Porto-Sinusoidal Vascular Disorder: An Under-Recognized Liver Manifestation in Turner Syndrome
Abstract
1. Introduction
2. Case Presentation
2.1. Case 1
2.2. Case 2
2.3. Case 3
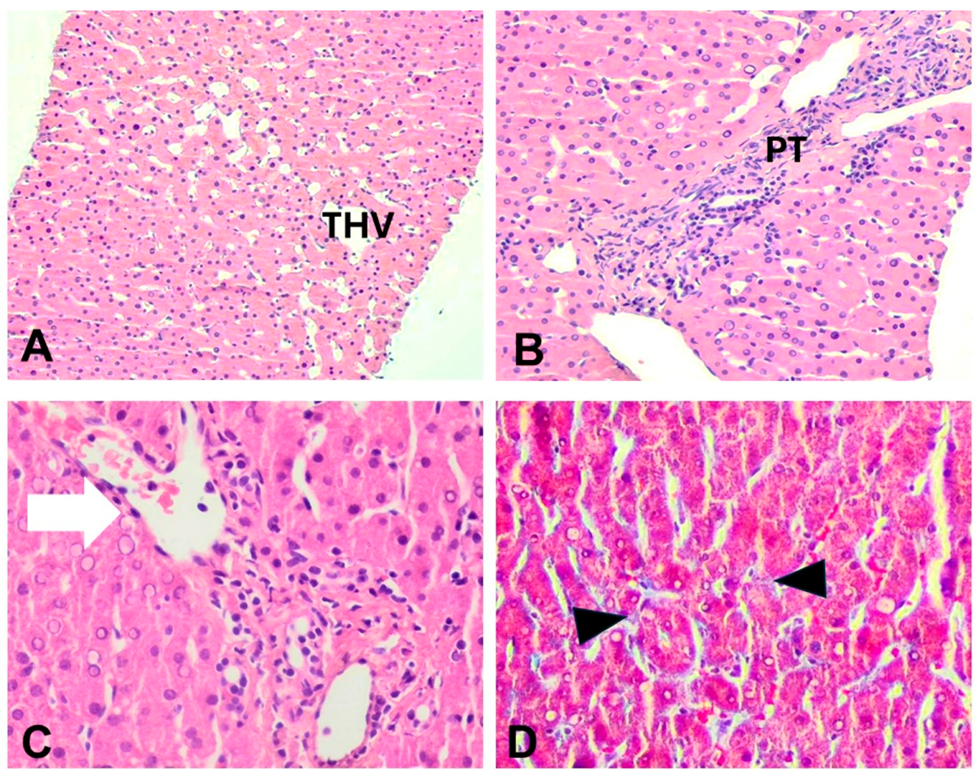
2.4. Diagnostic Assessment
3. Discussion
4. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Gravholt, C.H.; Andersen, N.H.; Conway, G.S.; Dekkers, O.M.; Geffner, M.E.; Klein, K.O.; Lin, A.E.; Mauras, N.; Quigley, C.A.; Rubin, K.; et al. Clinical Practice Guidelines for the Care of Girls and Women with Turner Syndrome: Proceedings from the 2016 Cincinnati International Turner Syndrome Meeting. Eur. J. Endocrinol. 2017, 177, G1–G70. [Google Scholar] [CrossRef] [PubMed]
- Gravholt, C.H.; Viuff, M.; Just, J.; Sandahl, K.; Brun, S.; van der Velden, J.; Andersen, N.H.; Skakkebaek, A. The Changing Face of Turner Syndrome. Endocr. Rev. 2023, 44, 33–69. [Google Scholar] [CrossRef]
- Gravholt, C.H.; Viuff, M.H.; Brun, S.; Stochholm, K.; Andersen, N.H. Turner Syndrome: Mechanisms and Management. Nat. Rev. Endocrinol. 2019, 15, 601–614. [Google Scholar] [CrossRef]
- Cameron-Pimblett, A.; La Rosa, C.; King, T.F.J.; Davies, M.C.; Conway, G.S. The Turner Syndrome Life Course Project: Karyotype-phenotype Analyses across the Lifespan. Clin. Endocrinol. 2017, 87, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Calanchini, M.; Moolla, A.; Tomlinson, J.W.; Cobbold, J.F.; Grossman, A.; Fabbri, A.; Turner, H.E. Liver Biochemical Abnormalities in Turner Syndrome: A Comprehensive Characterization of an Adult Population. Clin. Endocrinol. 2018, 89, 667–676. [Google Scholar] [CrossRef] [PubMed]
- El-Mansoury, M.; Berntorp, K.; Bryman, I.; Hanson, C.; Innala, E.; Karlsson, A.; Landin-Wilhelmsen, K. Elevated Liver Enzymes in Turner Syndrome during a 5-year Follow-up Study. Clin. Endocrinol. 2008, 68, 485–490. [Google Scholar] [CrossRef]
- Bourcigaux, N.; Dubost, E.; Buzzi, J.-C.; Donadille, B.; Corpechot, C.; Poujol-Robert, A.; Christin-Maitre, S. Focus on Liver Function Abnormalities in Patients with Turner Syndrome: Risk Factors and Evaluation of Fibrosis Risk. J. Clin. Endocrinol. Metab. 2023, 108, 2255–2261. [Google Scholar] [CrossRef]
- Viuff, M.H.; Stochholm, K.; Grønbæk, H.; Berglund, A.; Juul, S.; Gravholt, C.H. Increased Occurrence of Liver and Gastrointestinal Diseases and Anaemia in Women with Turner Syndrome—A Nationwide Cohort Study. Aliment. Pharmacol. Ther. 2021, 53, 821–829. [Google Scholar] [CrossRef]
- Singh, I.; Noel, G.; Barker, J.M.; Chatfield, K.C.; Furniss, A.; Khanna, A.D.; Nokoff, N.J.; Patel, S.; Pyle, L.; Nahata, L.; et al. Hepatic Abnormalities in Youth with Turner Syndrome. Liver Int. 2022, 42, 2237–2246. [Google Scholar] [CrossRef]
