The Hungry Heart: Managing Cardiogenic Shock in Patients with Severe Anorexia Nervosa—A Case Report Series
Abstract
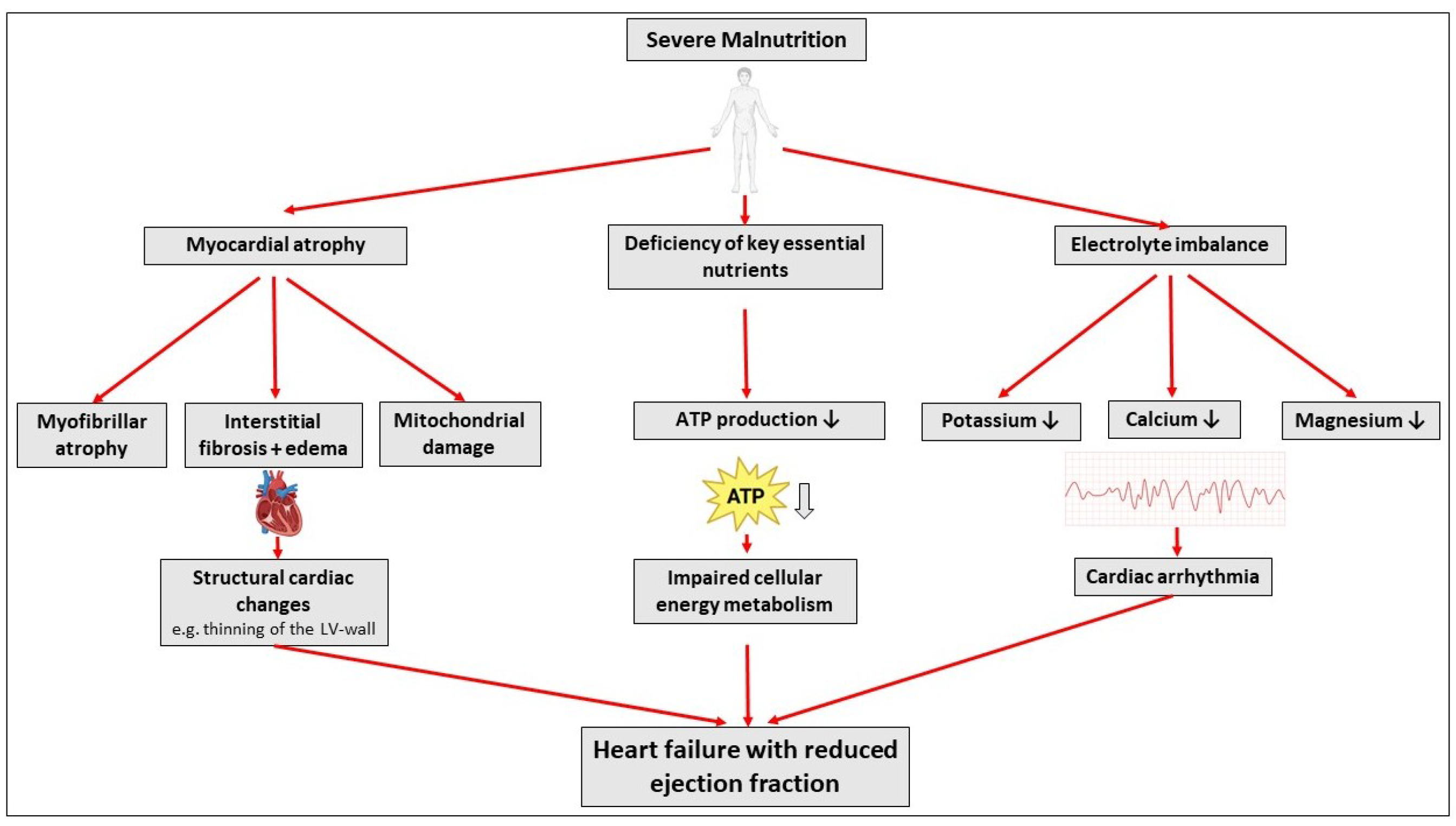
1. Introduction
2. Cases
2.1. Case 1
2.2. Case 2
2.3. Case 3
3. Therapy Recommendation
- -
- Continuous hemodynamic and ECG monitoring.
