Platelet-Rich Plasma for Knee Osteoarthritis: A Comprehensive Narrative Review of the Mechanisms, Preparation Protocols, and Clinical Evidence
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Extraction
2.3. Synthesis
3. Results
3.1. Summary of Included Studies
3.2. Treatment Effects
3.3. Functional Outcomes
3.4. Data on Adverse Events
3.5. Disease Severity
3.6. Risk of Bias Assessment
3.7. GRADE Assessment
3.8. Evidence Map of PRP Formulations and Comparators
4. Discussion
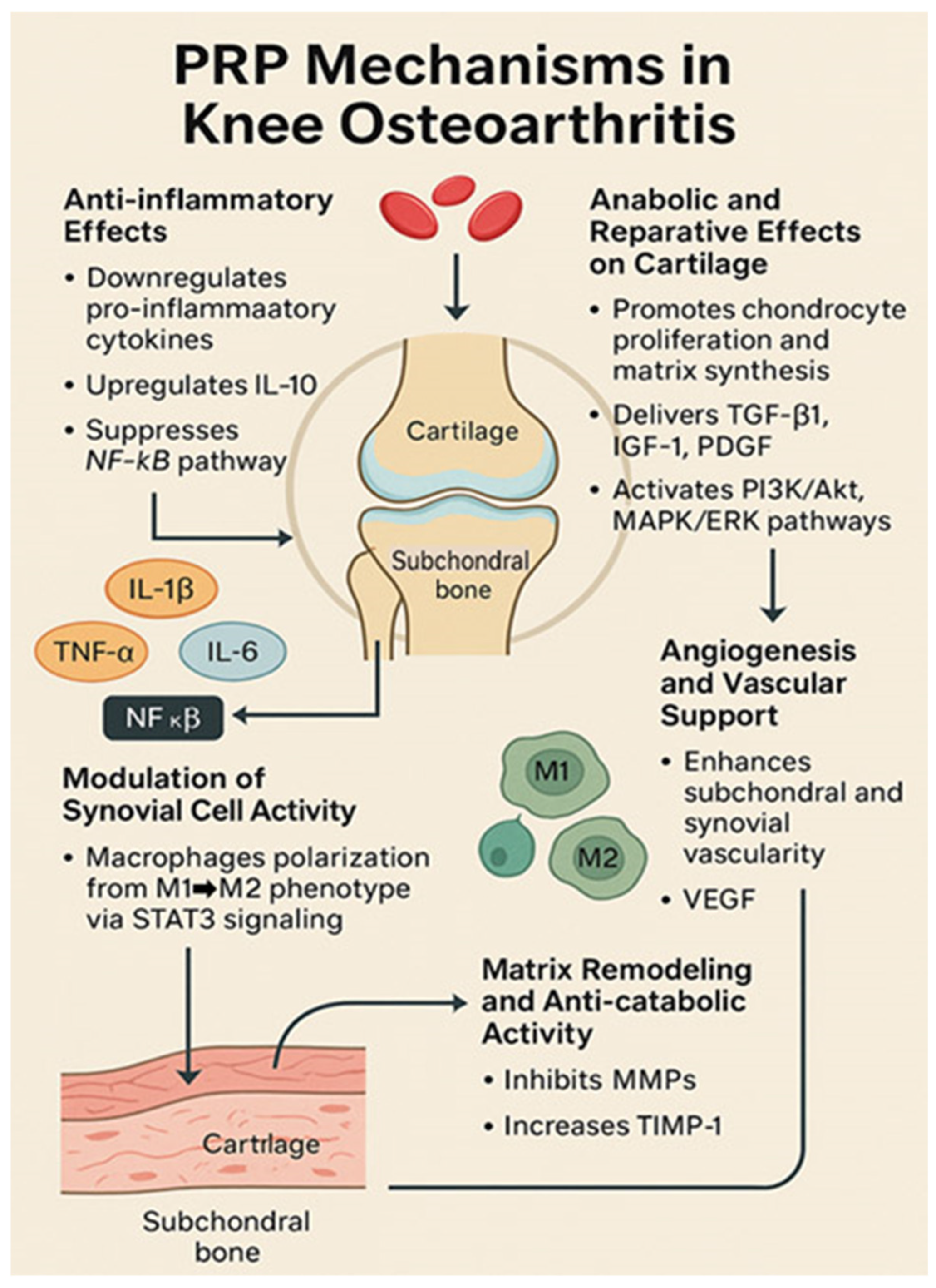
4.1. Mechanisms of Action of PRP in Osteoarthritis
4.2. PRP Preparation and Formulations
4.3. Clinical Efficacy of PRP in KOA
4.3.1. PRP vs. Placebo (Saline)
4.3.2. PRP vs. Hyaluronic Acid
4.3.3. PRP vs. Corticosteroids
4.3.4. PRP vs. Other Biologics (e.g., BMAC)
4.4. Magnitude of Clinical Effects of PRP
4.5. Systematic Reviews and Meta-Analyses
4.6. Impact of PRP Formulation
4.6.1. Leukocyte Content (LR-PRP vs. LP-PRP)
4.6.2. Platelet Activation
4.7. Safety and Adverse Events
4.8. Practical Considerations and Implementation
Repeat Treatments
4.9. Current Guidelines and Recommendations
4.10. Limitations and Heterogeneity
4.11. Addressing Heterogeneity: Proposed Standards for PRP Preparation and Outcome Measures
- Consensus on PRP Classification:
- Clearly categorize PRP based on the following variables:
- Platelet Concentration: Standardized reporting as the fold increase relative to baseline platelet counts.
- Leukocyte Content: Explicitly define PRP as leukocyte-rich (LR-PRP) or leukocyte-poor (LP-PRP) with precise numeric thresholds.
- Activation Method: Consistent reporting of the activation status (activated vs. non-activated) along with activating agents, concentrations, and volumes used.
- Standardized Preparation and Injection Protocols:
- Studies should implement the following:
- Validated, commercially available PRP preparation systems approved by regulatory bodies (FDA and EMA) to ensure reproducibility.
- Uniform injection protocols specifying the number, volume, interval, and delivery method (preferably ultra-sound-guided).
- Uniform Outcome Measurement:
- Adopt core outcome sets to standardize reporting:
- Pain: VAS/NRS and the WOMAC pain subscale.
- Function: WOMAC function, IKDC, and KOOS.
- Imaging: Standardized MRI protocols and cartilage scoring systems.
- Patient-Reported Outcomes: Patient Global Assessment (PGA) and Quality-of-Life metrics (EQ-5D and SF-36).
- Adverse Events: Clearly defined and consistently reported.
- Enhanced Reporting Guidelines:
- Establishment of a Centralized Registry:
- Regular Expert Consensus Meetings:
4.12. Guidance on Patient Selection Criteria for PRP Therapy in KOA
- Mild-to-moderate KOA: Patients classified as Kellgren–Lawrence grades II–III typically show better outcomes than those with advanced disease.
- Younger age (<60 years): Younger patients tend to have more responsive cartilage and synovial tissue, potentially resulting in greater symptomatic relief.
- Lower BMI (<30 kg/m2): Patients with lower body mass indices generally experience more noticeable improvement, possibly due to lower mechanical stress on joints.
- Shorter disease duration: Patients with earlier disease stages or shorter symptom durations (under 5 years) may benefit more significantly.
- Minimal joint deformity and preserved joint mechanics: Patients without substantial varus or valgus malalignment respond more favorably.
- Previous inadequate response to conservative therapies: Patients who have not benefited sufficiently from NSAIDs, exercise, physical therapy, or hyaluronic acid injections might find added relief from PRP.
- Contraindications to steroid or surgical interventions: Patients unable or unwilling to pursue steroid injections or surgical options may particularly benefit from PRP.
- Advanced KOA (Kellgren–Lawrence grade IV), marked joint space narrowing, severe cartilage loss.
- Significant knee malalignment or instability requiring surgical correction.
- Severe obesity (BMI > 35 kg/m2), as high mechanical loading may diminish therapeutic outcomes.
