Corporal Composition and Gut Microbiome Modification Through Exclusion Dietary Intervention in Adult Patients with Crohn’s Disease: Protocol for a Prospective, Interventional, Controlled, Randomized Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Objectives
- To assess the improvement of clinical and inflammatory parameters related to the progression of the disease.
- To evaluate the incidence of sarcopenia through the assessment of muscle strength, function, and composition using functional tests, dynamometry, bioelectrical impedance analysis (BIA), and nutritional ultrasound.
- To determine the rate of improvement in the quality of life of patients and the disease remission by evaluating the Crohn’s Disease Activity Index (using the CVEII9 quality of life questionnaire and the Harvey–Bradshaw Index).
- To analyze the modifications in the intestinal microbiota resulting from the implementation of the ED.
- To examine the progression of clinical, anthropometric, analytical, and body composition parameters.
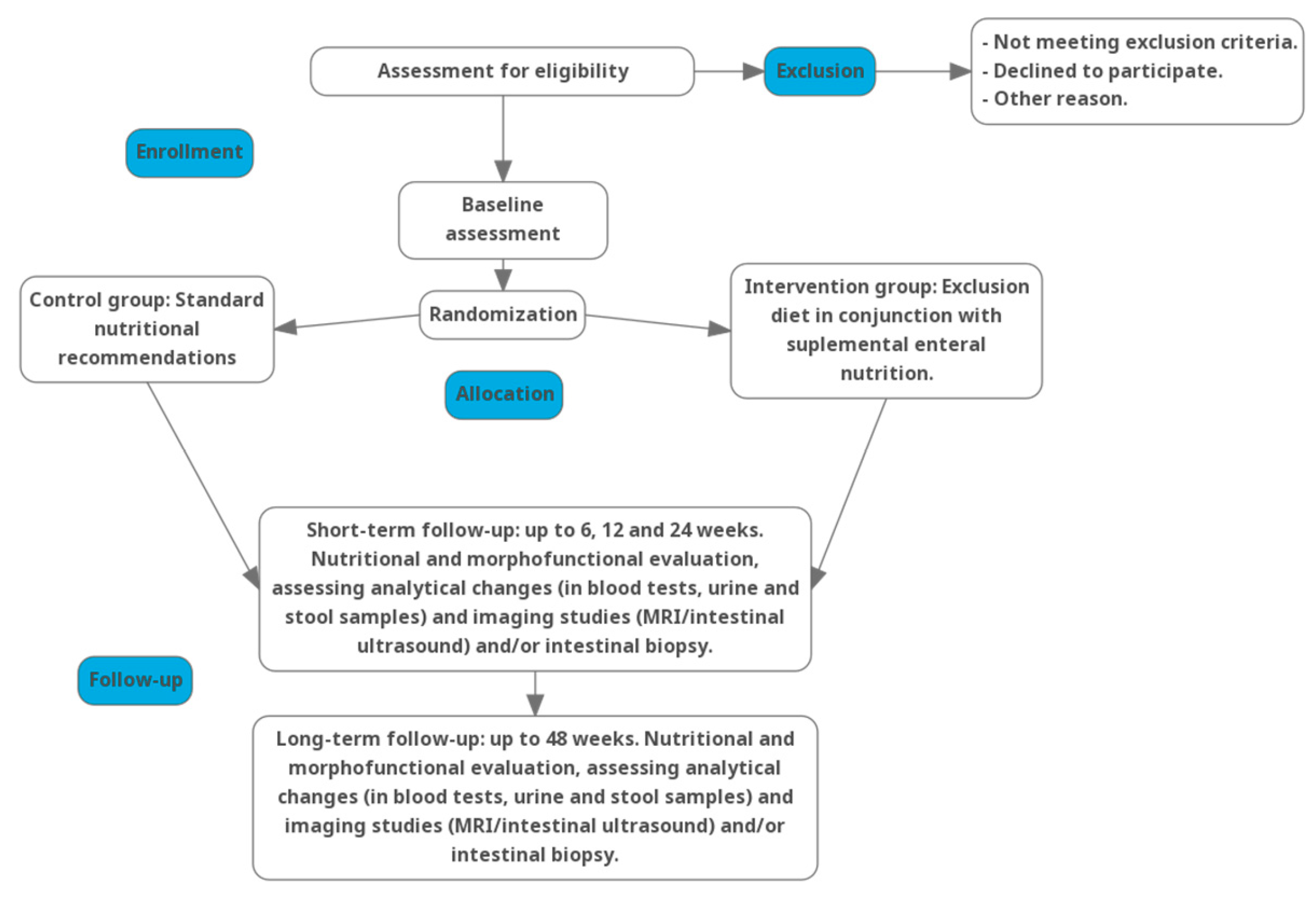
2.2. Design
2.3. Study Setting
2.4. Eligibility Criteria
2.4.1. Inclusion Criteria
- Subjects of both sexes over 18 years of age.
