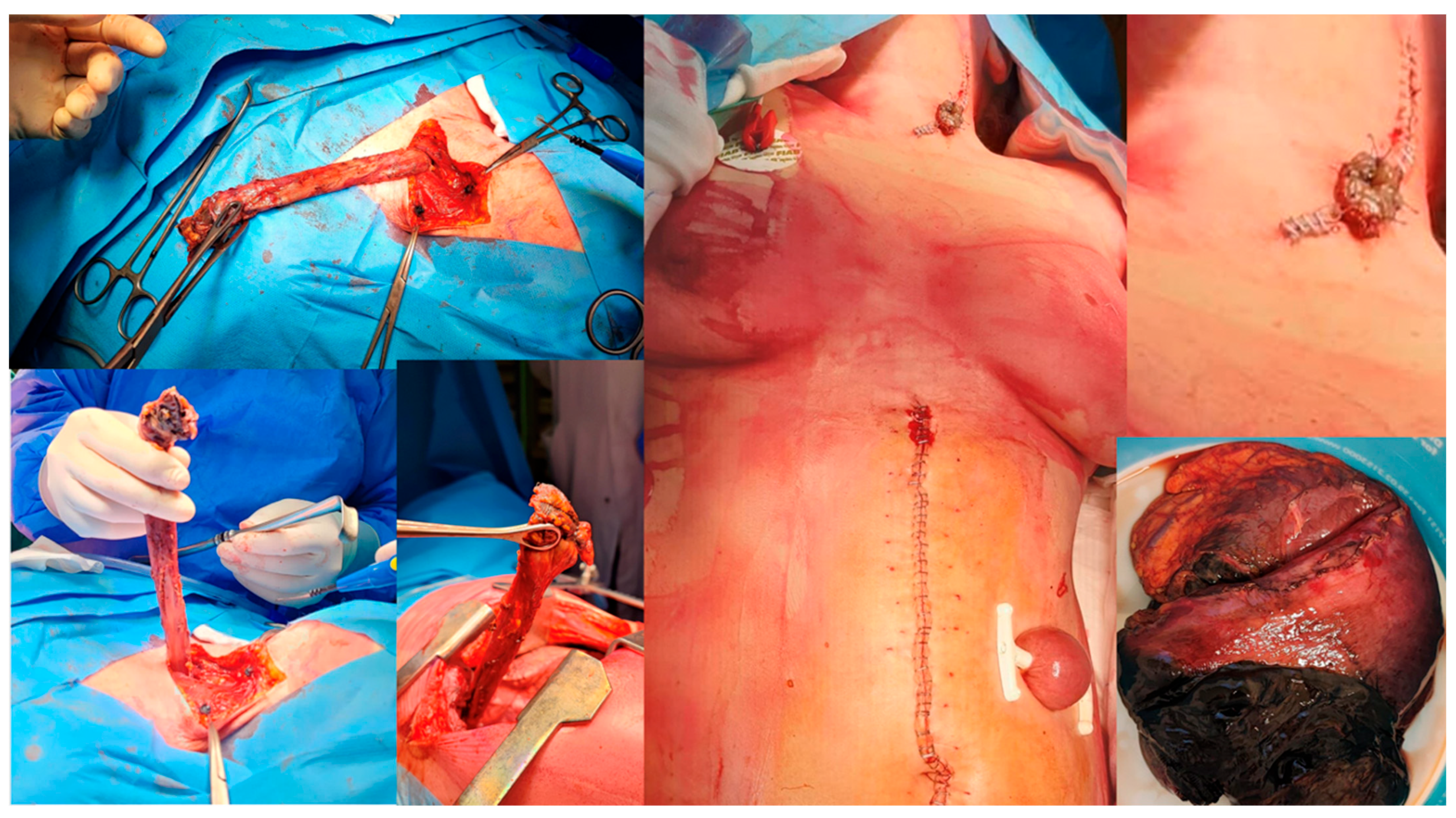
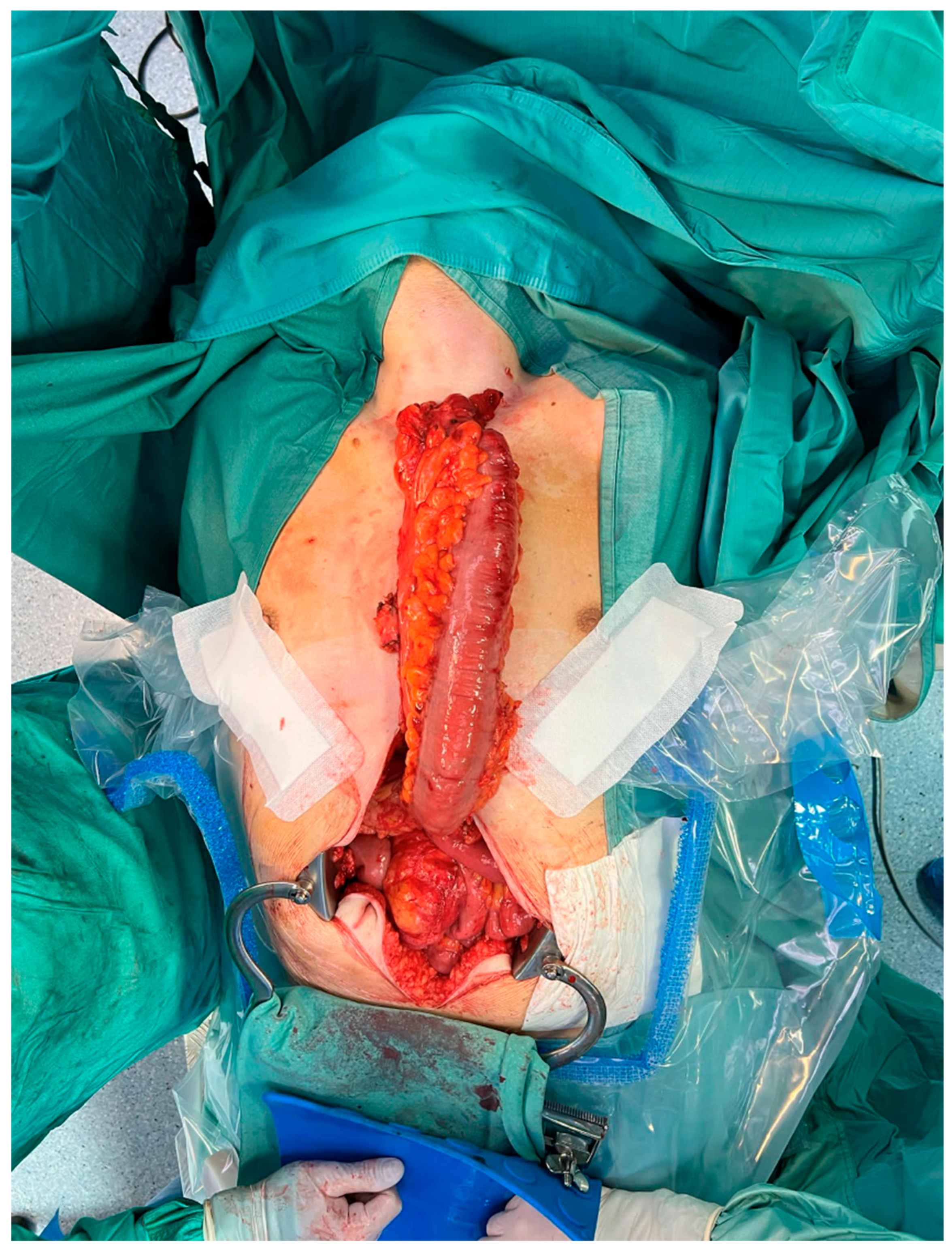

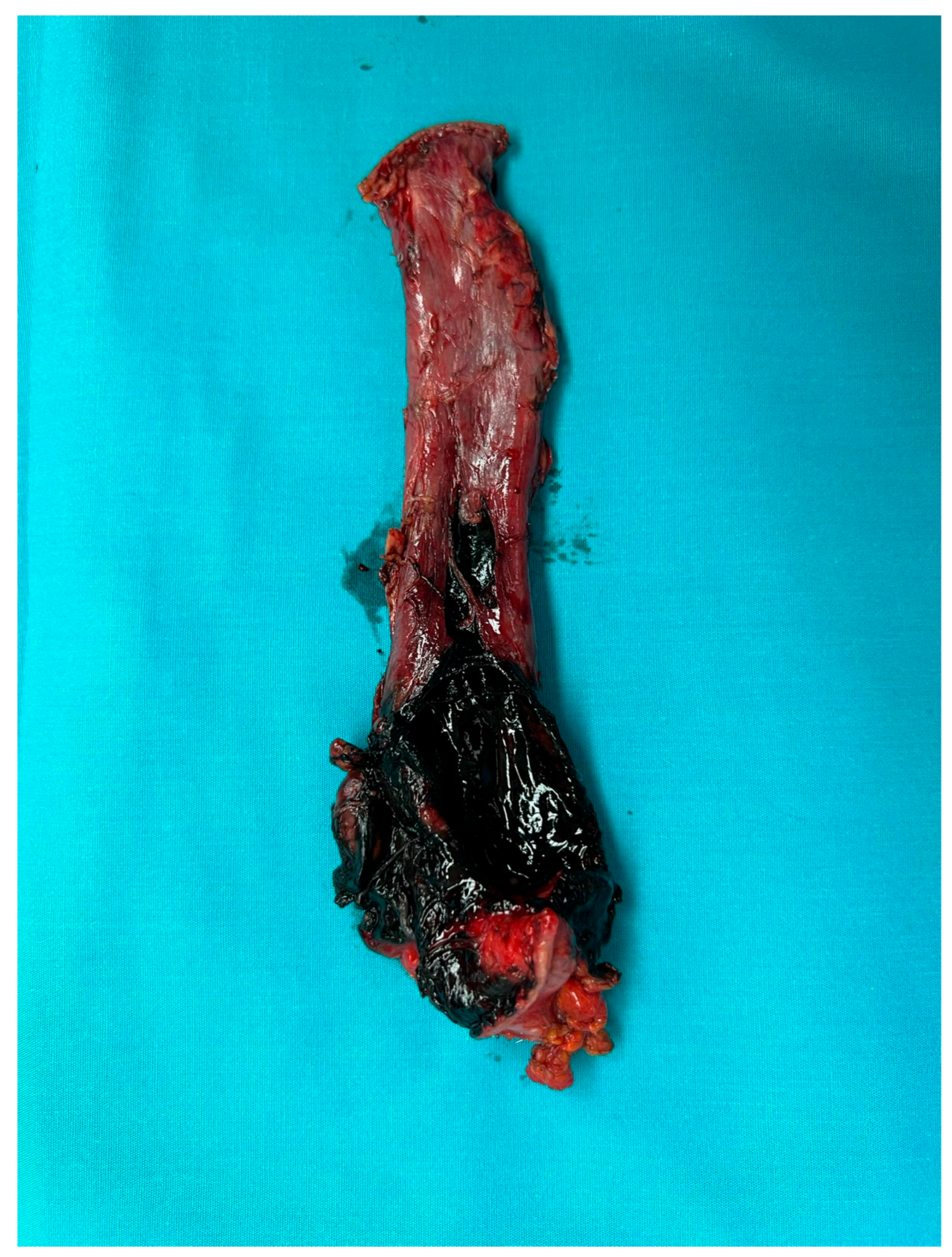
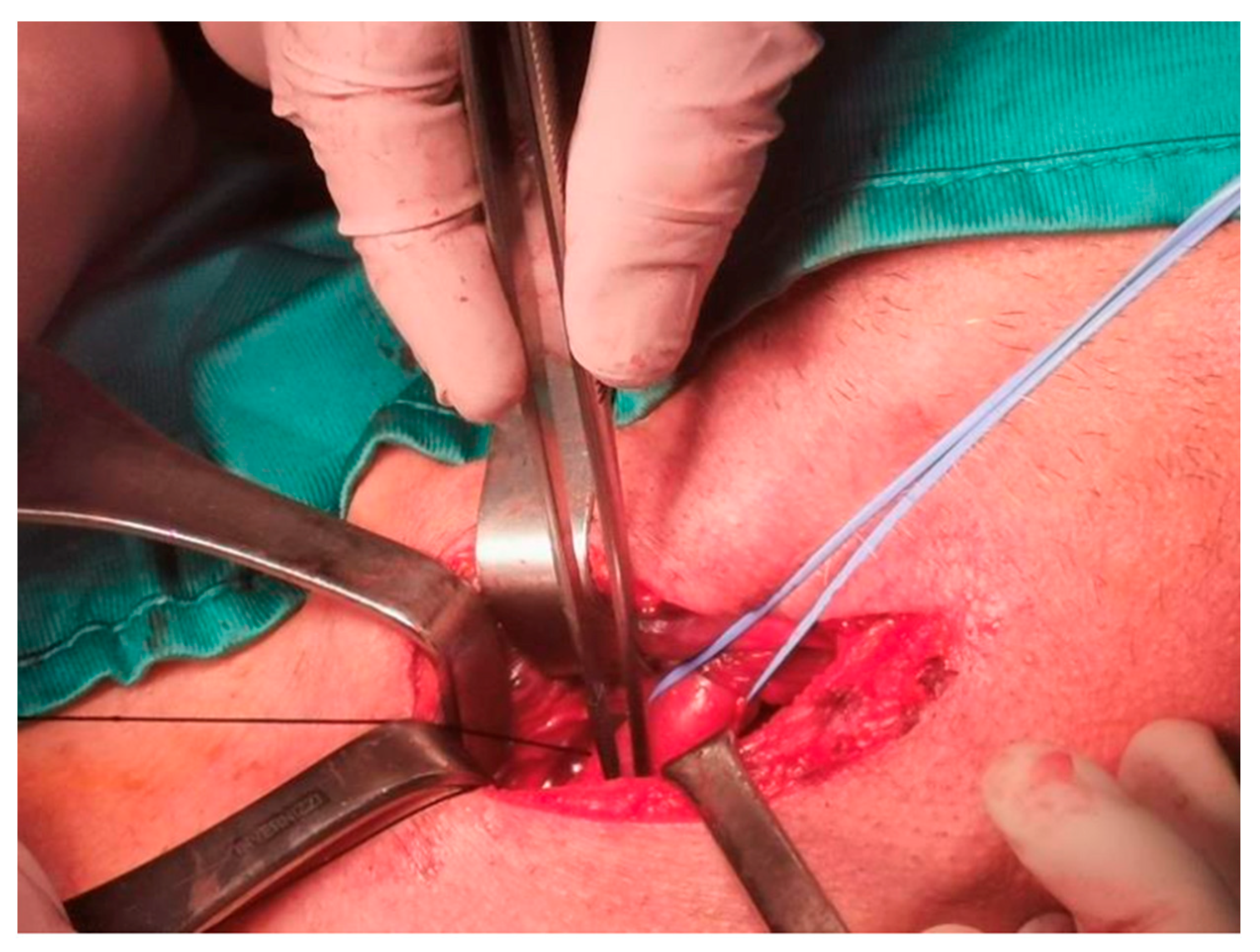
| Demographic Characteristics of Cohort | |
| Age (years) | 66 ± 17.75 |
| Gender (male) | 31 |
| Smoking | 13 |
| Cardiovascular disease | 6 |
| Diabetes | 2 |
| Neurological and psychiatric disorders | 10 |
| Oncological diseases | 2 |
| Frequent clinical symptoms onset | N° Patients |
| Chest pain | 26 |
| Vomiting | 12 |
| Fever | 10 |
| Abdominal pain | 8 |
| Dysphagia | 5 |
| Subcutaneous emphysema | 5 |
| Cervical pain | 3 |
| Sialorrhea | 3 |
| Respiratory failure | 3 |
| Dyspnoea | 3 |
| Hemodynamic instability | 1 |
| Xerostomy | 1 |
| Dysphonia | 1 |
| Rhinolalia | 1 |
| Melaena | 1 |
| FB (n%) | Boerhaave S (n%) | Caustic Ing. (n%) | Traumatic (n%) | Iatrogenic (n%) | |
|---|---|---|---|---|---|