- Using these criteria can help clinicians carefully select patients who are most likely to derive symptomatic benefit from PRP injections, ensuring an optimal balance of patient expectations, treatment effectiveness, and clinical resource use.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Yao, Q.; Wu, X.; Tao, C.; Gong, W.; Chen, M.; Qu, M.; Zhong, Y.; He, T.; Chen, S.; Xiao, G. Osteoarthritis: Pathogenic signaling pathways and therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 56. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Li, Z.; Alexander, P.G.; Ocasio-Nieves, B.D.; Yocum, L.; Lin, H.; Tuan, R.S. Pathogenesis of Osteoarthritis: Risk Factors, Regulatory Pathways in Chondrocytes, and Experimental Models. Biology 2020, 9, 194. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Yu, H.; Huang, X.; Shen, J.; Xiao, G.; Chen, L.; Wang, H.; Xing, L.; Chen, D. Current understanding of osteoarthritis pathogenesis and relevant new approaches. Bone Res. 2022, 10, 60. [Google Scholar] [CrossRef] [PubMed]
- Ng, M.; Gakidou, E.; Lo, J.; Abate, Y.H.; Abbafati, C.; Abbas, N.; Abbasian, M.; ElHafeez, S.A.; Abdel-Rahman, W.M.; Abd-Elsalam, S.; et al. Global, regional, and national prevalence of adult overweight and obesity, 1990–2021, with forecasts to 2050: A forecasting study for the Global Burden of Disease Study 2021. Lancet 2025, 405, 813–838. [Google Scholar] [CrossRef]
- Loeser, R.F.; Collins, J.A.; Diekman, B.O. Ageing and the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 412–420. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Z.; Li, T.; Xu, H.; Zhang, H. Senescence in osteoarthritis: From mechanism to potential treatment. Arthritis Res. Ther. 2022, 24, 174. [Google Scholar] [CrossRef]
- Zheng, L.; Zhang, Z.; Sheng, P.; Mobasheri, A. The role of metabolism in chondrocyte dysfunction and the progression of osteoarthritis. Ageing Res. Rev. 2021, 66, 101249. [Google Scholar] [CrossRef]
- Jiang, W.; Chen, H.; Lin, Y.; Cheng, K.; Zhou, D.; Chen, R.; Song, C.; Zeng, L.; Yu, H. Mechanical stress abnormalities promote chondrocyte senescence—The pathogenesis of knee osteoarthritis. Biomed. Pharmacother. 2023, 167, 115552. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, P.; Xue, X.; Zhang, Z.; Wang, L.; Jiang, Y.; Zhang, C.; Zhou, H.; Lv, S.; Shen, W.; et al. The role of platelet-rich plasma in biomedicine: A comprehensive overview. iScience 2025, 28, 111705. [Google Scholar] [CrossRef]
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis: A disease of the joint as an organ. Arthritis Rheum. 2012, 64, 1697–1707. [Google Scholar] [CrossRef]
- Mehrabani, D.; Seghatchian, J.; Acker, J.P. Platelet rich plasma in treatment of musculoskeletal pathologies. Transfus. Apher. Sci. 2019, 58, 102675. [Google Scholar] [CrossRef] [PubMed]
- Everts, P.; Onishi, K.; Jayaram, P.; Lana, J.F.; Mautner, K. Platelet-Rich. Plasma: New Performance Understandings and Therapeutic Considerations in 2020. Int. J. Mol. Sci. 2020, 21, 7794. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Zhang, C.; Tuan, R.S. Biology of platelet-rich plasma and its clinical application in cartilage repair. Arthritis Res. Ther. 2014, 16, 204. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, M.I.; Whitney, K.; Evans, T.; LaPrade, R.F. Platelet-Rich Plasma and Cartilage Repair. Curr. Rev. Musculoskelet. Med. 2018, 11, 573–582. [Google Scholar] [CrossRef]
- Da Fonseca, L.; Santos, G.S.; Huber, S.C.; Setti, T.M.; Setti, T.; Lana, J.F. Human platelet lysate—A potent (and overlooked) orthobiologic. J. Clin. Orthop. Trauma 2021, 21, 101534. [Google Scholar] [CrossRef]
- Testa, G.; Giardina, S.M.C.; Culmone, A.; Vescio, A.; Turchetta, M.; Cannavò, S.; Pavone, V. Intra-Articular Injections in Knee Osteoarthritis: A Review of Literature. J. Funct. Morphol. Kinesiol. 2021, 6, 15. [Google Scholar] [CrossRef]
- Gopinath, G. Efficiency of intraarticular injection of PRP and hyaluronic acid combination in osteoarthritis knee: A systematic review and meta-analysis. J. Orthop. Rep. 2025, 4 (Suppl. S2), 100554. [Google Scholar] [CrossRef]
- Lopes-Silva, R.; Santos, M.; Sequeira, M.L.; Silva, A.; Antunes, T.; Valejo-Coelho, P.; Neiva-Sousa, M. Platelet-Rich Plasma Effectiveness in Treating Androgenetic Alopecia: A Comprehensive Evaluation. Cureus 2025, 17, e77371. [Google Scholar] [CrossRef]
- Berrigan, W.; Tao, F.; Kopcow, J.; Park, A.L.; Allen, I.; Tahir, P.; Reddy, A.; Bailowitz, Z. The Effect of Platelet Dose on Outcomes after Platelet Rich Plasma Injections for Musculoskeletal Conditions: A Systematic Review and Meta-Analysis. Curr. Rev. Musculoskelet. Med. 2024, 17, 570–588. [Google Scholar] [CrossRef]
- Mariani, E.; Pulsatelli, L. Platelet Concentrates in Musculoskeletal Medicine. Int. J. Mol. Sci. 2020, 21, 1328. [Google Scholar] [CrossRef]
- Chahla, J.; Cinque, M.E.; Piuzzi, N.S.; Mannava, S.; Geeslin, A.G.; Murray, I.R.; Dornan, G.J.; Muschler, G.F.; LaPrade, R.F. A Call for Standardization in Platelet-Rich Plasma Preparation Protocols and Composition Reporting: A Systematic Review of the Clinical Orthopaedic Literature. J. Bone Jt. Surg. 2017, 99, 1769–1779. [Google Scholar] [CrossRef] [PubMed]
- Magalon, J.; Chateau, A.L.; Bertrand, B.; Louis, M.L.; Silvestre, A.; Giraudo, L.; Veran, J.; Sabatier, F. DEPA classification: A proposal for standardising PRP use and a retrospective application of available devices. BMJ Open Sport. Exerc. Med. 2016, 2, e000060. [Google Scholar] [CrossRef] [PubMed]
- Kon, E.; Di Matteo, B.; Delgado, D.; Cole, B.J.; Dorotei, A.; Dragoo, J.L.; Filardo, G.; A Fortier, L.; Giuffrida, A.; Jo, C.H.; et al. Platelet-rich plasma for the treatment of knee osteoarthritis: An expert opinion and proposal for a novel classification and coding system. Expert Opin. Biol. Ther. 2020, 20, 1447–1460. [Google Scholar] [CrossRef] [PubMed]
- Yaman, R.; Kinard, T.N. Platelet rich plasma: Hope or hype? Ann. Blood 2021, 7, 6. [Google Scholar] [CrossRef]
- Abrams, G.; Cole, B. Hyaluronic Acid and Platelet-Rich Plasma, Intra-Articular Infiltration in the Treatment of Gonarthrosis: Letter to the Editor. Am. J. Sports Med. 2013, 41, NP27–NP28. [Google Scholar] [CrossRef]
- Filardo, G.; Kon, E.; Di Martino, A.; Di Matteo, B.; Merli, M.; Cenacchi, A.; Fornasari, P.; Marcacci, M. Platelet-Rich Plasma vs. Hyaluronic Acid to Treat Knee Degenerative Pathology: Study Design and Preliminary Results of a Randomized Controlled Trial. BMC Musculoskelet. Disord. 2012, 13, 229. [Google Scholar] [CrossRef]
- Say, F.; Gürler, D.; Yener, K.; Bülbül, M.; Malkoc, M. Platelet-Rich Plasma Injection Is More Effective Than Hyaluronic Acid in the Treatment of Knee Osteoarthritis. Acta Chir. Orthop. Traumatol. Cechoslov. 2013, 80, 278–283. [Google Scholar] [CrossRef]
- Khoshbin, A.; Leroux, T.; Wasserstein, D.; Marks, P.; Theodoropoulos, J.; Ogilvie-Harris, D.; Gandhi, R.; Takhar, K.; Lum, G.; Chahal, J. The Efficacy of Platelet-Rich Plasma in the Treatment of Symptomatic Knee Osteoarthritis: A Systematic Review with Quantitative Synthesis. Arthrosc. J. Arthrosc. Relat. Surg. 2013, 29, 2037–2048. [Google Scholar] [CrossRef]