- A diagnosis of active luminal CD with small bowel involvement, with or without colonic involvement, prior to study inclusion.
- Active symptoms of CD at the time of initiation of the nutritional intervention.
- Active disease, defined as a Harvey–Bradshaw Index (HBI) > 4 and an objective measure of disease activity, such as an elevated inflammatory marker (CRP > 5 mg/L or 0.5 mg/dL, or calprotectin ≥ 250 µg/g) and/or a radiological imaging test (MR enterography or intestinal ultrasound) or an endoscopic test (ileocolonoscopy or capsule endoscopy).
- Ability and willingness to adhere to one of the nutritional interventions.
- Capacity to complete and sign the informed consent form.
2.4.2. Exclusion Criteria
- Patients experiencing a severe flare associated with fistulizing tracts or strictures during the study period.
- Hospitalized patients.
- Patients with known intolerance or hypersensitivity to the components of the nutritional supplement Modulen IBD.
- Patients following another diet or who are participating in other nutritional trials.
- Patients scheduled for surgical intervention during the study period.
- Patients with active malignancy.
- Patients under treatment with antibiotics or probiotics.
- Patients with other clinical conditions that may interfere with the implementation of the planned nutritional interventions (such as heart disease, celiac disease, uncontrolled diabetes, active infections, tuberculosis, or a positive stool test for Clostridium difficile toxin).
2.5. Withdrawal and Replacement of Study Subjects
2.6. Withdrawal Criteria
- The occurrence of an adverse event which, in the judgment of the investigator, necessitates the withdrawal of the subject.
- A protocol deviation that compromises the interpretation of the study results and the scientific validity of the study.
- The voluntary decision of the subject.
- The explicit refusal of the subject to continue his/her participation in the study.
- Loss to follow-up.
2.7. Consent
2.8. Research Ethics Approval
2.9. Protocol Amendments
2.10. Intervention
- Control Group: Patients will receive modifications to their pharmacological treatment alongside standard nutritional recommendations. The control group will follow a Mediterranean diet with increased caloric and protein intake during the acute flare. The diet will emphasize a balanced nutritional intake without unnecessary food restrictions, along with adequate fiber and hydration (as tolerated), and a reduction in ultra-processed food consumption.
- Experimental Group: Patients will receive modifications to their pharmacological treatment and will be assigned to an intervention consisting of an exclusion diet in conjunction with supplemental enteral nutrition. This nutritional strategy will involve a progressive increase in the caloric intake derived from the diet, coupled with a corresponding reduction in supplemental enteral nutrition.
2.11. Sample Size and Recruitment
2.12. Randomization and Blinding
2.13. Data and Sample Collection and Analysis of Variables
2.13.1. Data Collection
2.13.2. Sample Collection and Storage
2.13.3. Characterization of Quantifiable Analytical Parameters in Serum, Urine, and Stool
- Hematological and coagulation parameters: complete blood count (CBC) and coagulation profile.
- General biochemical profile: fasting glucose, electrolytes (sodium, potassium, chloride), nitrogenous compounds (urea, creatinine), mineral metabolism markers (calcium, phosphorus, magnesium), hepatic function enzymes (aspartate aminotransferase [AST/GOT], alanine aminotransferase [ALT/GPT], gamma-glutamyl transferase [GGT], alkaline phosphatase, total and direct bilirubin, lactate dehydrogenase [LDH], creatine kinase).