- -
- Central venous access.
- -
- Daily 12-lead ECG.
- -
- Daily transthoracic echocardiography.
- -
- Regular checks of glucose, electrolytes (potassium, sodium, phosphorus, magnesium, and calcium), kidney tests (blood urea nitrogen, creatinine), liver tests (AST, ALT, bilirubin), thyroid function tests (TSH, fT3, fT4) complete blood count, albumin, total proteins, C-reactive protein, 25-hydroxyvitamin D3 levels.
- -
- Regular checks of hydration status.
- -
- Mean arterial pressure aim of ≥ 65 mmHg.
- -
- First line catecholamines: dobutamine and norepinephrine.
- -
- Mechanical circulatory support for patients with progressive cardiogenic shock.
- -
- Initial phase with high catecholamine support: parenteral nutrition, in case of reduced catecholamine support enteral nutrition.
- -
- Calorie intake:
- ○
- Day 1–3: 5–10 kcal/kg BW/d.
- ○
- Day 4–6: 10–20 kcal/kg BW/d.
- ○
- Day 7–9: 20–30 kcal/kg BW/d.
- ○
- Day 10 and more: Full recommended calorie intake.
- -
- Fluid replacement:
- ○
- Day 1–3: 20–25 mL/kg BW/d.
- ○
- Day 4–6: 25–30 mL/kg BW/d.
- ○
- Day 7 and more: 30–35 mL/kg BW/d.
- -
- Thiamine substitution (200–300 mg) on days 1–5.
- -
- Phosphorus substitution: 750–1500 mg daily.
- -
- Daily substitution of polyvitamine and multi-trace element solution.
- -
- Electrolyte substitution if necessary.
- -
- Psychotherapeutic interventions if possible.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AN | Anorexia nervosa |
| BMI | Body mass index |
| CPAP | Continuous positive airway pressure |
| CS | Cardiogenic shock |
| CVVHD | Continuous venovenous hemodialysis |
| LV-EF | Left ventricular ejection fraction |
| MCS | Mechanical circulatory support |
| VA-ECMO | Venoarterial extracorporeal membrane oxygenation |
References
- Romano, C.; Chinali, M.; Pasanisi, F.; Greco, R.; Celentano, A.; Rocco, A.; Palmieri, V.; Signorini, A.; Contaldo, F.; de Simone, G. Reduced hemodynamic load and cardiac hypotrophy in patients with anorexia nervosa. Am. J. Clin. Nutr. 2003, 77, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Lamzabi, I.; Syed, S.; Reddy, V.B.; Jain, R.; Harbhajanka, A.; Arunkumar, P. Myocardial changes in a patient with anorexia nervosa: A case report and review of literature. Am. J. Clin. Pathol. 2015, 143, 734–737. [Google Scholar] [CrossRef] [PubMed]
- Vignaud, M.; Constantin, J.-M.; Ruivard, M.; Villemeyre-Plane, M.; Futier, E.; Bazin, J.-E.; Annane, D.; AZUREA Group (AnorexieRea Study Group). Refeeding syndrome influences outcome of anorexia nervosa patients in intensive care unit: An observational study. Crit Care 2010, 14, R172. [Google Scholar] [CrossRef]
- Lüsebrink, E.; Binzenhöfer, L.; Adamo, M.; Lorusso, R.; Mebazaa, A.; Morrow, D.A.; Price, S.; Jentzer, J.C.; Brodie, D.; Combes, A.; et al. Cardiogenic shock. Lancet 2024, 404, 2006–2020. [Google Scholar] [CrossRef]