- Holguin, E. Platelet-Rich Plasma Injection Is More Effective Than Hyaluronic Acid in the Treatment of Knee Osteoarthritis. Orthop. J. Sports Med. 2014, 2, 2325967114S00240. [Google Scholar] [CrossRef]
- Laudy, A.B.M.; Bakker, E.W.P.; Rekers, M.; Moen, M.H. Efficacy of Platelet-Rich Plasma Injections in Osteoarthritis of the Knee: A Systematic Review and Meta-Analysis. Br. J. Sports Med. 2014, 49, 657–672. [Google Scholar] [CrossRef]
- Raeissadat, S.A.; Rayegani, S.M.; Hassanabadi, H.; Fathi, M.; Ghorbani, E.; Babaee, M.; Azma, K. Knee Osteoarthritis Injection Choices: Platelet-Rich Plasma (PRP) Versus Hyaluronic Acid (A One-Year Randomized Clinical Trial). Clin. Med. Insights Arthritis Musculoskelet. Disord. 2015, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Montanez-Heredia, E.; Irízar, S.; Huertas, P.; Otero, E.; del Valle, M.; Prat, I.; Díaz-Gallardo, M.S.; Perán, M.; Marchal, J.; Hernández-Lamas, M.C. Intra-Articular Injections of Platelet-Rich Plasma Versus Hyaluronic Acid in the Treatment of Osteoarthritic Knee Pain: A Randomized Clinical Trial in the Context of the Spanish National Health Care System. Int. J. Mol. Sci. 2016, 17, 1064. [Google Scholar] [CrossRef] [PubMed]
- Filardo, G.; Di Matteo, B.; Di Martino, A.; Merli, M.; Cenacchi, A.; Fornasari, P.; Marcacci, M.; Kon, E. Platelet-Rich Plasma Intra-Articular Knee Injections Show No Superiority Versus Viscosupplementation. Am. J. Sports Med. 2015, 43, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Cole, B.; Fortier, L.; Karas, V.; Hussey, K.; Tilton, A.; Merkow, D.B.; Verma, N.; Bach, B.; Forsythe, B. Hyaluronic Acid Versus Platelet-Rich Plasma. Orthop. J. Sports Med. 2015, 3, 2325967115S00123. [Google Scholar] [CrossRef]
- Lana, J.F.; Weglein, A.; Sampson, S.; Vicente, E.F.; Huber, S.C.; Souza, C.V.; Ambach, M.A.; Vincent, H.; Urban-Paffaro, A.; Onodera, C.M.K.; et al. Randomized Controlled Trial Comparing Hyaluronic Acid, Platelet-Rich Plasma and the Combination of Both in the Treatment of Mild and Moderate Osteoarthritis of the Knee. J. Stem Cells Regen. Med. 2016, 12, 69. [Google Scholar]
- Cerza, F.; Carnì, S.; Carcangiu, A.; Di Vavo, I.; Schiavilla, V.; Pecora, A.; De Biasi, G.; Ciuffreda, M. Comparison between hyaluronic acid and platelet-rich plasma, intra-articular infiltration in the treatment of gonarthrosis. Am. J. Sports Med. 2012, 40, 2822–2827. [Google Scholar] [CrossRef]
- Dai, W.L.; Zhou, A.G.; Zhang, H.; Zhang, J. Efficacy of Platelet-Rich Plasma in the Treatment of Knee Osteoarthritis: A Meta-analysis of Randomized Controlled Trials. Arthrosc. J. Arthrosc. Relat. 2017, 33, 659–670. [Google Scholar] [CrossRef]
- Shen, L.; Yuan, T.; Chen, S.; Xie, X.; Zhang, C. The Temporal Effect of Platelet-Rich Plasma on Pain and Physical Function in the Treatment of Knee Osteoarthritis: Systematic Review and Meta-analysis of Randomized Controlled Trials. J. Orthop. Surg. Res. 2017, 12, 16. [Google Scholar] [CrossRef]
- Di Martino, A.; Di Matteo, B.; Papio, T.; Tentoni, F.; Selleri, F.; Cenacchi, A.; Kon, E.; Filardo, G. Platelet-Rich Plasma Versus Hyaluronic Acid Injections for the Treatment of Knee Osteoarthritis: Results at 5 Years of a Double-Blind, Randomized Controlled Trial. Am. J. Sports Med. 2018, 47, 347–354. [Google Scholar] [CrossRef]
- Zhang, H.F.; Wang, C.G.; Li, H.; Huang, Y.T.; Li, Z.J. Intra-Articular Platelet-Rich Plasma Versus Hyaluronic Acid in the Treatment of Knee Osteoarthritis: A Meta-Analysis. Drug Des. Dev. Ther. 2018, 12, 445–453. [Google Scholar] [CrossRef]
- Lin, K.Y.; Yang, C.C.; Hsu, C.J.; Yeh, M.L.; Renn, J.H. Intra-Articular Injection of Platelet-Rich Plasma Is Superior to Hyaluronic Acid or Saline Solution in the Treatment of Mild to Moderate Knee Osteoarthritis: A Randomized, Double-Blind, Triple-Parallel, Placebo-Controlled Clinical Trial. Arthrosc. J. Arthrosc. Relat. Surg. 2019, 35, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Meheux, C.J.; McCulloch, P.C.; Lintner, D.M.; Varner, K.E.; Harris, J.D. Efficacy of Intra-Articular Platelet-Rich Plasma Injections in Knee Osteoarthritis: A Systematic Review. Arthrosc. J. Arthrosc. Relat. Surg. 2016, 32, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; He, Z.X.; Shu, L.P.; Li, X.; Ma, M.; Ye, C. Intra-Articular Platelet-Rich Plasma Combined with Hyaluronic Acid Injection for Knee Osteoarthritis Is Superior to PRP or HA Alone in Inhibiting Inflammation and Improving Pain and Function. Arthrosc. J. Arthrosc. Relat. Surg. 2020, 37, 903–915. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Luo, X.; Xiong, Y.; Liu, G.; Wang, J.W.; Chen, X.; Mi, B. Platelet-Rich Plasma Versus Hyaluronic Acid in Knee Osteoarthritis: A Meta-Analysis with the Consistent Ratio of Injection. J. Orthop. Surg. 2020, 28, 2309499019887660. [Google Scholar] [CrossRef]
- Gong, H.; Li, K.; Xie, R.; Du, G.; Li, L.; Wang, S.; Yin, J.; Gu, J.; Wang, P.; Chen, M.; et al. Clinical therapy of platelet-rich plasma vs hyaluronic acid injections in patients with knee osteoarthritis: A system-atic review and meta-analysis of randomized double-blind controlled trials. Medicine 2021, 100, e25168. [Google Scholar] [CrossRef]
- Karasavvidis, T.; Totlis, T.; Gilat, R.; Cole, B.J. Platelet-Rich Plasma Combined with Hyaluronic Acid Improves Pain and Function Compared with Hyaluronic Acid Alone in Knee Osteoarthritis: A Systematic Review and Meta-Analysis. Arthrosc. J. Arthrosc. Relat. Surg. 2020, 37, 1277–1287. [Google Scholar] [CrossRef]
- Tang, J.; Nie, M.; Zhao, J.; Zhang, G.; Zhang, Q.; Wang, B. Platelet-Rich Plasma Versus Hyaluronic Acid in the Treatment of Knee Osteoarthritis: A Meta-Analysis. J. Orthop. Surg. Res. 2020, 15, 403. [Google Scholar] [CrossRef]
- Tan, J.; Chen, H.; Zhao, L.; Huang, W. Platelet Rich Plasma Versus Hyaluronic Acid in the Treatment of Knee Osteoarthritis: A Meta-Analysis of 26 Randomized Controlled Trials. Arthrosc. J. Arthrosc. Relat. Surg. 2020, 36, 233–246. [Google Scholar] [CrossRef]
- Filardo, G.; Previtali, D.; Napoli, F.; Candrian, C.; Zaffagnini, S.; Grassi, A. PRP Injections for the Treatment of Knee Osteoarthritis: A Meta-Analysis of Randomized Controlled Trials. Cartilage 2021, 13, 364S–375S. [Google Scholar] [CrossRef]
- Belk, J.W.; Lim, J.J.; Keeter, C.L.; McCulloch, P.; Houck, D.A.; McCarty, E.; Frank, R.; Kraeutler, M. Patients with Knee Osteoarthritis Who Receive Platelet-Rich Plasma or Bone-Marrow Aspirate Concentrate Injections Have Better Outcomes Than Patients Who Receive Hyaluronic Acid: Systematic Review and Meta-Analysis. Arthrosc. J. Arthrosc. Relat. Surg. 2023, 39, 1714–1734. [Google Scholar] [CrossRef]
- Jivan, S.J.; Monzavi, S.M.; Zargaran, B.; Alamdari, D.H.; Afshari, J.T.; Etemad-Rezaie, A.; Sakhmaresi, T.A.; Shariati-Sarabi, Z. Comparative Analysis of the Effectiveness of Intra-Articular Injection of Platelet-Rich Plasma Versus Hyaluronic Acid for Knee Osteoarthritis: Results of an Open-Label Trial. Arch. Bone Jt. Surg. 2021, 9, 487–495. [Google Scholar]
- McLarnon, M.; Heron, N. Intra-Articular Platelet-Rich Plasma Injections Versus Intra-Articular Corticosteroid Injections for Symptomatic Management of Knee Osteoarthritis: Systematic Review and Meta-Analysis. BMC Musculoskelet. Disord. 2021, 22, 550. [Google Scholar] [CrossRef] [PubMed]