- Metabolic and endocrine markers: insulin, homeostatic model assessment for insulin resistance (HOMA-IR), C-peptide, and glycated hemoglobin (HbA1c).
- Lipid profile: total cholesterol, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and triglycerides.
- Nutritional biomarkers: prealbumin, albumin, fat-soluble vitamins (A, D, E), trace elements (zinc, selenium), vitamin B12, iron, folate, ferritin, transferrin, and transferrin saturation index.
- Inflammatory biomarkers: C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and interleukin-6 (IL-6). Inflammatory markers will be quantified using high-sensitivity multiplex immunoassays to enhance precision and reduce inter-assay variability.
- Fecal calprotectin: a surrogate marker of intestinal inflammation, measured using enzyme-linked immunosorbent assay (ELISA) or equivalent high-sensitivity immunoassays.
- Analysis of the gut microbiota composition will be performed by 16S rRNA gene sequencing in stools. DNA extraction from stool samples will be performed using 200 mg of sample using the QIAamp DNA Stool Mini kit (Qiagen, Manchester, UK), following the manufacturer’s instructions. The concentration and quality of the DNA will be determined by the Agilent 2200 TapeStation system. To create the libraries, DNA samples (5 ng/µL) will be amplified using a primer, encoding the hypervariable regions V2-4-8 and V3-6, 7-9 of the bacterial 16S rRNA using the 16S Metagenomics Kit (Thermo Fisher, Waltham, MA, USA). The amplicons obtained will be purified using AMPure® XP beads (Beckman Coulter, Pasadena, CA, USA) and, subsequently, the libraries will be created using the Ion Plus Fragment Library kit (Thermo Fisher, Waltham, MA, USA) and the Ion Xpress Barcode Adapters 1–16 kit (Thermo Fisher, Waltham, MA, USA) to add barcodes to purified amplicons. The libraries will be purified using AMPure® XP beads (Beckman Coulter, Pasadena, CA, USA) and quantified by fluorescence. Sequencing of the libraries will be carried out using an Ion S5 platform. The results of composition, diversity, and richness will be analyzed using the QIIME2 platform, and functionality will be analyzed through the PICRUST software package (Version v2.6.2).
2.14. Data Management
2.15. Statistical Methods
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roda, G.; Ng, S.C.; Kotze, P.G.; Argollo, M.; Panaccione, R.; Spinelli, A.; Kaser, A.; Peyrin-Biroulet, L.; Danese, S. Crohn’s Disease. Nat. Rev. Dis. Primers 2020, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Boyapati, R.; Satsangi, J.; Ho, G.T. Pathogenesis of Crohn’s Disease. F1000Prime Rep. 2015, 7, 44. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Sánchez, M.A.; Melgar, S.; O’Donoghue, K.; Martínez-Sánchez, M.A.; Fernández-Ruiz, V.E.; Ferrer-Gómez, M.; Ruiz-Alcaraz, A.J.; Ramos-Molina, B. Crohn’s Disease, Host–Microbiota Interactions, and Immunonutrition: Dietary Strategies Targeting Gut Microbiome as Novel Therapeutic Approaches. Int. J. Mol. Sci. 2022, 23, 8361. [Google Scholar] [CrossRef] [PubMed]
- Nitescu, M.; Istratescu, D.; Preda, C.M.; Manuc, T.E.; Louis, E.; Manuc, M.; Stroie, T.; Catrinoiu, M.; Tieranu, C.G.; Badea, L.E.; et al. Role of an Exclusion Diet (Reduced Disaccharides, Saturated Fats, Emulsifiers, Red and Ultra-Processed Meats) in Maintaining the Remission of Chronic Inflammatory Bowel Diseases in Adults. Medicina 2023, 59, 329. [Google Scholar] [CrossRef] [PubMed]
- Platero-Rodrigo, E.; García-Manzanares Vázquez de Agredos, A.; Gil-Fournier, N.; Álvarez-Hernández, J. Soporte nutricional en la enfermedad inflamatoria. In Dietoterapia, Nutrición Clínica y Metabolismo; Román, A.L., Bellido-Guerrero, D., García-Luna, P., Oliveira-Fuster, G., Eds.; Aulamédica: Toledo, España, 2017; pp. 481–493. [Google Scholar]