| Location | |||||
| Cervical | 10 (66.66%) | 1 (50%) | 2 (40%) | ||
| Distal | 5 (33.33%) | 11 (73.33%) | 4 (50.00%) | 1 (50%) | 3 (60%) |
| Oesophagogastric | 4 (26.66%) | 4 (50.00%) | |||
| Surgery | |||||
| Debridement, drainage | 5 (33.33%) | 3 (20%) | 1 (50%) | 1 (25%) | |
| Oesophagotomy/Direct suture | 10 (66.66%) | 7 (46.66%) | 1 (50%) | 4 (80%) | |
| Muscle/Tissue flap | 6 (40%) | 3 (20%) | 1 (50%) | 1 (20%) | |
| Oesophagectomy | 4 (26.66%) | 4 (50%) | |||
| Oesophagectomy and gastrectomy | 3 (37.50%) | ||||
| Direct bipolar exclusion | 1 (15.55%) | ||||
| Bipolar oesophageal diversion | 2 (13.33%) | ||||
| Dietary jejunostomy | 1 (6.66%) | 6 (40%) | 7 (87.50%) | 1 (20%) | |
| Dietary gastrostomy | 1 (14.28%) | ||||
| Reconstructive Surgery | |||||
| Gastroplasty in same operative session | 2 (13.33%) | ||||
| Two-stage retrosternal gastroplasty | 2 (13.33%) | ||||