- Sdeek, M.; Sabry, D.; El-Sdeek, H.; Darweash, A. Intra-Articular Injection of Platelet Rich Plasma Versus Hyaluronic Acid for Moderate Knee Osteoarthritis. Acta Orthop. Belg. 2021, 87, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Knapik, D.M.; Polce, E.M.; Eikani, C.; Bjornstad, A.H.; Gursoy, S.; Perry, A.K.; Westrick, J.C.; Yanke, A.B.; Verma, N.N.; et al. Relative Efficacy of Intra-Articular Injections in the Treatment of Knee Osteoarthritis: A Systematic Review and Network Meta-Analysis. Am. J. Sports Med. 2021, 50, 3140–3148. [Google Scholar] [CrossRef]
- Wang, Y.C.; Lee, C.L.; Chen, Y.J.; Tien, Y.C.; Lin, S.Y.; Chen, C.H.; Chou, P.P.; Huang, H.T. Comparing the Efficacy of Intra-Articular Single Platelet-Rich Plasma (PRP) versus Novel Crosslinked Hyaluronic Acid for Early-Stage Knee Osteoarthritis: A Prospective, Double-Blind, Randomized Controlled Trial. Medicina 2022, 58, 1028. [Google Scholar] [CrossRef]
- Branch, E.A.; Cook, J.; Cohen, A.; Plummer, H.; Emami, A.; Truett, J.; Anz, A.W. Platelet Rich Plasma Is Similar to Platelet Rich Plasma Plus Hyaluronic Acid for the Treatment of Knee Osteoarthritis at 2 Years: A Randomized Controlled Trial. J. Cartil. Jt. Preserv. 2023, 3, 100129. [Google Scholar] [CrossRef]
- Karas, F.; Essmat, E.; el Tregy, S. Comperative Study Between Effect of Hyalouronic Acid and Platelet Rich Plasma in Treatment of Knee Osteoarthritis. Benha Med. J. 2023, 40, 23–30. [Google Scholar] [CrossRef]
- Li, S.; Xing, F.; Yan, T.; Zhang, S.; Chen, F. Multiple Injections of Platelet-Rich Plasma Versus Hyaluronic Acid for Knee Osteoarthritis: A Systematic Review and Meta-Analysis of Current Evidence in Randomized Controlled Trials. J. Pers. Med. 2023, 13, 429. [Google Scholar] [CrossRef]
- Ivander, G.; Anggono, Y. A Comparison of Intra-Articular Hyaluronic Acid and Platelet-Rich Plasma for Knee Osteoarthritis: A Systematic Review. Orthop. Rev. 2024, 16, 94236. [Google Scholar] [CrossRef]
- Jawanda, H.; Khan, Z.A.; Warrier, A.A.; Acuna, A.J.; Allahabadi, S.; Kaplan, D.; Ritz, E.; Jackson, G.R.; Mameri, E.S.; Batra, A.; et al. Platelet Rich Plasma, Bone Marrow Aspirate Concentrate and Hyaluronic Acid Injections Outperform Corticosteroids in Pain and Function Scores at a Minimum of 6 Months as Intra-Articular Injections for Knee Osteoarthritis: A Systematic Review and Network Meta-Analysis. Arthrosc. J. Arthrosc. Relat. Surg. 2024, 40, 1623–1636. [Google Scholar]
- Indra, A.; Das, C.; Das, P. Comparative Study Between Hyaluronic Acid & Platelet Rich Plasma in Treatment of Knee Osteoarthritis. IP Int. J. Orthop. Rheumatol. 2024, 10, 30–35. [Google Scholar]
- Dório, M.; Pereira, R.M.R.; Luz, A.G.B.; Deveza, L.A. Efficacy of platelet-rich plasma and plasma for symptomatic treatment of knee osteoarthritis: A double-blinded placebo-controlled randomized clinical trial. BMC Musculoskelet Disord. 2021, 22, 822. [Google Scholar] [CrossRef] [PubMed]
- Bennell, K.L.; Paterson, K.L.; Metcalf, B.R.; Wrigley, T.V.; O’Donnell, J.; Wagn, Y.; Harris, A.; Forbes, A.; Connell, D.; Linklater, J.; et al. Effect of Intra-Articular Platelet-Rich Plasma vs. Saline Injections on Knee Cartilage Volume and Pain in Patients with Knee Osteoarthritis: The RESTORE Randomized Clinical Trial. JAMA 2021, 326, 2021–2030. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Park, Y.B.; Ha, C.W.; Roh, Y.J.; Park, J.G. Adverse Reactions and Clinical Outcomes for Leukocyte-Poor Versus Leukocyte-Rich Platelet-Rich Plasma in Knee Osteoarthritis: A Systematic Review and Meta-analysis. Orthop. J. Sports Med. 2021, 9, 23259671211011948. [Google Scholar] [CrossRef]
- Cole, B.; Karas, V.; Hussey, K.; Merkow, D.B.; Pilz, K.; Fortier, L. Hyaluronic Acid Versus Platelet-Rich Plasma: A Prospective, Double-Blind Randomized Controlled Trial Comparing Clinical Outcomes and Effects on Intra-Articular Biology for the Treatment of Knee Osteoarthritis. Am. J. Sports Med. 2017, 45, 339–346. [Google Scholar] [CrossRef]
- Kon, E.; Mandelbaum, B.; Buda, R.; Filardo, G.; Delcogliano, M.; Timoncini, A.; Fornasari, P.; Giannini, S.; Marcacci, M. Platelet-Rich Plasma Intra-Articular Injection Versus Hyaluronic Acid Viscosupplementation as Treatments for Cartilage Pathology: From Early Degeneration to Osteoarthritis. Arthrosc. J. Arthrosc. Relat. Surg. 2011, 27, 1490–1501. [Google Scholar] [CrossRef]
- De Cassai, A.; Boscolo, A.; Zarantonello, F.; Pettenuzzo, T.; Sella, N.; Geraldini, F.; Munari, M.; Navalesi, P. Enhancing study quality assessment: An in-depth review of risk of bias tools for meta-analysis-a comprehensive guide for anesthesiologists. J. Anesth. Analg. Crit. Care. 2023, 3, 44. [Google Scholar] [CrossRef]
- Nejadghaderi, S.A.; Balibegloo, M.; Rezaei, N. The Cochrane risk of bias assessment tool 2 (RoB 2) versus the original RoB: A perspective on the pros and cons. Health Sci. Rep. 2024, 7, e2165. [Google Scholar] [CrossRef]
- Patel, S.; Dhillon, M.; Aggarwal, R.; Marwaha, S.; Jain, S. Treatment with Platelet-Rich Plasma Is More Effective Than Placebo for Knee Osteoarthritis. Am. J. Sports Med. 2013, 41, 356–364. [Google Scholar] [CrossRef]
- Cook, C.; Smith, P. Platelet-Rich Plasma Mechanisms in Osteoarthritis: Revisiting the Protein Cascade. J. Orthop. Res. 2018, 36, 207–214. [Google Scholar]
- Szwedowski, D.; Nitek; Popko, J. Platelet-Rich Plasma in Orthopaedic Applications: Evidence-Based Update and Analysis. Pol. Ann. Med. 2021, 28, 91–97. [Google Scholar]
- Zhuo, F.; Li, J.; Wang, Y.H.; Li, M.; Song, F.F.; Liu, Y.L.; Tao, Z.Y. Platelet-rich plasma inhibits inflammation, apoptosis, and the NLRP3/Caspase-1 pathway and induces matrix metalloproteinases and proliferation of IL-1β-induced articular chondrocytes by downregulating T-box transcription factor 3. Eur. J. Inflamm. 2022, 20, 1721727X221093056. [Google Scholar] [CrossRef]
- Sohrobion, B.; Abri, A.; Roustaei, N.; Parsa, H. Cartilage-Protective Effects of Platelet-Rich Plasma in a Rabbit Model of Osteoarthritis. J. Exp. Orthop. 2019, 6, 21. [Google Scholar]
- Sanchez, M.; Fiz, N.; Azofra, J.; Usabiaga, J.; Recalde, E.; Gutierrez, A.G.G.; Albillos, J.; Garate, R.; Aguirre, J.J.; Padilla, S.; et al. A Randomized Clinical Trial Evaluating Plasma Rich in Growth Factors (PRGF-Endoret) Versus Hyaluronic Acid in the Short-Term Treatment of Symptomatic Knee Osteoarthritis. Arthroscopy 2018, 34, 1920–1927. [Google Scholar] [CrossRef]
- Dhillon, R.S.; Beale, S.J.; Sengir, F.; McCarthy, M.B.; LaPrade, R.F. Current Concepts for Platelet-Rich Plasma in the Management of Cartilage Defects and Osteoarthritis of the Knee. Cartilage 2017, 8, 263–278. [Google Scholar]
- Kim, J.D.; Sung, C.M.; Jang, J.D.; Hyun, C.; Lee, S.W. Leukocyte-Poor Platelet-Rich Plasma Is Superior to Leukocyte-Rich Platelet-Rich Plasma in Knee Osteoarthritis Patients: A Systematic Review and Meta-analysis. Orthop. J. Sports Med. 2021, 9, 2325967121992786. [Google Scholar]
- Zhang, B.; Dong, B.; Wang, L.; Wang, Y.; Gao, Z.; Li, Y.; Wang, H.; Lu, X. Comparison of the efficacy of autologous Lp-PRP and Lr-PRP for treating intervertebral disc degeneration: In vitro and in vivo study. J. Orthop. Surg. Res. 2024, 19, 731. [Google Scholar] [CrossRef]