- Adolph, T.E.; Zhang, J. Diet Fuelling Inflammatory Bowel Diseases: Preclinical and Clinical Concepts. Gut 2022, 71, 2574–2586. [Google Scholar] [CrossRef] [PubMed]
- Martín-Masot, R.; Herrador-López, M.; Navas-López, V.M. Dietary Habit Modifications in Paediatric Patients After One Year of Treatment with the Crohn’s Disease Exclusion Diet. Nutrients 2023, 15, 554. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, S.C.; Bager, P.; Escher, J.; Forbes, A.; Hébuterne, X.; Hvas, C.L.; Joly, F.; Klek, S.; Krznaric, Z.; Ockenga, J.; et al. ESPEN Guideline on Clinical Nutrition in Inflammatory Bowel Disease. Clin. Nutr. 2023, 42, 352–379. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.; El-Matary, W.; Van Limbergen, J. A Case-Based Approach to New Directions in Dietary Therapy of Crohn’s Disease: Food for Thought. Nutrients 2020, 12, 880. [Google Scholar] [CrossRef] [PubMed]
- Ding, N.S.; Tassone, D.; al Bakir, I.; Wu, K.; Thompson, A.J.; Connell, W.R.; Malietzis, G.; Lung, P.; Singh, S.; Choi, C.H.R.; et al. Systematic Review: The Impact and Importance of Body Composition in Inflammatory Bowel Disease. J. Crohn’s Colitis 2022, 16, 1475–1492. [Google Scholar] [CrossRef] [PubMed]
- Szczubełek, M.; Pomorska, K.; Korólczyk-Kowalczyk, M.; Lewandowski, K.; Kaniewska, M.; Rydzewska, G. Effectiveness of Crohn’s Disease Exclusion Diet for Induction of Remission in Crohn’s Disease Adult Patients. Nutrients 2021, 13, 4112. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.; Wine, E.; Assa, A.; Sigall Boneh, R.; Shaoul, R.; Kori, M.; Cohen, S.; Peleg, S.; Shamaly, H.; On, A.; et al. Crohn’s Disease Exclusion Diet Plus Partial Enteral Nutrition Induces Sustained Remission in a Randomized Controlled Trial. Gastroenterology 2019, 157, 440–450.e8. [Google Scholar] [CrossRef] [PubMed]
- Djuric, Z.; Ren, J.; Blythe, J.; VanLoon, G.; Sen, A. A Mediterranean Dietary Intervention in Healthy American Women Changes Plasma Carotenoids and Fatty Acids in Distinct Clusters. Nutr. Res. 2009, 29, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Jabłońska, B.; Mrowiec, S. Nutritional Status and Its Detection in Patients with Inflammatory Bowel Diseases. Nutrients 2023, 15, 1991. [Google Scholar] [CrossRef] [PubMed]
- Sigall-Boneh, R.; Shabat, S.; Yanai, C. Dietary Therapy with the Crohn’s Disease Exclusion Diet is a Successful Strategy for Induction of Remission in Children and Adults Failing Biological Therapy. J. Crohn’s Colitis 2017, 11, 1205–1212. [Google Scholar] [CrossRef] [PubMed]
- Sigall-Boneh, R.; Pfeffer-Gik, T.; Segal, I.; Zangen, T.; Boaz, M.; Levine, A. Partial Enteral Nutrition with a Crohn’s Disease Exclusion Diet is Effective for Induction of Remission in Children and Young Adults with Crohn’s Disease. Inflamm. Bowel Dis. 2014, 20, 1353–1360. [Google Scholar] [CrossRef] [PubMed]
- Miele, E.; Shamir, R.; Aloi, M.; Assa, A.; Braegger, C.; Bronsky, J.; de Ridder, L.; Escher, J.C.; Hojsak, I.; Kolaček, S.; et al. Nutrition in Pediatric Inflammatory Bowel Disease: A Position Paper on Behalf of the Porto Inflammatory Bowel Disease Group of the European Society of Pediatric Gastroenterology, Hepatology and Nutrition. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 687–708. [Google Scholar] [CrossRef]
- Greco, S.; Bonsi, B.; Fabbri, N. Diet and Nutrition Against Inflammatory Bowel Disease: Trick or Treat(ment)? World J. Exp. Med. 2022, 12, 104–107. [Google Scholar] [CrossRef] [PubMed]



| Study Period | ||||||
|---|---|---|---|---|---|---|