- Duquesnoy, M.; Tric, L.; Hanachi, M.; Godart, N.; Lamer, C. Renutrition and management of severe anorexia nervosa in intensive care: Review and multidisciplinary approach. J. Food Nutr. Diet Sci. 2024, 2, 107–121. [Google Scholar] [CrossRef]
- Bischoff, S.C.; Arends, J.; Decker-Baumann, C.; Hütterer, E.; Koch, S.; Mühlebach, S.; Roetzer, I.; Schneider, A.; Seipt, C.; Simanek, R.; et al. S3-Leitlinie Heimenterale und heimparenterale Ernährung der Deutschen Gesellschaft für Ernährungsmedizin (DGEM). Aktuelle Ernährungsmedizin 2024, 49, 73–155. [Google Scholar]
- da Silva, J.S.V.; Seres, D.S.; Sabino, K.; Adams, S.C.; Berdahl, G.J.; Citty, S.W.; Cober, M.P.; Evans, D.C.; Greaves, J.R.; Gura, K.M.; et al. ASPEN Consensus Recommendations for Refeeding Syndrome. Nutr. Clin. Pract. 2020, 35, 178–195. [Google Scholar] [CrossRef] [PubMed]
- van Hoeken, D.; Hoek, H.W. Review of the burden of eating disorders: Mortality, disability, costs, quality of life, and family burden. Curr. Opin. Psychiatry 2020, 33, 521–527. [Google Scholar] [CrossRef]
- Birmingham, C.L.; Gritzner, S. Heart failure in anorexia nervosa: Case report and review of the literature. Eat. Weight Disord. 2007, 12, e7–e10. [Google Scholar] [CrossRef]
- Ono, T.; Kasaoka, S.; Fujita, M.; Yamashita, S.; Kumagai, K.; Kaneda, K.; Tsuruta, R.; Maekawa, T. Complete recovery from severe myocardial dysfunction in a patient with anorexia nervosa. J. Cardiol. 2009, 54, 480–484. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Kioka, H.; Hashimoto, R.; Takeda, S.; Momose, K.; Ohtani, T.; Yamaguchi, O.; Wasa, M.; Nakatani, S.; Sakata, Y. Cardiogenic shock caused by a left midventricular obstruction during refeeding in a patient with anorexia nervosa. Nutrition 2017, 35, 148–150. [Google Scholar] [CrossRef] [PubMed]
- St John Sutton, M.G.; Plappert, T.; Crosby, L.; Douglas, P.; Mullen, J.; Reichek, N. Effects of reduced left ventricular mass on chamber architecture, load, and function: A study of anorexia nervosa. Circulation 1985, 72, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- Winston, A.P.; Jamieson, C.P.; Madira, W.; Gatward, N.M.; Palmer, R.L. Prevalence of thiamin deficiency in anorexia nervosa. Int. J. Eat. Disord. 2000, 28, 451–454. [Google Scholar] [CrossRef] [PubMed]
- Cappelli, V.; Bottinelli, R.; Polla, B.; Reggiani, C. Altered contractile properties of rat cardiac muscle during experimental thiamine deficiency and food deprivation. J. Mol. Cell. Cardiol. 1990, 22, 1095–1106. [Google Scholar] [CrossRef]
- O’Connor, G.; Nicholls, D. Refeeding hypophosphatemia in adolescents with anorexia nervosa: A systematic review. Nutr. Clin. Pract. 2013, 28, 358–364. [Google Scholar] [CrossRef]