- Shim, J.W.; Lee, J.-S.; Park, Y.-B.; Cho, H.-C.; Jung, H.-S. The effect of leucocyte concentration of platelet-rich plasma on outcomes in patients with lateral epicondylitis: A systematic review and meta-analysis. J. Shoulder Elb. Surg. 2022, 31, 634–645. [Google Scholar] [CrossRef]
- Lana, J.F.; Huber, S.C.; Purita, J.; Tambeli, C.H.; Santos, G.S.; Paulus, C.; Annichino-Bizzacchi, J.M. Leukocyte-rich PRP versus leukocyte-poor PRP—The role of monocyte/macrophage function in the healing cascade. J. Clin. Orthop. Trauma 2019, 10 (Suppl. S1), S7–S12. [Google Scholar] [CrossRef]
- Nishio, H.; Saita, Y.; Kobayashi, Y.; Takaku, T.; Fukusato, S.; Uchino, S.; Wakayama, T.; Ikeda, H.; Kaneko, K. Platelet-rich plasma promotes recruitment of macrophages in the process of tendon healing. Regen. Ther. 2020, 14, 262–270. [Google Scholar] [CrossRef]
- Marques, L.F.; Stessuk, T.; Camargo, I.C.; Sabeh Junior, N.; dos Santos, L.; Ribeiro-Paes, J.T. Platelet-rich plasma (PRP): Methodological aspects and clinical applications. Platelets 2015, 26, 101–103. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, A.; Di Matteo, B.; Papio, T.; Tentoni, F.; Selleri, F.; Cenacchi, A.; Kon, E.; Filardo, G. Leukocyte-Rich Versus Leukocyte-Poor Platelet-Rich Plasma Injections for Knee Osteoarthritis: A Double-Blind Randomized Trial. Am. J. Sports Med. 2022, 50, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Zhou, Y.; Zhang, H.; Lin, J.; Xu, T. Activated Versus Non-Activated Platelet-Rich Plasma for Knee Osteoarthritis: A Meta-analysis of Randomized Controlled Trials. BMC Musculoskelet. Disord. 2023, 24, 222. [Google Scholar]
- Qiao, X.C.; Yan, L.; Feng, Y.; Li, X.Y.; Zhang, K.; Lv, Z.; Xu, C.J.; Zhao, S.; Liu, F.R.; Yang, X.H.; et al. Efficacy and safety of corticosteroids, hyaluronic acid, and PRP and combination therapy for knee osteoarthritis: A systematic review and network meta-analysis. BMC Musculoskelet. Disord. 2023, 24, 926. [Google Scholar] [CrossRef]
- Bensa, A.; Previtali, D.; Sangiorgio, A.; Boffa, A.; Salerno, M.; Filardo, G. PRP Injections for the Treatment of Knee Osteoarthritis: The Improvement Is Clinically Significant and Influenced by Platelet Concentration: A Meta-analysis of Randomized Controlled Trials. Am. J. Sports Med. 2025, 53, 745–754. [Google Scholar] [CrossRef]
- Murali, A.; Khan, I.; Tiwari, S. Navigating the treatment landscape: Choosing between platelet-rich plasma (PRP) and hyaluronic acid (HA) for knee osteoarthritis management—A narrative review. J. Orthop. Rep. 2024, 3, 100248. [Google Scholar] [CrossRef]
- Tiwari, V.; Raina, D.B.; Khan, W.; Malhotra, R.; Ismail, H. Platelet-Rich Plasma Versus Corticosteroid Injections in the Treatment of Knee Osteoarthritis: A Systematic Review and Meta-analysis. Arch. Orthop. Trauma Surg. 2022, 142, 3625–3637. [Google Scholar]
- McAlindon, T.E.; LaValley, M.P.; Harvey, W.F.; Price, L.L.; Driban, J.B.; Zhang, M.; Ward, R. Effect of Intra-Articular Triamcinolone vs. Saline on Knee Cartilage Volume and Pain in Patients with Knee Osteoarthritis: A Randomized Clinical Trial. JAMA 2017, 317, 1967–1975. [Google Scholar] [CrossRef]
- Shapiro, S.A.; Kazmerchak, S.E.; Heckman, M.G.; Zubair, A.C.; O’Connor, M.I. A Prospective, Single-Blind, Placebo-Controlled Trial of Bone Marrow Aspirate Concentrate for Knee Osteoarthritis. Am. J. Sports Med. 2017, 45, 82–90. [Google Scholar] [CrossRef]
- Elksnis, A.; Vallence, B. PRP vs. BMAC in Knee Osteoarthritis: A Systematic Review and Meta-analysis. Clin. J. Sport. Med. 2022, 32, e245–e255. [Google Scholar]
- Tanguilig, G.; Dhillon, J.; Kraeutler, M.J. Platelet-Rich Plasma for Knee and Hip Osteoarthritis Pain: A Scoping Review. Curr. Rev. Musculoskelet. Med. 2024, 17, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Pabinger, C.; Kobinia, G.S.; Dammerer, D. Injection therapy in knee osteoarthritis: Cortisol, hyaluronic acid, PRP, or BMAC (mesenchymal stem cell therapy)? Front. Med. 2024, 11, 1463997. [Google Scholar] [CrossRef] [PubMed]
- Mende, E.; Love, R.J.; Young, J.L. A Comprehensive Summary of the Meta-Analyses and Systematic Reviews on Platelet-Rich Plasma Therapies for Knee Osteoarthritis. Mil Med. 2024, 189, 2347–2356. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Gong, C.; Peng, X.; Liu, X.; Su, X.; Tao, X.; Li, Y.; Wen, Y.; Li, W. Efficacy and safety of platelet-rich plasma injections for the treatment of osteoarthritis: A systematic review and meta-analysis of randomized controlled trials. Front. Med. 2023, 10, 1204144. [Google Scholar] [CrossRef]
- Khalid, S.; Ali, A.; Deepak, F.; Zulfiqar, M.S.; Malik, L.U.; Fouzan, Z.; Nasr, R.A.; Qamar, M.; Bhattarai, P. Comparative effectiveness of intra-articular therapies in knee osteoarthritis: A meta-analysis comparing platelet-rich plasma (PRP) with other treatment modalities. Ann. Med. Surg. 2024, 86, 361–372. [Google Scholar] [CrossRef]
- Rodríguez-Merchán, E.C. Intra-Articular Platelet-Rich Plasma Injections in Knee Osteoarthritis: A Review of Their Current Molecular Mechanisms of Action and Their Degree of Efficacy. Int. J. Mol. Sci. 2022, 23, 1301. [Google Scholar] [CrossRef]
- Liu, Q.; Ye, H.; Yang, Y.; Chen, H. The efficacy and safety of intra-articular platelet-rich plasma versus sodium hyaluronate for the treatment of osteoarthritis: Meta-analysis. PLoS ONE 2025, 20, e0314878. [Google Scholar] [CrossRef]
- Khalilizad, M.; Emadian, S.T.; Marzban Abbas Abadi, M. Comparative efficacy of different doses of platelet-rich plasma injection in the treatment of knee osteoarthritis: A systematic review and network meta-analysis. J. Orthop. Surg. Res. 2025, 20, 221. [Google Scholar] [CrossRef]
- Orchard, J.W.; Read, J.W.; Anderson, I.J. The Use and Abuse of NSAIDs and Corticosteroid Injections in Sports Medicine. Clin. Sports Med. 2022, 41, 599–611. [Google Scholar]
- Samuelson, E.M.; Ebel, J.A.; Reynolds, S.B.; Arnold, R.M.; Brown, D.E. The Cost-Effectiveness of Platelet-Rich Plasma Compared With Hyaluronic Acid Injections for the Treatment of Knee Osteoarthritis. Arthrosc. J. Arthrosc. Relat. Surg. 2020, 36, 3072–3078. [Google Scholar] [CrossRef]
- Kolasinski, S.L.; Neogi, T.; Hochberg, M.C.; Oatis, C.; Guyatt, G.; Block, J.; Callahan, L.; Copenhaver, C.; Dodge, C.; Felson, D.; et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthritis Care Res. 2020, 72, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Kraus, V.B.; Blanco, F.J.; Englund, M.; Karsdal, M.A.; Lohmander, L.S. OARSI Clinical Trials Recommendations: Soluble Biomarkers in OA. Osteoarthr. Cartil. 2019, 27, 572–578. [Google Scholar]
- Bannuru, R.R.; Osani, M.C.; Vaysbrot, E.E.; Arden, N.K.; Bennell, K.; Bierma-Zeinstra, S.M.A.; Kraus, V.B.; Lohmander, L.S.; Abbott, J.H.; Bhandari, M.; et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr. Cartil. 2019, 27, 1578–1589. [Google Scholar] [CrossRef] [PubMed]
- Murray, M.M. Management of Osteoarthritis of the Knee (Non-Arthroplasty), Evidence-Based Clinical Practice Guideline, 2nd ed.; American Academy of Orthopaedic Surgeons: Rosemont, IL, USA, 2021; Available online: https://www.aaos.org/globalassets/quality-and-practice-resources/biologics/technology-overview_prp-for-knee-oa.pdf (accessed on 15 March 2025).