| Timepoint | Pre-DE | t0 | t1 (6 w) | t2 (12 w) | t3 (24 w) | t4 (48 w) |
| Enrollment | ||||||
| Diagnosis (Index, Harvey–Bradshaw Index, MUST) | X | |||||
| Inclusion criteria and exclusion criteria | X | |||||
| Informed consent signature | X | |||||
| Intervention | ||||||
| Standard nutritional recommendations vs. ED + enteral nutrition | X | X | X | X | X | |
| Assessments | ||||||
| Quality of life questionnaire (CVEII-9) | X | X | X | X | X | |
| Harvey–Bradshaw activity index | X | X | X | X | X | |
| Physical activity questionnaire (PAQ) | X | X | X | |||
| Food Frequency Questionnaire (FFQ) | X | X | X | |||
| 24 h dietary recall | X | X | X | X | X | |
| Mediterranean diet adherence questionnaire (PREDIMED) | X | X | X | |||
| Anthropometry (weight, height, BMI) | X | X | X | X | ||
| Sarcopenia screening (SARC-F) | X | X | X | X | ||
| Functional sarcopenia tests (dynamometry) | X | X | X | X | ||
| Bioelectrical Impedance Analysis (BIA) | X | X | X | X | X | |
| Nutritional ultrasound | X | X | X | X | ||
| Blood tests, urine and stool samples | X | X | X | X | X | |
| Intestinal imaging (MRI/intestinal ultrasound). | X | X | X | |||
| Intestinal biopsy. Optional. | X | X | X | |||
| Phase | Type of Food | |
|---|---|---|
| Phase 1 (0–6 weeks) | Obligatory | Protein-rich foods: fresh chicken breast (at least 150–200 g/day), 2 eggs/day. Carbohydrate-rich foods: 2 fresh potatoes/day peeled, cooked and cooled before consumption, 2 bananas/day, 1 apple/day peeled. |
| Permitted | Protein-rich foods: 100–150 g of fresh white fish once a week as a substitute for chicken. Carbohydrate-rich foods: white rice (unlimited), rice noodles without preservatives (unlimited), rice flour for baking (unlimited). Fruits: 1 avocado/day (no more 1/2 avocado per meal), 5 ripe strawberries/day, 1 cantaloupe slice/day. Vegetables: 2 tomatoes/day (or 6 cherry tomatoes), 2 cucumbers/day peeled, 1 carrot/day, fresh spinach (225 g raw leaves/day), 3 lettuce leaves once a day. Condiments: olive oil, canola oil, salt, pepper, paprika, cinnamon, cumin, turmeric, mint, oregano, coriander, rosemary, sage, basil, thyme, dill, parsley, onion (all types), garlic, ginger, natural lemon juice, honey (3 tbsp/day), sugar (3 teaspoons/day). Drinks: water, sparkling water, infusions, 1 glass of freshly squeezed orange juice per day. | |
| Prohibited | Protein-rich foods: pre-cooked or smoked processed meat and fish, seafood, red meat, pork, pork, turkey, and other poultry parts, soy products, dairy products, ice cream, vegetable milks (soy, rice, almond). Carbohydrate-rich foods: wheat products (breakfast cereals, bread and baked goods of any kind), baker’s yeast and other flours, gluten-free products not mentioned above, soya products, pulses (lentils, peas, chickpeas and beans), maize, frozen potatoes. Fruits: dried fruits, all other fruits. Vegetables: frozen vegetables, kale, leek, asparagus, artichokes, all other vegetables not mentioned as permitted. Condiments: margarine, sauces, salad dressings, syrups (maple syrup, corn syrup, etc.), jam of any kind, artificial sweeteners, spice mixtures, other oils (soybean oil, sunflower oil, corn oil, etc.), marmalade of any kind, artificial sweeteners and oil spray. Beverages: soft drinks, fruit juice, other sweetened beverages, alcoholic beverages, coffee, chocolate milk, milkshakes, all types of tea (leaf and tea bags). Nuts and dried fruit. Others: canned goods, packaged snacks (crisps, crackers, popcorn, etc.), candy, chocolate, cakes, biscuits, chewing gum. Foods that are not on the list of recommended or permitted foods are prohibited. | |