- Murialdo, G.; Casu, M.; Falchero, M.; Brugnolo, A.; Patrone, V.; Cerro, P.F.; Ameri, P.; Andraghetti, G.; Briatore, L.; Copello, F.; et al. Alterations in the autonomic control of heart rate variability in patients with anorexia or bulimia nervosa: Correlations between sympathovagal activity, clinical features, and leptin levels. J. Endocrinol. Investig. 2007, 30, 356–362. [Google Scholar] [CrossRef]
- Fonseca, V.; Havard, C.W. Electrolyte disturbances and cardiac failure with hypomagnesaemia in anorexia nervosa. Br. Med. J. (Clin. Res. Ed.) 1985, 291, 1680–1682. [Google Scholar] [CrossRef]
- Facchini, M.; Sala, L.; Malfatto, G.; Bragato, R.; Redaelli, G.; Invitti, C. Low-K+ dependent QT prolongation and risk for ventricular arrhythmia in anorexia nervosa. Int. J. Cardiol. 2006, 106, 170–176. [Google Scholar] [CrossRef]
- Kanbur, N.O.; Goldberg, E.; Pinhas, L.; Hamilton, R.M.; Clegg, R.; Katzman, D.K. Second-degree atrioventricular block (Mobitz Type I) in an adolescent with anorexia nervosa: Intrinsic or acquired conduction abnormality. Int. J. Eat. Disord. 2009, 42, 575–578. [Google Scholar] [CrossRef]
- Räisänen, U.; Hunt, K. The role of gendered constructions of eating disorders in delayed help-seeking in men: A qualitative interview study. BMJ Open 2014, 4, e004342. [Google Scholar] [CrossRef]
- Gueguen, J.; Godart, N.; Chambry, J.; Brun-Eberentz, A.; Foulon, C.; Divac, S.M.; Guelfi, J.; Rouillon, F.; Falissard, B.; Huas, C. Severe anorexia nervosa in men: Comparison with severe AN in women and analysis of mortality. Int. J. Eat. Disord. 2012, 45, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Strobel, C.; Quadflieg, N.; Naab, S.; Voderholzer, U.; Fichter, M.M. Long-term outcomes in treated males with anorexia nervosa and bulimia nervosa-A prospective, gender-matched study. Int. J. Eat. Disord. 2019, 52, 1353–1364. [Google Scholar] [CrossRef] [PubMed]
- Møller, J.E.; Engstrøm, T.; Jensen, L.O.; Eiskjær, H.; Mangner, N.; Polzin, A.; Schulze, P.C.; Skurk, C.; Nordbeck, P.; Clemmensen, P.; et al. Microaxial Flow Pump or Standard Care in Infarct-Related Cardiogenic Shock. N. Engl. J. Med. 2024, 390, 1382–1393. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contribution of the Heart Failure Association (HFA) of the ESC. Rev. Esp. Cardiol. (Engl. Ed.) 2022, 75, 523. [Google Scholar]
| Patient 1 | Patient 2 | Patient 3 | |
|---|---|---|---|
| Age (years) | 54 | 25 | 55 |
| Gender | female | male | male |
| Pre-existing conditions | stroke | schizophrenia | dilated cardiomyopathy, invasive aspergillosis |
| Type of anorexia (restricting/binge eating) | restricting | restricting | restricting |
| Medication on admission | - | - | acetylsalicylic acid, mirtazapine |
| BMI on admission (kg/m2) | 12.0 | 15.6 | 12.5 |
| SOFA II score on admission | 20 | 16 | 13 |
| SCAI stage admission | D | D | E |
| SCAI stage max ICU stay | D | D | E |
| Cardiopulmonary resuscitation (OHCA/IHCA) | IHCA | OHCA, IHCA | - |
