Novel Putative Effectors Identified in the Arrhythmogenesis of Idiopathic Outflow Tract Ventricular Arrhythmias: A Novel Concept Beyond Triggered Activity
Abstract
1. Introduction
2. Methods
3. Results
4. Discussion
4.1. Clinical Relevance of OT-VA
4.2. Arrhythmogenic Substrate for OT-VA: Current Concepts
4.3. Conduction Tissue Remnants: Could They Have a Role in Arrhythmogenesis?
4.4. PVC Ablation from the Atrium?
4.5. Atrial Pacing-Induced OT-PVCs: Implications on Novel Mechanistic Insights into Arrhythmogenesis
4.6. The Limitations of the Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AVNRT | atrioventricular nodal reentrant tachycardia |
| CS | coronary sinus |
| cAMP | cyclic adenosine monophosphate |
| DAD | delayed afterdepolarization |
| IVA | idiopathic ventricular arrhythmias |
| ECG | Electrocardiogram |
| L/RVOT | left/right ventricular outflow tract |
| OT | outflow tract |
| LV | left ventricle |
| LBBB | left bundle branch block |
| RBBB | right bundle branch block |
| BBB | bundle branch block |
| PVC | premature ventricular contraction |
| EP | electrophysiology |
| AP | accessory pathway |
| VA/T | ventricular arrhythmia/tachycardia |
References
- Lerman, B.B. Mechanism of outflow tract tachycardia. Heart Rhythm. 2007, 4, 973–976. [Google Scholar] [CrossRef] [PubMed]
- Priori, S.G.; Blomstrom-Lundqvist, C.; Mazzanti, A.; Blom, N.; Borggrefe, M.; Camm, J.; Elliott, P.M.; Fitzsimons, D.; Hatala, R.; Hindricks, G.; et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur. Heart J. 2015, 36, 2793–2867. [Google Scholar] [PubMed]
- Al-Khatib, S.M.; Stevenson, W.G.; Ackerman, M.J.; Bryant, W.J.; Callans, D.J.; Curtis, A.B.; Deal, B.J.; Dickfeld, T.; Field, M.E.; Fonarow, G.C.; et al. 2017 AHA/ACC/HRS Guideline for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation 2018, 72, e91–e220. [Google Scholar]
- Pedersen, C.T.; Kay, G.N.; Kalman, J.; Borggrefe, M.; Della-Bella, P.; Dickfeld, T.; Dorian, P.; Huikuri, H.; Kim, Y.H.; Knight, B.; et al. EHRA/HRS/APHRS expert consensus on ventricular arrhythmias. Europace 2014, 16, 1257–1283. [Google Scholar] [CrossRef]
- Lerman, B.B. Outflow tract ventricular arrhythmias: An update. Trends Cardiovasc. Med. 2015, 25, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Tanawuttiwat, T.; Nazarian, S.; Calkins, H. The role of catheter ablation in the management of ventricular tachycardia. Eur. Heart J. 2016, 37, 594–609. [Google Scholar] [CrossRef]
- Hasdemir, C.; Alp, A.; Simsek, E.; Kose, N.; Aydin, M.; Payzin, S. Spontaneous atrioventricular nodal reentrant tachycardia in patients with idiopathic ventricular arrhythmias: The incidence, clinical, and electrophysiologic characteristics. J. Cardiovasc. Electrophysiol. 2013, 24, 1370–1374. [Google Scholar] [CrossRef]
- Kautzner, J.; Cihak, R.; Vancura, V.; Bytesnik, J. Coincidence of idiopathic ventricular outflow tract tachycardia and atrioventricular nodal reentrant tachycardia. Europace 2003, 5, 215–220. [Google Scholar] [CrossRef]
- Chen, J.; Hoff, P.I.; Rossvoll, O.; De Bortoli, A.; Solheim, E.; Sun, L.Z.; Schuster, P.; Larsen, T.; Ohm, O.J. Ventricular arrhythmias originating from the aortomitral continuity: An uncommon variant of left ventricular outflow tract tachycardia. Europace 2012, 14, 388–395. [Google Scholar] [CrossRef]
- Shirai, Y.; Goya, M.; Isobe, M.; Hirao, K. Preferential Pathway Pacing within the Aortic Sinus of Valsalva: Strong Evidence for the Existence of Preferential Conduction with Different Exit Sites Traversing the Ventricular Septum. J. Cardiovasc. Electrophysiol. 2015, 26, 805–808. [Google Scholar] [CrossRef]
- Yamada, T.; Murakami, Y.; Yoshida, N.; Okada, T.; Shimizu, T.; Toyama, J.; Yoshida, Y.; Tsuboi, N.; Muto, M.; Inden, Y.; et al. Preferential conduction across the ventricular outflow septum in ventricular arrhythmias originating from the aortic sinus cusp. J. Am. Coll. Cardiol. 2007, 50, 884–891. [Google Scholar] [CrossRef] [PubMed]
- Hai, J.J.; Chahal, A.A.; Friedman, P.A.; Vaidya, V.R.; Syed, F.F.; DeSimone, C.V.; Nanda, S.; Brady, P.A.; Madhavan, M.; Cha, Y.M.; et al. Electrophysiologic characteristics of ventricular arrhythmias arising from the aortic mitral continuity-potential role of the conduction system. J. Cardiovasc. Electrophysiol. 2015, 26, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Hachiya, H.; Yamauchi, Y.; Iesaka, Y.; Yagishita, A.; Sasaki, T.; Higuchi, K.; Kawabata, M.; Sugiyama, K.; Tanaka, Y.; Kusa, S.; et al. Discrete prepotential as an indicator of successful ablation in patients with coronary cusp ventricular arrhythmia. Circ. Arrhythm. Electrophysiol. 2013, 6, 898–904. [Google Scholar] [CrossRef]