- Koulouri, O.; Ostberg, J.; Conway, G.S. Liver Dysfunction in Turner’s Syndrome: Prevalence, Natural History and Effect of Exogenous Oestrogen. Clin. Endocrinol. 2008, 69, 306–310. [Google Scholar] [CrossRef]
- Gravholt, C.H.; Juul, S.; Naeraa, R.W.; Hansen, J. Morbidity in Turner Syndrome. J. Clin. Epidemiol. 1998, 51, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, M.M.; Attenhofer Jost, C.; Babovic-Vuksanovic, D.; Connolly, H.M.; Egbe, A. Long-Term Outcomes in Patients with Turner Syndrome: A 68-Year Follow-Up. J. Am. Heart Assoc. 2019, 8, e011501. [Google Scholar] [CrossRef]
- Fedor, I.; Zold, E.; Barta, Z. Liver Abnormalities in Turner Syndrome: The Importance of Estrogen Replacement. J. Endocr. Soc. 2022, 6, e011501. [Google Scholar] [CrossRef]
- De Gottardi, A.; Sempoux, C.; Berzigotti, A. Porto-Sinusoidal Vascular Disorder. J. Hepatol. 2022, 77, 1124–1135. [Google Scholar] [CrossRef] [PubMed]
- De Gottardi, A.; Rautou, P.-E.; Schouten, J.; Rubbia-Brandt, L.; Leebeek, F.; Trebicka, J.; Murad, S.D.; Vilgrain, V.; Hernandez-Gea, V.; Nery, F.; et al. Porto-Sinusoidal Vascular Disease: Proposal and Description of a Novel Entity. Lancet Gastroenterol. Hepatol. 2019, 4, 399–411. [Google Scholar] [CrossRef]
- Roulot, D.; Degott, C.; Chazouillères, O.; Oberti, F.; Calès, P.; Carbonell, N.; Benferhat, S.; Bresson-Hadni, S.; Valla, D. Vascular Involvement of the Liver in Turner’s Syndrome. Hepatology 2004, 39, 239–247. [Google Scholar] [CrossRef]
- Gioia, S.; Baiocchini, A.; d’Amati, G.; Tavano, D.; Ridola, L.; Nardelli, S.; de Felice, I.; Lapenna, L.; Merli, M.; Pellicelli, A.; et al. Porto-Sinusoidal Vascular Disorder (PSVD): Application of New Diagnostic Criteria in a Multicenter Cohort of Patients. Dig. Liver Dis. 2024, 56, 291–296. [Google Scholar] [CrossRef]
- Guido, M.; Alves, V.A.F.; Balabaud, C.; Bathal, P.S.; Bioulac-Sage, P.; Colombari, R.; Crawford, J.M.; Dhillon, A.P.; Ferrell, L.D.; Gill, R.M.; et al. Histology of Portal Vascular Changes Associated with Idiopathic Non-cirrhotic Portal Hypertension: Nomenclature and Definition. Histopathology 2019, 74, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Ciriaci, N.; Bertin, L.; Rautou, P.-E. Genetic Predisposition to Porto-Sinusoidal Vascular Disorder. Hepatology 2024. [Google Scholar] [CrossRef]
- Jørgensen, K.T.; Rostgaard, K.; Bache, I.; Biggar, R.J.; Nielsen, N.M.; Tommerup, N.; Frisch, M. Autoimmune Diseases in Women with Turner’s Syndrome. Arthritis Rheum. 2010, 62, 658–666. [Google Scholar] [CrossRef]