| Duration of ICU stay (days) | 12 | 58 | 32 |
| Duration of hospital stay (days) | 12 | 58 | 32 |
| Systolic blood pressure on admission (mmHg) | 97 | 82 | 83 |
| Diastolic blood pressure on admission (mmHg) | 72 | 52 | 73 |
| Heart rate on admission (bpm) | 89 | 79 | 109 |
| LVEF on admission (%) | 15 | 20 | 5 |
| LVEF on ICU discharge (%) | 60 | 45 | 60 |
| Mechanical ventilation (duration in days) | 13 | 18 | 12 |
| Laboratory values on admission | |||
| Hemoglobin (g/dL) | 9.4 | 4.8 | 7.8 |
| pH | 7.31 | 7.65 | 7.39 |
| Lactate (mmol/L) | 5.3 | 2.5 | 3.4 |
| Potassium (mmol/L) | 4.5 | 3.9 | 5 |
| Sodium (mmol/L) | 136 | 132 | 140 |
| Phosphorus (mg/dL) | 0.94 | 3.6 | 5.4 |
| Magnesium (mmol/L) | 4.7 | 3.28 | 0.96 |
| Calcium (mmol/L) | 2.21 | 1.65 | 1.89 |
| Blood urea nitrogen (mg/dL) | 11 | 4.4 | 3.1 |
| Creatinine (mg/dL) | 0.9 | 0.6 | 0.8 |
| AST (U/L) | 85 | 918 | 1208 |
| ALT (U/L) | 39 | 681 | 309 |
| Bilirubin (md/dL) | 1.1 | 0.5 | 0.6 |
| TSH (µg/mL) | 0.65 | 2.04 | 0.75 |
| fT3 (pg/mL) | 1.1 | 1.2 | 1.1 |
| fT4 (ng/dl) | 0.9 | 0.5 | 1.4 |
| Albumin (g/dL) | 2.5 | 2.3 | 2.2 |
| Total protein (g/dL) | 3.8 | 2.8 | 3.3 |
| CRP (mg/dL) | 14.5 | <0.1 | 6.2 |
| Vitamin D (ng/mL) | <3 | <3 | 11.3 |
| VA-ECMO duration | - | 10 days | 8 days |
| Impella (duration) | - | - | 16 days |
| Renal replacement therapy (duration) | 5 days | 40 days | 2 days |
| Laboratory values day 1–7 | |||
| Lactate max on day 1 (mmol/L) | 6.5 | 4.1 | 3.4 |
| Lactate max on day 2 (mmol/L) | 4.3 | 4.5 | 3.3 |
| Lactate max on day 3 (mmol/L) | 3.2 | 3.2 | 1.3 |
| Lactate max on day 4 (mmol/L) | 2.3 | 1.8 | 1.4 |
| Lactate max on day 5 (mmol/L) | 2 | 1.6 | 1.8 |
| Lactate max on day 6 (mmol/L) | 1.7 | 1.7 | 1.3 |
| Lactate max on day 7 (mmol/L) | 1.6 | 1.3 | 1.9 |
| Mean Potassium day 1 (mmol/L) | 4.1 | 3.5 | 4.4 |
| Mean Potassium day 2 (mmol/L) | 3.7 | 4.3 | 4.6 |
| Mean Potassium day 3 (mmol/L) | 3.8 | 4 | 4.2 |
| Mean Potassium day 4 (mmol/L) | 3.7 | 4.5 | 4.3 |
| Mean Potassium day 5 (mmol/L) | 3.9 | 4.2 | 4.1 |
| Mean Potassium day 6 (mmol/L) | 4.6 | 4.3 | 4 |
| Mean Potassium day 7 (mmol/L) | 4.2 | 4.4 | 4.5 |
| Mean phosphorus day 1 (mg/dL) | 4.7 | 3.3 | 4.95 |
| Mean phosphorus day 2 (mg/dL) | 2.85 | 3.8 | 5.35 |
| Mean phosphorus day 3 (mg/dL) | 1.95 | 4 | 5.1 |
| Mean phosphorus day 4 (mg/dL) | 1.6 | 3.4 | 4.9 |
| Mean phosphorus day 5 (mg/dL) | 2.35 | 2.3 | 4.6 |
| Mean phosphorus day 6 (mg/dL) | 2.2 | 4.1 | 2.6 |
| Mean phosphorus day 7 (mg/dL) | 3.6 | 5 | 1.7 |
| Mean albumin day 1 (g/dL) | 2.5 | 2.65 | 2.45 |
| Mean albumin day 2 (g/dL) | 3.1 | n.a. | 2.6 |
| Mean albumin day 3 (g/dL) | 2.7 | n.a. | 2.6 |
| Mean albumin day 4 (g/dL) | 3.4 | 3.2 | 2.3 |
| Mean albumin day 5 (g/dL) | 3.2 | 3.3 | 2.3 |
| Mean albumin day 6 (g/dL) | n.a. | 3.6 | 2.3 |
| Mean albumin day 7 (g/dL) | 3.2 | 3.2 | n.a. |