| Phase 2 (7–12 weeks) | Obligatory | Same as phase 1. |
| Permitted | Protein-rich foods: 100–150 g of fresh white fish once a week as a substitute for chicken, one can of tuna in olive or canola oil once a week. Eating red meat is not recommended, but if you wish, you should limit yourself to a fresh fillet of unprocessed lean meat, up to 200 g per week (only once a week). Carbohydrate-rich foods: white rice (unlimited), rice noodles without preservatives (unlimited), rice flour for baking (unlimited), ½ sweet potato/day, bread 1 slice/day (preferably homemade), lentils, peas, chickpeas or dried beans, half a cup/day uncooked, quinoa (unlimited), 50 g oats (one serving of oatmeal or crackers is allowed 1 or 2 times a day). Fruits: 1 avocado/day (no more 1/2 avocado per meal), 5 ripe strawberries/day, 1 cantaloupe slice/day, one pear, peach or kiwi fruit per day, 10 blueberries (25 g) can replace the allowed strawberries. From week 10 onwards, all fruits can be introduced in small quantities (except those on the off-limits list). Vegetables: 2 tomatoes/day (or 6 cherry tomatoes), 2 cucumbers/day peeled, 1 carrot/day, fresh spinach (225 g raw leaves/day), 3 lettuce leaves once a day, courgette (1 large or 2 small), 4–6 fresh mushrooms, 2 broccoli or cauliflower florets (but not all at the same time). From week 10 onwards, all vegetables can be introduced in small quantities (except those on the off-limits list). Condiments: olive oil, canola oil, salt, pepper, paprika, cinnamon, cumin, turmeric, mint, oregano, coriander, rosemary, sage, basil, thyme, dill, parsley, onion (all types), garlic, ginger, natural lemon juice, honey (3 tbsp/day), sugar (3 teaspoons/day), baking soda or baking powder. Drinks: water, sparkling water, infusions, 1 glass of freshly squeezed orange juice per day, chamomile tea (permitted from week 7 onwards). Nuts: unsalted, unroasted, and unprocessed almonds or walnuts, 36 g/day Tahini (without emulsifiers and sulfites). | |
| Prohibited | Protein-rich foods: pre-cooked or smoked processed meat and fish, seafood, red meat, pork, pork, turkey, and other poultry parts, soy products, dairy products, ice cream, vegetable milks (soy, rice, almond). Carbohydrate-rich foods: wheat products (breakfast cereals, bread and baked goods of any kind), baker’s yeast and other flours, gluten-free products not mentioned above, soya products, pulses (lentils, peas, chickpeas and beans), maize ((permitted from week 10), frozen potatoes. Fruits: dried fruits, all other fruits (permitted from week 10 onwards). Fruit is not permitted under any circumstances: persimmon, passion fruit, pomegranate, prickly pear. Vegetables: frozen vegetables, kale, leek, asparagus, artichokes. Condiments: margarine, sauces, salad dressings, syrups (maple syrup, corn syrup, etc.), jam of any kind, artificial sweeteners, spice mixtures, other oils (soybean oil, sunflower oil, corn oil, etc.), marmalade of any kind, artificial sweeteners and oil spray. Beverages: soft drinks, fruit juice, other sweetened beverages, alcoholic beverages, coffee, chocolate milk, milkshakes, all types of tea (leaf and tea bags). Nuts and dried fruit. Others: canned goods, packaged snacks (crisps, crackers, popcorn, etc.), candy, chocolate, cakes, biscuits, chewing gum. Foods that are not on the list of recommended or permitted foods are prohibited. | |