- Lleo, A.; Moroni, L.; Caliari, L.; Invernizzi, P. Autoimmunity and Turner’s Syndrome. Autoimmun. Rev. 2012, 11, A538–A543. [Google Scholar] [CrossRef] [PubMed]
- Gatti, S.; Gelzoni, G.; Catassi, G.N.; Catassi, C. The Clinical Spectrum of Inflammatory Bowel Disease Associated with Specific Genetic Syndromes: Two Novel Pediatric Cases and a Systematic Review. Front. Pediatr. 2021, 9, 742830. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, N.; Tokushige, K.; Haruta, I.; Yamauchi, K.; Hayashi, N. Analysis of Adhesion Molecules in Patients with Idiopathic Portal Hypertension. J. Gastroenterol. Hepatol. 1999, 14, 364–369. [Google Scholar] [CrossRef]
- Kotani, K.; Kawabe, J.; Morikawa, H.; Akahoshi, T.; Hashizume, M.; Shiomi, S. Comprehensive Screening of Gene Function and Networks by DNA Microarray Analysis in Japanese Patients with Idiopathic Portal Hypertension. Mediators Inflamm. 2015, 2015, 349215. [Google Scholar] [CrossRef]
- Roulot, D. Liver Involvement in Turner Syndrome. Liver Int. 2013, 33, 24–30. [Google Scholar] [CrossRef]
- Gravholt, C.H. Turner Syndrome and the Heart. Am. J. Cardiovasc. Drugs 2002, 2, 401–413. [Google Scholar] [CrossRef]
- Kopacek Zilz, C.; Keller Brenner, J.; Elnecave, R.H. Portal Vein Thrombosis and High Factor VIII in Turner Syndrome. Horm. Res. Paediatr. 2006, 66, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Jobe, S.; Donohoue, P.; Di Paola, J. Deep Venous Thrombosis and Turner Syndrome. J. Pediatr. Hematol. Oncol. 2004, 26, 272. [Google Scholar] [CrossRef]
- Pinto, R.B.; Silveira, T.R.; Bandinelli, E.; Röhsig, L. Portal Vein Thrombosis in Children and Adolescents: The Low Prevalence of Hereditary Thrombophilic Disorders. J. Pediatr. Surg. 2004, 39, 1356–1361. [Google Scholar] [CrossRef]
- Hutchison, A.L.; Tavaglione, F.; Romeo, S.; Charlton, M. Endocrine Aspects of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): Beyond Insulin Resistance. J. Hepatol. 2023, 79, 1524–1541. [Google Scholar] [CrossRef]
- Singeap, A.-M.; Stanciu, C.; Huiban, L.; Muzica, C.M.; Cuciureanu, T.; Girleanu, I.; Chiriac, S.; Zenovia, S.; Nastasa, R.; Sfarti, C.; et al. Association between Nonalcoholic Fatty Liver Disease and Endocrinopathies: Clinical Implications. Can. J. Gastroenterol. Hepatol. 2021, 1, 6678142. [Google Scholar] [CrossRef] [PubMed]
- Piantanida, E.; Ippolito, S.; Gallo, D.; Masiello, E.; Premoli, P.; Cusini, C.; Rosetti, S.; Sabatino, J.; Segato, S.; Trimarchi, F.; et al. The Interplay between Thyroid and Liver: Implications for Clinical Practice. J. Endocrinol. Investig. 2020, 43, 885–899. [Google Scholar] [CrossRef] [PubMed]