- Dubin, J.; Leucht, P.; Murray, M.; Pezold, R. American Academy of Orthopaedic Surgeons Technology Overview Summary: Platelet-Rich Plasma (PRP) for Knee Osteoarthritis. J. Am. Acad. Orthop. Surg. 2024, 32, 296–301. [Google Scholar] [CrossRef]
- The Non-Surgical Management of Hip & Knee Osteoarthritis Work Group; The Office of Quality and Patient Safety; Office of Evidence Based Practice. VA/DoD Clinical Practice Guideline for the Non-Surgical Management of Hip and Knee Osteoarthritis; Department of Veterans Affairs and Department of Defense: Washington, DC, USA, 2020.
- Laver, L.; Filardo, G.; Sanchez, M.; Magalon, J.; Tischer, T.; Abat, F.; Bastos, R.; Cugat, R.; Iosifidis, M.; Kocaoglu, B.; et al. The use of injectable orthobiologics for knee osteoarthritis: A European ESSKA-ORBIT consensus. Part 1-Blood-derived products (platelet-rich plasma). Knee Surg. Sports Traumatol. Arthrosc. 2024, 32, 783–797. [Google Scholar] [CrossRef]
- Eymard, F.; Oubaya, N.; Ornetti, P.; Sellam, J.; Richette, P.; Chevalier, X. Protocol for a multicentre randomised triple-blind controlled trial assessing the clinical efficacy of intra-articular platelet-rich plasma injections versus placebo in symptomatic knee osteoarthritis (PIKOA). BMJ Open 2024, 14, e085025. [Google Scholar] [CrossRef]
- Kon, E.; de Girolamo, L.; Laver, L.; Andriolo, L.; Andia, I.; Bastos, R.; Beaufils, P.; Biant, L.; Bøe, B.; Boffa, A.; et al. Platelet-rich plasma injections for the management of knee osteoarthritis: The ESSKA-ICRS consensus. Recommendations using the RAND/UCLA appropriateness method for different clinical scenarios. Knee Surg. Sports Traumatol. Arthrosc. 2024, 32, 2938–2949. [Google Scholar] [CrossRef]
- NICE National Institute for Health and Care Excellence. Platelet-Rich Plasma Injections for Knee Osteoarthritis; Interventional Procedures Guidance; Reference number: IPG637; NICE National Institute for Health and Care Excellence: London, UK, 2019; Available online: https://www.nice.org.uk/guidance/ipg637 (accessed on 23 January 2019).
- Phillips, M.; Bhandari, M.; Grant, J.; Bedi, A.; Trojian, T.; Johnson, A.; Schemitsch, E. A Systematic Review of Current Clinical Practice Guidelines on Intra-articular Hyaluronic Acid, Corticosteroid, and Platelet-Rich Plasma Injection for Knee Osteoarthritis: An International Perspective. Orthop. J. Sports Med. 2021, 9, 23259671211030272. [Google Scholar] [CrossRef]

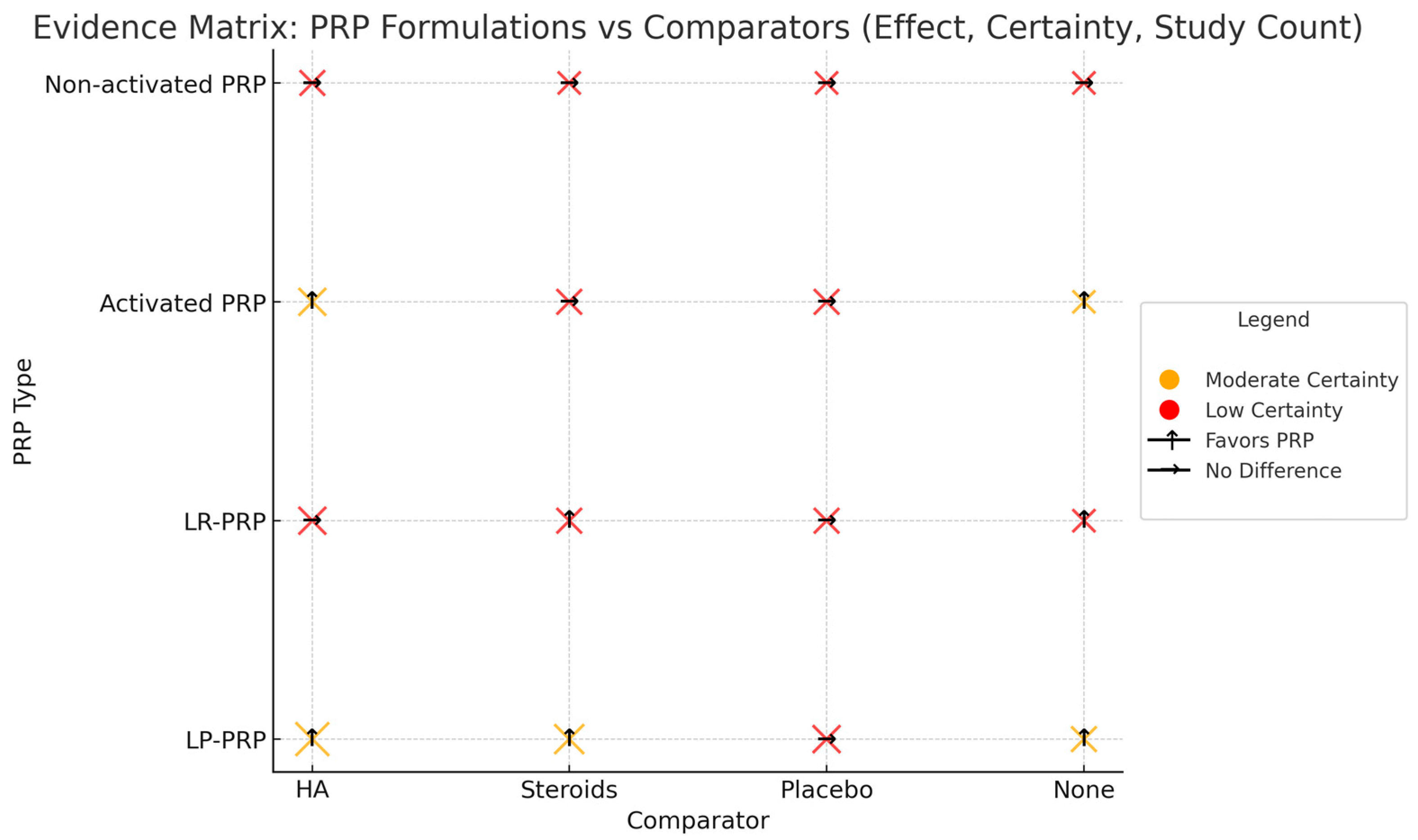
| Study | Study Design | Sample Size | Treatment Protocol | Follow-Up Duration | Outcomes |
|---|---|---|---|---|---|
| Kon et al., 2011 [23] | Prospective comparative study | 150 (PRP: 50 and HA: 100 | PRP: 3 injections every 14 days | 6 months | Both groups improved, but PRP produced higher IKDC and EQ-VAS scores and a lower re-intervention rate than HA at 6–24 months. |
| Filardo et al., 2012 [26] | Randomized double-blind prospective trial | 109 (PRP: 54 and HA: 55) | 3 weekly injections | 12 months | PRP and HA both improved pain and function; no significant overall difference at 12 months, although PRP trended better in low-grade OA. |
| Say et al., 2013 [27] | Prospective, comparative clinical study | 90 (PRP: 45 and HA: 45) | PRP: 1 and HA: 3 injections | 6 months | Single PRP injection yielded greater reductions in pain (VAS) and WOMAC scores than HA at 6 months. |
| Khoshbin et al., 2013 [28] | Systematic review | 577 (PRP: 264 and Control: 313) | 2, 3, or 4 injections | 24 weeks | Meta-analysis found PRP reduced WOMAC pain and improved function more than saline or HA at 6 and 12 months without increasing adverse events. |
| Holguin, 2014 [29] | Prospective study | 150 (PRP: 55; HA: 55) | 3 weekly injections | 12 months | PRP provided significantly greater improvements in WOMAC total and pain vs. HA at 12 months. |
| Laudy et al., 2014 [30] | Systematic review | 1110 (PRP~50% HA ~50%) 10 studies | Three intra-articular injections | 6 to 12 months | Systematic review concluded evidence is limited; PRP may offer symptomatic benefit over HA, but heterogeneity prevents firm conclusions. |
| Raeissadat et al., 2015 [31] | Non-placebo-controlled randomized clinical trial | 160 (PRP: 87 and HA: 73) | PRP: 2 injections at 4-week interval and HA: 3 injections at 1-week interval | 12 months | At 12 months PRP produced larger decreases in WOMAC pain and stiffness and higher patient satisfaction than HA. |
| Montañez-Heredia et al., 2015 [32] | Double-blind randomized controlled trial | 95 | PRP-1: 1, PRP-2: 2, and HA: 3 injections | 3 months | No significant difference among single- or double-dose PRP and HA at 3 months; all groups improved similarly. |
| Filardo et al., 2015 [33] | Randomized double-blind trial | 192 (PRP: 94, HA: 89) | 3 weekly injections | 12 months | Both PRP and HA improved outcomes over 12 months; PRP did not achieve superiority except in younger, less degenerated knees. |
| Cole et al., 2015 [34] | Double-masked prospective randomized controlled trial | 111 | 3 weekly injections | 24 weeks | PRP resulted in lower pain (VAS) and higher IKDC than HA at 24 and 52 weeks; primary WOMAC pain not different. |
| Lana et al., 2016 [35] | Multi-center, randomized, controlled, double-blind, prospective trial | 105 (HA: 36, PRP: 36, and HA + PRP: 33) | 3 injections at 2-week intervals | 12 months | Combination PRP + HA achieved the greatest reduction in WOMAC pain; PRP alone also outperformed HA at 12 months. |
| Cunningham, 2017 [36] | Randomized controlled trial | 120 (PRP: 60 and HA: 60) | 4 weekly injections | 24 weeks | PRP produced significantly better WOMAC pain and function scores than HA at 24 weeks. |
| Dai et al., 2017 [37] | Meta-analysis | 1069 | Varied among studies | 12 months | Meta-analysis: PRP superior to HA for pain and function at 12 months (WOMAC pain MD: −2.83). |
| Shen et al., 2017 [38] | Systematic review and meta-analysis (17 RCT) | 1423 (HA Ozone and Saline) | Varied among studies | 12 weeks to 12 months | PRP injections provided greater short-term (≤6 months) pain relief than HA or ozone in pooled RCTs. |