| Two-stage retrosternal coloplasty | 2 (13.33%) | 3 (37.50%) | |||
| Time of onset | |||||
| <24 h | 8 (53.33%) | 7 (46.66%) | 4 (50.00%) | 2 (100%) | 4 (80%) |
| >24–72 h | 3 (20%) | 3 (20%) | 3 (37.50%) | 1 (20%) | |
| >72 h | 4 (26.66%) | 5 (33.33%) | 1 (12.50%) |
| Post-Operative Complications | N° Patients | |
|---|---|---|
| Fever | 17 | |
| Fistula | 7 | |
| Respiratory failure | 6 | |
| Surgical wound infection | 2 | |
| Pleural and mediastinal effusion | 2 | |
| Oesophageal sub-stenosis | 2 | |
| Sepsis | 1 | |
| Mediastinitis | 1 | |
| DVT (deep vein thrombosis) | 1 | |
| Epistaxis | 1 | |
| Abdomen compartment syndrome | 1 | |
| Flap group | Complications in N° of patients | p-value |
| Cervical | (1/4) 25% | 0.303 |
| Thoracic | (4/7) 57.14% | |
| Flap group | Mortality in N° of patients | p-value |
| Cervical | (0/4) 0% | 0.428 |
| Thoracic | (1/7) 14.28% | |