- Nicoară-Farcău, O.; Rusu, I.; Stefănescu, H.; Tanțău, M.; Badea, R.I.; Procopeț, B. Diagnostic Challenges in Non-Cirrhotic Portal Hypertension—Porto Sinusoidal Vascular Disease. World. J. Gastroenterol. 2020, 26, 3000–3011. [Google Scholar] [CrossRef] [PubMed]
- Lampichler, K.; Semmler, G.; Wöran, K.; Simbrunner, B.; Jachs, M.; Hartl, L.; Bauer, D.J.M.; Balcar, L.; Burghart, L.; Trauner, M.; et al. Imaging Features Facilitate Diagnosis of Porto-Sinusoidal Vascular Disorder. Eur. Radiol. 2022, 33, 1422–1432. [Google Scholar] [CrossRef]
- Giri, S.; Singh, A.; Roy, A.; Patel, R.K.; Tripathy, T.; Angadi, S. Noninvasive Differentiation of Porto-Sinusoidal Vascular Disorder from Cirrhosis: A Systematic Review. Abdom. Radiol. 2023, 48, 2340–2348. [Google Scholar] [CrossRef]
- Korkmaz, Ö.; Savaş, R.; Levent, E.; Özen, S.; Mecidov, İ.; Gökşen, D.; Darcan, Ş. The Role of Cardiac Magnetic Resonance Imaging in the Determination of Cardiovascular Anomalies in Children and Young Adults with Turner Syndrome. J. Pediatr. Res. 2019, 6, 203–207. [Google Scholar] [CrossRef]
- Obara-Moszynska, M.; Rajewska-Tabor, J.; Rozmiarek, S.; Karmelita-Katulska, K.; Kociemba, A.; Rabska-Pietrzak, B.; Janus, M.; Siniawski, A.; Mrozinski, B.; Graczyk-Szuster, A.; et al. The Usefulness of Magnetic Resonance Imaging of the Cardiovascular System in the Diagnostic Work-Up of Patients with Turner Syndrome. Front. Endocrinol. 2018, 9, 609. [Google Scholar] [CrossRef]
- Dakua, S.; Sahambi, J. Detection of Left Ventricular Myocardial Contours from Ischemic Cardiac MR Images. IETE J. Res. 2011, 57, 372. [Google Scholar] [CrossRef]
- Burt, A.D.; Gouw, A.S.H.; Callea, F.; Clouston, A.D.; Dienes, H.; Goodman, Z.D.; Kakar, S.; Kleiner, D.E.; Lackner, C.; Park, Y.N.; et al. Making Sense of ‘Porto-Sinusoidal Vascular Disorder’: What Does It Mean for the Pathologist and the Patient? Liver Int. 2025, 45, e16196. [Google Scholar] [CrossRef]
- Wöran, K.; Semmler, G.; Jachs, M.; Simbrunner, B.; Bauer, D.J.M.; Binter, T.; Pomej, K.; Stättermayer, A.F.; Schwabl, P.; Bucsics, T.; et al. Clinical Course of Porto-Sinusoidal Vascular Disease Is Distinct from Idiopathic Noncirrhotic Portal Hypertension. Clin. Gastroenterol. Hepatol. 2022, 20, e251–e266. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiong, Q.; Zhong, Y.; Liu, D.; Liu, H.; Wang, L.; Du, Z.; Chen, M.; Zheng, Y.; Yang, Y. Clinical Characteristics and Natural History of Porto-sinusoidal Vascular Disease: A Cohort Study of 234 Patients in China. Liver Int. 2024, 44, 2329–2340. [Google Scholar] [CrossRef] [PubMed]