| Di Martino et al., 2018 [39] | Double-blind randomized controlled trial | 192 | 3 weekly injections | Mean 64.3 months | Early PRP benefit over HA faded; at mean 5-year follow-up, no significant difference in IKDC or KOOS. |
| Zhang et al., 2018 [40] | Meta-analysis (RCTs) | 1524 (PRP: 788 and HA: 736) | PRP 3 and HA 3 (injections once a week) | 12 months | Pooled RCTs showed that PRP reduced WOMAC pain and VAS more effectively than HA at 6 and 12 months. |
| Lin et al., 2019 [41] | Randomized, dose-controlled, placebo-controlled, double-blind, triple-parallel clinical trial | 87 knees (53 patients) | 3 weekly injections | 12 months | Dose-controlled RCT confirmed that PRP improved WOMAC pain and IKDC vs. placebo; benefits sustained for 12 months. |
| Meheux et al., 2020 [42] | Systematic review | 739 patients (817 knees) | No mention found | Up to 12 months | Systematic review found that PRP yielded clinically significant pain and function improvements lasting up to 12 months and exceeded HA in most trials. |
| Xu et al., 2020 [43] | Prospective cohort study | 122 (PRP: 40, HA: 34, and PRP + HA: 48) | PRP: 3 and HA: 3 (injections once a week) | 24 months | The PRP + HA combination had greatest WOMAC improvement; PRP alone was better than HA and benefits sustained for up to 24 months. |
| Wu et al., 2020 [44] | Meta-analysis | 1063 (PRP: 526 and HA: 537. | PRP: 3 and HA: 3 (injections once a week) | No mention | Meta-analysis: PRP was superior to HA for VAS and WOMAC pain at 6–12 months, with more mild post-injection pain events. |
| Li et al., 2020 [45] | Systematic review and meta-analysis | 661 (PRP: 338 and A: 323) | Varied among studies | 12 months | PRP showed better pain relief and functional scores than HA at 3–12 months in pooled analysis. |
| Karasavvidis et al., 2020 [46] | Systematic review and meta-analysis | 377 (PRP-HA: 193 and HA: 184) | Varied among studies | 6 to 12 months | Network meta-analysis indicated that the PRP + HA combination ranked highest for pain reduction, ahead of PRP or HA alone. |
| Tang et al., 2020 [47] | Meta-analysis (20 RCT) | 1281 (PRP: 654 and HA: 627) | Varied among studies (1–4 injections) | 3 to 12 months | PRP outperformed HA on VAS and WOMAC across 20 RCTs, with effects persisting for 12 months. |
| Tan et al., 2020 [48] | Meta-analysis | 2430 | Varied among studies | 12 months | Meta-analysis of 2430 knees showed that PRP provided superior pain relief vs. HA at 6 and 12 months with comparable safety. |
| Filardo et al., 2020 [49] | Systematic review and meta-analysis | 2829 (PRP: 1403 and Control: 1426) | Varied among studies | 12 months | Review concluded that PRP offers small-to-moderate clinical benefit over HA, especially in younger patients. |
| Belk et al., 2023 [50] | Systematic review and meta-analysis | 2396 (PRP: 1042, BMAC: 226, and HA: 1128) | Varied among studies | PRP: 13.5 months, BMAC: 17.5 months, and HA: 14.4 months | PRP and BMAC yielded larger mean improvements in WOMAC pain than HA at ~14 months; adverse events similar. |
| Jivan et al., 2021 [51] | Phase I open-label clinical trial | 20 (PRP: 10 and HA: 10) | Varied among studies | 12 months | The phase I trial showed a 60% pain reduction with PRP vs. 40% with HA at 12 months; no severe adverse events. |
| McLarnon and Heron, 2021 [52] | Systematic review and meta-analysis | 648 | Single or triple injections | 12 months | Systematic review: The majority of RCTs favored PRP over HA for WOMAC pain/function at 12 months. |
| Sdeek et al., 2021 [53] | Prospective, double-blind, randomized controlled trial | 189 | 3 injections every 2 weeks | 36 months | PRP maintained significant WOMAC and VAS improvements over HA throughout the 36 months of follow-up. |
| Singh et al., 2021 [54] | Meta-analysis | PRP, HA, and corticosteroids found | Varied among studies | Minimum 6 months | Network meta-analysis ranked PRP highest for pain relief over HA and corticosteroids at ≥6 months. |
| Wang et al., 2022 [55] | Prospective, double-blind, parallel, randomized controlled trial | 110 (PRP: 54 and HA: 56) | Single injection | 6 months | Single-injection RCT found no significant superiority of PRP over HA on WOMAC pain at 6 months. |
| Branch et al., 2023 [56] | Randomized controlled trial | 64 | 3 injections | 24 months | PRP superior to HA for WOMAC at 6 and 12 months, but groups converged by 24 months; safety profiles comparable. |
| Karas et al., 2023 [57] | Systematic review | No mention found | Varied among studies | 12 months | Systematic review affirms consistent PRP advantage over HA across the majority of the included trials. |
| Li et al., 2023 [58] | Systematic review and meta-analysis | 1512 | Varied among studies | 12 months | Updated meta-analysis showed that PRP offered clinically meaningful reductions in VAS and WOMAC vs. HA at 12 months. |
| Belk et al., 2023 [50] | Systematic review and meta-analysis | 2396 (PRP: 1042, BMAC: 226, and HA: 1128) | Varied among studies | PRP: 13.5 months, BMAC: 17.5 months, and HA: 14.4 months | PRP and BMAC yielded larger mean improvements in WOMAC pain than HA at ~14 months; adverse events similar. |
| Ivander and Anggono, 2024 [59] | Systematic review | 447 (PRP: 198 and HA: 194) | Varied (single and multiple injections) | Varied (1–24 months) | The systematic review found that PRP reduced VAS more than HA at 6 months; combination therapy most effective. |
| Jawanda et al., 2024 [60] | Systematic review and network meta-analysis | 9338 knees | Varied among studies | Minimum 6 months | Network meta-analysis ranked PRP as most efficacious for pain and function at ≥6 months among injectables. |
| Indra et al., 2024 [61] | Comparative study | No mention found | No mention found | 12 months | Comparative study reported significantly greater WOMAC and VAS improvements with PRP over HA at 12 months. |
| Outcome Measure | 3 Months | 6 Months | 12 Months |
|---|---|---|---|
| Pain reduction (VAS/WOMAC pain) | Significant improvement in most RCTs vs. HA/steroids; some vs. placebo | Sustained benefit in most studies, especially LP-PRP vs. HA | Mixed results; some RCTs show parity with placebo |