| Complication rates by time of onset | N° of patients | p-value |
| <24 h | (15/25) 60% | 0.246 |
| >24 h | (14/20) 70% | |
| Mortality | ||
| Mortality rates by surgery timing | N° of patients | p-value |
| <24 | (6/25) 24% | 0.030 |
| >24 | (0/20) 0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Napolitano, A.G.; Nachira, D.; Petracca Ciavarella, L.; Coviello, E.; Pourmolkara, D.; Vaz Sousa, R.; Meacci, E.; De Giacomo, T.; Venuta, F.; Porziella, V.; et al. Oesophageal Perforation Surgical Treatment: What Affects the Outcome? A Multicenter Experience. J. Clin. Med. 2025, 14, 4019. https://doi.org/10.3390/jcm14124019
Napolitano AG, Nachira D, Petracca Ciavarella L, Coviello E, Pourmolkara D, Vaz Sousa R, Meacci E, De Giacomo T, Venuta F, Porziella V, et al. Oesophageal Perforation Surgical Treatment: What Affects the Outcome? A Multicenter Experience. Journal of Clinical Medicine. 2025; 14(12):4019. https://doi.org/10.3390/jcm14124019
Chicago/Turabian StyleNapolitano, Antonio Giulio, Dania Nachira, Leonardo Petracca Ciavarella, Eleonora Coviello, Domenico Pourmolkara, Rita Vaz Sousa, Elisa Meacci, Tiziano De Giacomo, Federico Venuta, Venanzio Porziella, and et al. 2025. "Oesophageal Perforation Surgical Treatment: What Affects the Outcome? A Multicenter Experience" Journal of Clinical Medicine 14, no. 12: 4019. https://doi.org/10.3390/jcm14124019
APA StyleNapolitano, A. G., Nachira, D., Petracca Ciavarella, L., Coviello, E., Pourmolkara, D., Vaz Sousa, R., Meacci, E., De Giacomo, T., Venuta, F., Porziella, V., Margaritora, S., Puma, F., & Vannucci, J. (2025). Oesophageal Perforation Surgical Treatment: What Affects the Outcome? A Multicenter Experience. Journal of Clinical Medicine, 14(12), 4019. https://doi.org/10.3390/jcm14124019