- Cazals-Hatem, D.; Hillaire, S.; Rudler, M.; Plessier, A.; Paradis, V.; Condat, B.; Francoz, C.; Denninger, M.-H.; Durand, F.; Bedossa, P.; et al. Obliterative Portal Venopathy: Portal Hypertension Is Not Always Present at Diagnosis. J. Hepatol. 2011, 54, 455–461. [Google Scholar] [CrossRef]
- Siramolpiwat, S.; Seijo, S.; Miquel, R.; Berzigotti, A.; Garcia-Criado, A.; Darnell, A.; Turon, F.; Hernandez-Gea, V.; Bosch, J.; Garcia-Pagán, J.C. Idiopathic Portal Hypertension: Natural History and Long-Term Outcome. Hepatology 2014, 59, 2276–2285. [Google Scholar] [CrossRef]
- Hollande, C.; Mallet, V.; Darbeda, S.; Vallet-Pichard, A.; Fontaine, H.; Verkarre, V.; Sogni, P.; Terris, B.; Gouya, H.; Pol, S. Impact of Obliterative Portal Venopathy Associated with Human Immunodeficiency Virus. Medicine 2016, 95, e3081. [Google Scholar] [CrossRef]
- Schouten, J.N.L.; Van der Ende, M.E.; Koëter, T.; Rossing, H.H.M.; Komuta, M.; Verheij, J.; van der Valk, M.; Hansen, B.E.; Janssen, H.L.A. Risk Factors and Outcome of HIV-associated Idiopathic Noncirrhotic Portal Hypertension. Aliment. Pharmacol. Ther. 2012, 36, 875–885. [Google Scholar] [CrossRef] [PubMed]
- Guido, M.; Sarcognato, S.; Sonzogni, A.; Lucà, M.G.; Senzolo, M.; Fagiuoli, S.; Ferrarese, A.; Pizzi, M.; Giacomelli, L.; Colloredo, G. Obliterative Portal Venopathy without Portal Hypertension: An Underestimated Condition. Liver Int. 2016, 36, 454–460. [Google Scholar] [CrossRef]
- Kawabata, S.; Sakamoto, S.; Honda, M.; Hayashida, S.; Yamamoto, H.; Mikami, Y.; Inomata, Y. Liver Transplantation for a Patient with Turner Syndrome Presenting Severe Portal Hypertension: A Case Report and Literature Review. Surg. Case Rep. 2016, 2, 68. [Google Scholar] [CrossRef]
- Elsheikh, M.; Hodgson, H.J.F.; Wass, J.A.H.; Conway, G.S. Hormone Replacement Therapy May Improve Hepatic Function in Women with Turner’s Syndrome. Clin. Endocrinol. 2001, 55, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Wójcik, M.; Ruszała, A.; Januś, D.; Starzyk, J.B. Liver Biochemical Abnormalities in Adolescent Patients with Turner Syndrome. J. Clin. Res. Pediatr. Endocrinol. 2019, 11, 395–399. [Google Scholar] [CrossRef]
- Santi, M.; Flück, C.E.; Hauschild, M.; Kuhlmann, B.; Kuehni, C.E.; Sommer, G. Health Behaviour of Women with Turner Syndrome. Acta Paediatr. 2021, 110, 2424–2429. [Google Scholar] [CrossRef]

| Case 1 | Case 2 | Case 3 | |
|---|---|---|---|
| Age (years) | 22 | 30 | 23 |
| Labor (weeks) | 38 | 41 | 40 |
| Menarche (years) | 12 | 17 | 12 (induction) |