| Phase 3 (13 week) | Permitted | The phase 2 diet will be followed for 5 days of the week (Monday to Friday) with the following additions: Protein-rich foods: other parts of the chicken can be used, but the skin, wings, and offal should be avoided; fresh seafood, white fish or salmon, once a week, one serving per day of natural unprocessed yogurt with all-natural fat and no additives. Carbohydrate-rich foods: two slices of bread per day (preferably homemade), a small portion of pasta can be eaten instead of bread (200 g cooked). Fruits: all fruits and berries, including dehydrated fruits if they are sulfite-free, except for fruits that are expressly not allowed. Vegetables: all vegetables except those expressly not allowed (provided you do not suffer from stenosis). Drinks: one cup of black coffee or tea (no instant coffee or capsules and only 1 cup per day). Same restrictions for other foods, beverages, and condiments as in phase 2. Weekends (Saturdays and Sundays): foods that are not included in the ED are allowed at free meals. It is important not to overeat foods that are not included in the ED. Try to maintain a varied diet also during free meals at weekends. Homemade food is preferable to frozen or processed food. One free home-cooked breakfast and one free home-cooked meal (lunch or dinner) per day on Saturdays and Sundays (including wheat, dairy, fish and other types of meat). |
| Prohibited | Protein-rich foods: frozen doughs (cake or pizza bases), processed meats (such as hot dogs, sausages, bacon), ready meals (frozen from fresh produce), and soft drinks. Fruits: passion fruit, persimmon, prickly pear, pomegranate. Vegetables: raw celery, large quantities of kale, leeks. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cano-Mármol, R.P.; Fernández-Ruiz, V.E.; Martínez-Pascual, C.; Ros-Madrid, I.; Martín-Pozuelo, G.; Oliva-Bolarín, A.; Martínez-Sánchez, M.A.; Egea-Valenzuela, J.; Núñez-Sánchez, M.Á.; Ramos-Molina, B.; et al. Corporal Composition and Gut Microbiome Modification Through Exclusion Dietary Intervention in Adult Patients with Crohn’s Disease: Protocol for a Prospective, Interventional, Controlled, Randomized Clinical Trial. J. Clin. Med. 2025, 14, 3998. https://doi.org/10.3390/jcm14113998
Cano-Mármol RP, Fernández-Ruiz VE, Martínez-Pascual C, Ros-Madrid I, Martín-Pozuelo G, Oliva-Bolarín A, Martínez-Sánchez MA, Egea-Valenzuela J, Núñez-Sánchez MÁ, Ramos-Molina B, et al. Corporal Composition and Gut Microbiome Modification Through Exclusion Dietary Intervention in Adult Patients with Crohn’s Disease: Protocol for a Prospective, Interventional, Controlled, Randomized Clinical Trial. Journal of Clinical Medicine. 2025; 14(11):3998. https://doi.org/10.3390/jcm14113998
Chicago/Turabian StyleCano-Mármol, Rosario Paloma, Virginia Esperanza Fernández-Ruiz, Cristina Martínez-Pascual, Inmaculada Ros-Madrid, Gala Martín-Pozuelo, Alba Oliva-Bolarín, María Antonia Martínez-Sánchez, Juan Egea-Valenzuela, María Ángeles Núñez-Sánchez, Bruno Ramos-Molina, and et al. 2025. "Corporal Composition and Gut Microbiome Modification Through Exclusion Dietary Intervention in Adult Patients with Crohn’s Disease: Protocol for a Prospective, Interventional, Controlled, Randomized Clinical Trial" Journal of Clinical Medicine 14, no. 11: 3998. https://doi.org/10.3390/jcm14113998
APA StyleCano-Mármol, R. P., Fernández-Ruiz, V. E., Martínez-Pascual, C., Ros-Madrid, I., Martín-Pozuelo, G., Oliva-Bolarín, A., Martínez-Sánchez, M. A., Egea-Valenzuela, J., Núñez-Sánchez, M. Á., Ramos-Molina, B., Ruiz-Alcaraz, A. J., & Ferrer-Gómez, M. (2025). Corporal Composition and Gut Microbiome Modification Through Exclusion Dietary Intervention in Adult Patients with Crohn’s Disease: Protocol for a Prospective, Interventional, Controlled, Randomized Clinical Trial. Journal of Clinical Medicine, 14(11), 3998. https://doi.org/10.3390/jcm14113998