| AST day 1 (U/L) | 85 | 918 | 1208 |
| AST day 2 (U/L) | 74 | 825 | 504 |
| AST day 3 (U/L) | 56 | 333 | 423 |
| AST day 4 (U/L) | 290 | 164 | 340 |
| AST day 5 (U/L) | 121 | 82 | 259 |
| AST day 6 (U/L) | 81 | 52 | 159 |
| AST day 7 (U/L) | 86 | 32 | 87 |
| ALT day 1 (U/L) | 39 | 681 | 309 |
| ALT day 2 (U/L) | 32 | 570 | 195 |
| ALT day 3 (U/L) | 32 | 358 | 183 |
| ALT day 4 (U/L) | 90 | 282 | 157 |
| ALT day 5 (U/L) | 73 | 207 | 133 |
| ALT day 6 (U/L) | 61 | 147 | 97 |
| ALT day 7 (U/L) | 61 | 100 | 24 |
| Creatinine day 1 (mg/dL) | 0.9 | 0.6 | 0.9 |
| Creatinine day 2 (mg/dL) | 0.8 | 0.8 | 1.1 |
| Creatinine day 3 (mg/dL) | 0.6 | 1.2 | 1.1 |
| Creatinine day 4 (mg/dL) | 0.7 | 1.3 | 1.1 |
| Creatinine day 5 (mg/dL) | 0.7 | 1.2 | 0.9 |
| Creatinine day 6 (mg/dL) | 0.7 | 1.4 | 0.6 |
| Creatinine day 7 (mg/dL) | 0.7 | 1.9 | 0.6 |
| Vasopressors (median dose in mg/h or µg/h) | |||
| Norepinephrin day 1 | 1.5 | 0.4 | 1.1 |
| Norepinephrin day 2 | 1.2 | 0.4 | 1 |
| Norepinephrin day 3 | 1.1 | 0.5 | 1 |
| Norepinephrin day 4 | 0.9 | 0.5 | 0.8 |
| Norepinephrin day 5 | 0.3 | 0.2 | 1 |
| Norepinephrin day 6 | 0.2 | 0.002 | 0.6 |
| Norepinephrin day 7 | - | 0.002 | 0.2 |
| Epinephrin day 1 | 0.7 | 0.1 | - |
| Epinephrin day 2 | - | - | - |
| Epinephrin day 3 | - | - | - |
| Epinephrin day 4 | - | - | - |
| Epinephrin day 5 | - | - | - |
| Epinephrin day 6 | - | - | - |
| Epinephrin day 7 | - | - | - |
| Dobutamin day 1 | 25 | - | 25 |
| Dobutamin day 2 | 30 | - | 20 |
| Dobutamin day 3 | 30 | - | 20 |
| Dobutamin day 4 | 20 | 10 | 20 |
| Dobutamin day 5 | 15 | 10 | 20 |
| Dobutamin day 6 | 10 | 10 | 15 |
| Dobutamin day 7 | - | 10 | - |
| Vasopressin day 1 | - | - | 0.8 |
| Vasopressin day 2 | - | - | - |
| Vasopressin day 3 | - | - | - |
| Vasopressin day 4 | - | - | - |
| Vasopressin day 5 | - | - | - |
| Vasopressin day 6 | - | - | - |
| Vasopressin day 7 | - | - | - |
| Calorie intake (kcal) | |||
| Calorie intake day 1 | - | - | 1029 |
| Calorie intake day 2 | 135 | 1365 | 2016 |
| Calorie intake day 3 | 277.5 | 2415 | 1806 |
| Calorie intake day 4 | 2041.2 | 3120.09 | 2268 |
| Calorie intake day 5 | 2922.75 | 3580.74 | 2793 |
| Calorie intake day 6 | 2553.2 | 3537.165 | 2367.75 |
| Calorie intake day 7 | 2046 | 3591.255 | 3024 |
| Fluid intake (mL) | |||
| Fluid intake day 1 | 4808.72 | 8922.86 | 6215.14 |
| Fluid intake day 2 | 3233.43 | 5483.11 | 3118.8 |
| Fluid intake day 3 | 3969.16 | 2607.11 | 4553.32 |
| Fluid intake day 4 | 2591.91 | 2679.89 | 5310.33 |
| Fluid intake day 5 | 3101.56 | 4097.92 | 7415.82 |
| Fluid intake day 6 | 2499.09 | 3111.41 | 5010.53 |
| Fluid intake day 7 | 2607.26 | 3810.69 | 5270.51 |
| Potassium intake (mval) | |||
| Potassium intake day 1 | 24.8 | 157.5 | 25.5 |
| Potassium intake day 2 | 81 | 187.3 | 34 |
| Potassium intake day 3 | 144 | 59 | 74.4 |