| Puberty (Tanner stages) | AH = III, PH = V, B = V | AH = II, PH = IV, B = III | AH = III, PH = V, B = V |
| Body height (cm) | 145 | 153 | 150 |
| Medical history | astigmatism hypermetropia strabismus iron deficiency anemia | astigmatism hypermetropia strabismus osteopenia dyslipidemia | constriction of aorta chronic autoimmune thyroiditis gonadal dysgenesis osteopenia insulin resistance hearing loss |
| Karyotype test | 45,XO | 45,XO/46,XX (fragment 80%/20%) | 45,XO |
| Phenotype characteristics | short stature, broad short neck, low hairline, deformity of external ears, broad chest | short stature, short, webbed neck, low hairline, low-set ears | short stature, short, webbed neck, low hairline, ear deformity, shield-shaped thorax, widely spaced nipples |
| Ht (%) | 38 | 38.6 | 39.4 |
| Hb (g/dL) | 11.5 | 12.8 | 13.7 |
| WBC (cells/μL) | 7620 | 6930 | 4630 |
| PLTs (cells/μL) | 528,000 | 190,000 | 189,000 |
| AST (5–32U/L) | 86 | 34 | 53 |
| ALT (5–31 U/L) | 106 | 37 | 130 |
| γ-GT (5–36 U/L) | 98 | 58 | 87 |
| ALP (35–120 U/L) | 125 | 149 | 71 |
| Total bilirubin (mg/dL) | 0.8 | 0.5 | 0.4 |
| Ferritin (ng/mL) | 13 | 120 | 32 |
| Glucose (mg/dL) | 81 | 92 | 93 |
| HBsAg | (−) | (−) | (−) |
| Anti-HBc | (−) | (−) | (−) |
| Anti-HBs | (+) | (+) | (+) |
| Anti-HCV | (−) | (−) | (−) |
| ANA | (−) | (−) | (−) |
| AMA | (−) | (−) | (−) |
| ASMA | (−) | (−) | (−) |
| IgG | N | N | N |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siasiakou, S.M.; Stoupi, E.; Roumpou, A.; Papanikolopoulou, A.; Syrigos, N.; Tiniakos, D.; Peppa, M. Porto-Sinusoidal Vascular Disorder: An Under-Recognized Liver Manifestation in Turner Syndrome. J. Clin. Med. 2025, 14, 3979. https://doi.org/10.3390/jcm14113979
Siasiakou SM, Stoupi E, Roumpou A, Papanikolopoulou A, Syrigos N, Tiniakos D, Peppa M. Porto-Sinusoidal Vascular Disorder: An Under-Recognized Liver Manifestation in Turner Syndrome. Journal of Clinical Medicine. 2025; 14(11):3979. https://doi.org/10.3390/jcm14113979
Chicago/Turabian StyleSiasiakou, Sofia M., Eleni Stoupi, Afroditi Roumpou, Amalia Papanikolopoulou, Nikolaos Syrigos, Dina Tiniakos, and Melpomeni Peppa. 2025. "Porto-Sinusoidal Vascular Disorder: An Under-Recognized Liver Manifestation in Turner Syndrome" Journal of Clinical Medicine 14, no. 11: 3979. https://doi.org/10.3390/jcm14113979
APA StyleSiasiakou, S. M., Stoupi, E., Roumpou, A., Papanikolopoulou, A., Syrigos, N., Tiniakos, D., & Peppa, M. (2025). Porto-Sinusoidal Vascular Disorder: An Under-Recognized Liver Manifestation in Turner Syndrome. Journal of Clinical Medicine, 14(11), 3979. https://doi.org/10.3390/jcm14113979