| Functional improvement (WOMAC/IKDC) | Consistent improvement in early to moderate KOA | Functional gains persist in KL I–II; mixed for KL III | Diminishing effect in moderate to severe OA (KL III–IV) |
| Structural effect (imaging-based) | No structural changes typically detectable | No disease-modifying effect evident | No radiographic progression delay observed |
| Adverse events | Very few, mostly local, and mild (e.g., injection site pain) | No new safety signals | Safe long-term profiles reported in most studies |
| Outcome Measure | 3 Months | 6 Months | 12 Months |
|---|---|---|---|
| Pain reduction (VAS/WOMAC pain) | Greater reduction than HA in most RCTs, particularly LP-PRP | Sustained superiority over HA; some studies show comparable effects | Mixed findings; several trials show no significant difference from HA |
| Functional improvement (WOMAC/IKDC) | Significantly better improvement vs. HA, especially in KL I–II | PRP superior to HA in maintaining function in early OA | Functional scores converge in moderate-to-severe OA |
| Structural effect (imaging-based) | No significant differences vs. HA | No imaging evidence of disease-modifying effects for either | No radiographic progression delay in either group |
| Adverse events | Similar or lower incidence than HA; mostly mild local reactions | Favorable safety profiles; less post-injection swelling than HA in some studies | Both treatments well tolerated; no major differences |
| Functional Measure | 3 Months | 6 Months | 12 Months |
|---|---|---|---|
| WOMAC Function Score | PRP shows greater improvement than HA in most RCTs; difference typically 10–20 points | Difference maintained; PRP superior in KL I–II; minimal decline in the HA group | Improvements diminish; scores similar in KL III–IV across groups |
| IKDC Score | PRP-treated patients demonstrate better gains; a 5–10-point advantage vs. HA | Sustained difference in favor of PRP, especially in active individuals | Scores converge; no significant between-group difference in advanced OA |
| Lequesne Index | PRP significantly reduces the disability score vs. HA | Benefit persists at 6 months for PRP; slight regression in the HA group | Scores align in patients with KL III or higher |
| KOOS Function Subscale | Marked improvement with PRP vs. HA; a larger effect size in early OA | PRP maintains advantage; HA plateaus or slightly regresses | Minimal between-group difference; PRP slightly better in KL I–II |
| Study | Randomization | Deviations from Interventions | Missing Data | Outcome Measurement | Selective Reporting | Overall Bias |
|---|---|---|---|---|---|---|
| Filardo et al. [26] | Low | Low | Some concerns | Low | Low | Low |
| Meheux et al. [42] | Some concerns | Low | Low | Low | Low | Some concerns |
| Patel et al. [69] | Low | Low | Low | Low | Low | Low |
| Cerza et al. [36] | High | Some concerns | Low | Some concerns | Low | High |
| Outcome | No. of Studies | Consistency | Certainty of Evidence (GRADE) | Comments |
|---|---|---|---|---|
| Pain reduction (PRP vs. HA) | 20+ RCTs and six meta-analyses | Moderate to high | Moderate | Consistent benefit at 6–12 months; I2 ~ 60–80% |
| Function improvement (PRP vs. HA) | 18+ RCTs and five meta-analyses | Moderate | Moderate | WOMAC and IKDC improvement; some heterogeneity |
| Pain reduction (PRP vs. corticosteroids) | 12+ RCTs and three meta-analyses | Moderate | Low | The short-term effect is similar; the long-term benefit favors PRP |
| Adverse events (PRP vs. HA or steroids) | 10+ RCTs | High | High | Very few serious AEs; mostly mild injection-site pain |
| Structural improvement (MRI or biomarkers) | 5 RCTs and two pilot studies | Low | Low | Exploratory only; insufficient evidence for conclusions |
| Organization | Year | Recommendation for PRP | Certainty/Comment |
|---|---|---|---|
| ACR/AF [101] | 2019 | Not recommended | Limited evidence and high variability |
| OARSI [102,103] | 2019 | Uncertain | Heterogeneous studies and bias risk |
| NICE (UK) [110] | 2019 | Special arrangements only | Requires governance and consent |
| GRIP [108] | 2020 | Recommended as a second-line treatment | Cautious use after other treatments fail |
| VA/DoD [106] | 2020 | Not recommended | Insufficient evidence for use |
| AAOS [104,105] | 2021 | Inconclusive | Lack of standardized protocols |
| AOSSM [85] | 2022 | Promising option | Useful in active patients with KL I–II |
| ESSKA-ORBIT [107] | 2024 | Recommended (early OA) | Prefer LP-PRP; 1–3 injections |
| ESSKA-ICRS [109] | 2024 | Recommended (post-failure conservative/injective) | Appropriate for KL 0–3, ≤80 years; not a first-line therapy or KL 4 |
| ISAKOS | 2025 | Observational data | PRP less effective in older patients, males, KL IV, and poor alignment; KL grade strongest predictor |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Glinkowski, W.M.; Gut, G.; Śladowski, D. Platelet-Rich Plasma for Knee Osteoarthritis: A Comprehensive Narrative Review of the Mechanisms, Preparation Protocols, and Clinical Evidence. J. Clin. Med. 2025, 14, 3983. https://doi.org/10.3390/jcm14113983
Glinkowski WM, Gut G, Śladowski D. Platelet-Rich Plasma for Knee Osteoarthritis: A Comprehensive Narrative Review of the Mechanisms, Preparation Protocols, and Clinical Evidence. Journal of Clinical Medicine. 2025; 14(11):3983. https://doi.org/10.3390/jcm14113983
Chicago/Turabian StyleGlinkowski, Wojciech Michał, Grzegorz Gut, and Dariusz Śladowski. 2025. "Platelet-Rich Plasma for Knee Osteoarthritis: A Comprehensive Narrative Review of the Mechanisms, Preparation Protocols, and Clinical Evidence" Journal of Clinical Medicine 14, no. 11: 3983. https://doi.org/10.3390/jcm14113983
APA StyleGlinkowski, W. M., Gut, G., & Śladowski, D. (2025). Platelet-Rich Plasma for Knee Osteoarthritis: A Comprehensive Narrative Review of the Mechanisms, Preparation Protocols, and Clinical Evidence. Journal of Clinical Medicine, 14(11), 3983. https://doi.org/10.3390/jcm14113983