| Potassium intake day 4 | 106.2 | 72 | 49.5 |
| Potassium intake day 5 | 157 | 48 | 233 |
| Potassium intake day 6 | 124 | 95.7 | 284 |
| Potassium intake day 7 | 72 | 40.5 | 294 |
| Phosphorus intake (mmol) | |||
| Phosphorus intake day 1 | 0 | 0 | 0 |
| Phosphorus intake day 2 | 20 | 40 | 0 |
| Phosphorus intake day 3 | 48 | 24 | 0 |
| Phosphorus intake day 4 | 0 | 24 | 0 |
| Phosphorus intake day 5 | 96 | 24 | 0 |
| Phosphorus intake day 6 | 24 | 72 | 0 |
| Phosphorus intake day 7 | 48 | 48 | 36 |
| Polyvitamine and multi-trace element solution day 1–7 | yes | yes | yes |
| Vitamin B1 intake (mg) | |||
| Vitamin B1 intake day 1 | 100 | 200 | 400 |
| Vitamin B1 intake day 2 | 800 | 300 | 400 |
| Vitamin B1 intake day 3 | 2000 | 400 | 400 |
| Vitamin B1 intake day 4 | 1500 | 400 | 400 |
| Vitamin B1 intake day 5 | 1500 | 400 | 0 |
| Vitamin B1 intake day 6 | 1000 | 400 | 0 |
| Vitamin B1 intake day 7 | 0 | 400 | 0 |
| Vitamin D intake (IE) | |||
| Vitamin D intake day 1 | 0 | 0 | 20000 |
| Vitamin D intake day 2 | 0 | 0 | 1000 |
| Vitamin D intake day 3 | 0 | 0 | 1000 |
| Vitamin D intake day 4 | 0 | 1000 | 1000 |
| Vitamin D intake day 5 | 0 | 1000 | 1000 |
| Vitamin D intake day 6 | 0 | 1000 | 1000 |
| Vitamin D intake day 7 | 0 | 1000 | 1000 |
| CPC on discharge | 1 | 1 | 3 |
| Final diagnosis | cardiogenic shock due to severe malnutrition in the context of AN | OHCA with cardiogenic shock due to severe malnutrition in context of AN | cardiogenic shock due to severe malnutrition in context of AN |
| Last follow-up visit | alive | alive | dead |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thienel, M.; Kaiser, R.; Gmeiner, J.; Orban, M.; Kääb, S.; Petzold, T.; Massberg, S.; Scherer, C. The Hungry Heart: Managing Cardiogenic Shock in Patients with Severe Anorexia Nervosa—A Case Report Series. J. Clin. Med. 2025, 14, 4011. https://doi.org/10.3390/jcm14114011
Thienel M, Kaiser R, Gmeiner J, Orban M, Kääb S, Petzold T, Massberg S, Scherer C. The Hungry Heart: Managing Cardiogenic Shock in Patients with Severe Anorexia Nervosa—A Case Report Series. Journal of Clinical Medicine. 2025; 14(11):4011. https://doi.org/10.3390/jcm14114011
Chicago/Turabian StyleThienel, Manuela, Rainer Kaiser, Jonas Gmeiner, Martin Orban, Stefan Kääb, Tobias Petzold, Steffen Massberg, and Clemens Scherer. 2025. "The Hungry Heart: Managing Cardiogenic Shock in Patients with Severe Anorexia Nervosa—A Case Report Series" Journal of Clinical Medicine 14, no. 11: 4011. https://doi.org/10.3390/jcm14114011
APA StyleThienel, M., Kaiser, R., Gmeiner, J., Orban, M., Kääb, S., Petzold, T., Massberg, S., & Scherer, C. (2025). The Hungry Heart: Managing Cardiogenic Shock in Patients with Severe Anorexia Nervosa—A Case Report Series. Journal of Clinical Medicine, 14(11), 4011. https://doi.org/10.3390/jcm14114011