- Szili Torok, T.; LJ, D.E.V.; Ozcan, E.E.; Hasdemir, C.; Kis, Z.; Kardos, A.; Geczy, T.; Kovacs, I.; Benedek, I.; Oosterwerff, E.; et al. Disappearance of Idiopathic Outflow Tract Premature Ventricular Contractions After Catheter Ablation of Overt Accessory Pathways. J. Cardiovasc. Electrophysiol. 2017, 28, 78–84. [Google Scholar] [CrossRef]
- Latchamsetty, R.; Bogun, F. Premature Ventricular Complexes and Premature Ventricular Complex Induced Cardiomyopathy. Curr. Probl. Cardiol. 2015, 40, 379–422. [Google Scholar] [CrossRef]
- Brooks, R.; Burgess, J.H. Idiopathic ventricular tachycardia. A review. Medicine 1988, 67, 271–294. [Google Scholar] [CrossRef]
- Joshi, S.; Wilber, D.J. Ablation of idiopathic right ventricular outflow tract tachycardia: Current perspectives. J. Cardiovasc. Electrophysiol. 2005, 16 (Suppl. S1), S52–S58. [Google Scholar] [CrossRef]
- Tada, H.; Ito, S.; Naito, S.; Kurosaki, K.; Kubota, S.; Sugiyasu, A.; Tsuchiya, T.; Miyaji, K.; Yamada, M.; Kutsumi, Y.; et al. Idiopathic ventricular arrhythmia arising from the mitral annulus: A distinct subgroup of idiopathic ventricular arrhythmias. J. Am. Coll. Cardiol. 2005, 45, 877–886. [Google Scholar] [CrossRef]
- Tada, H.; Tadokoro, K.; Ito, S.; Naito, S.; Hashimoto, T.; Kaseno, K.; Miyaji, K.; Sugiyasu, A.; Tsuchiya, T.; Kutsumi, Y.; et al. Idiopathic ventricular arrhythmias originating from the tricuspid annulus: Prevalence, electrocardiographic characteristics, and results of radiofrequency catheter ablation. Heart Rhythm. 2007, 4, 7–16. [Google Scholar]
- Valk, S.D.; de Groot, N.M.; Szili-Torok, T.; Van Belle, Y.L.; Res, J.C.; Jordaens, L. Clinical characteristics and acute results of catheter ablation for outflow tract ventricular tachycardia or premature beats. J. Interv. Card. Electrophysiol. 2012, 35, 301–309; discussion 309. [Google Scholar] [CrossRef]
- Tandri, H.; Macedo, R.; Calkins, H.; Marcus, F.; Cannom, D.; Scheinman, M.; Daubert, J.; Estes, M.; Wilber, D.; Talajic, M.; et al. Role of magnetic resonance imaging in arrhythmogenic right ventricular dysplasia: Insights from the North American arrhythmogenic right ventricular dysplasia (ARVD/C) study. Am. Heart J. 2008, 155, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Markowitz, S.M.; Weinsaft, J.W.; Waldman, L.; Petashnick, M.; Liu, C.F.; Cheung, J.W.; Thomas, G.; Ip, J.E.; Lerman, B.B. Reappraisal of Cardiac Magnetic Resonance Imaging in Idiopathic Outflow Tract Arrhythmias. J. Cardiovasc. Electrophysiol. 2014, 25, 1328–1335. [Google Scholar] [CrossRef] [PubMed]
- Boukens, B.J.; Christoffels, V.M.; Coronel, R.; Moorman, A.F. Developmental basis for electrophysiological heterogeneity in the ventricular and outflow tract myocardium as a substrate for life-threatening ventricular arrhythmias. Circ. Res. 2009, 104, 19–31. [Google Scholar] [CrossRef]
- Santangeli, P.; Hutchinson, M.D.; Supple, G.E.; Callans, D.J.; Marchlinski, F.E.; Garcia, F.C. Right Atrial Approach for Ablation of Ventricular Arrhythmias Arising From the Left Posterior-Superior Process of the Left Ventricle. Circ. Arrhythm. Electrophysiol. 2016, 9, e004048. [Google Scholar] [CrossRef]
- Moorman, A.F.; de Jong, F.; Lamers, W.H. Development of the conduction system of the heart. Pacing Clin. Electrophysiol. 1997, 20, 2087–2092. [Google Scholar] [CrossRef] [PubMed]
- McGuire, M.A.; de Bakker, J.M.; Vermeulen, J.T.; Moorman, A.F.; Loh, P.; Thibault, B.; Vermeulen, J.L.; Becker, A.E.; Janse, M.J. Atrioventricular junctional tissue. Discrepancy between histological and electrophysiological characteristics. Circulation 1996, 94, 571–577. [Google Scholar] [CrossRef]
- Boukens, B.J.; Coronel, R.; Christoffels, V.M. Embryonic development of the right ventricular outflow tract and arrhythmias. Heart Rhythm. 2016, 13, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.-Y.; Chen, Y.-C.; Lin, Y.-K.; Chen, S.-A.; Chen, Y.-J. Electrical and Structural Insights into Right Ventricular Outflow Tract Arrhythmogenesis. Int. J. Mol. Sci. 2023, 24, 11795. [Google Scholar] [CrossRef]
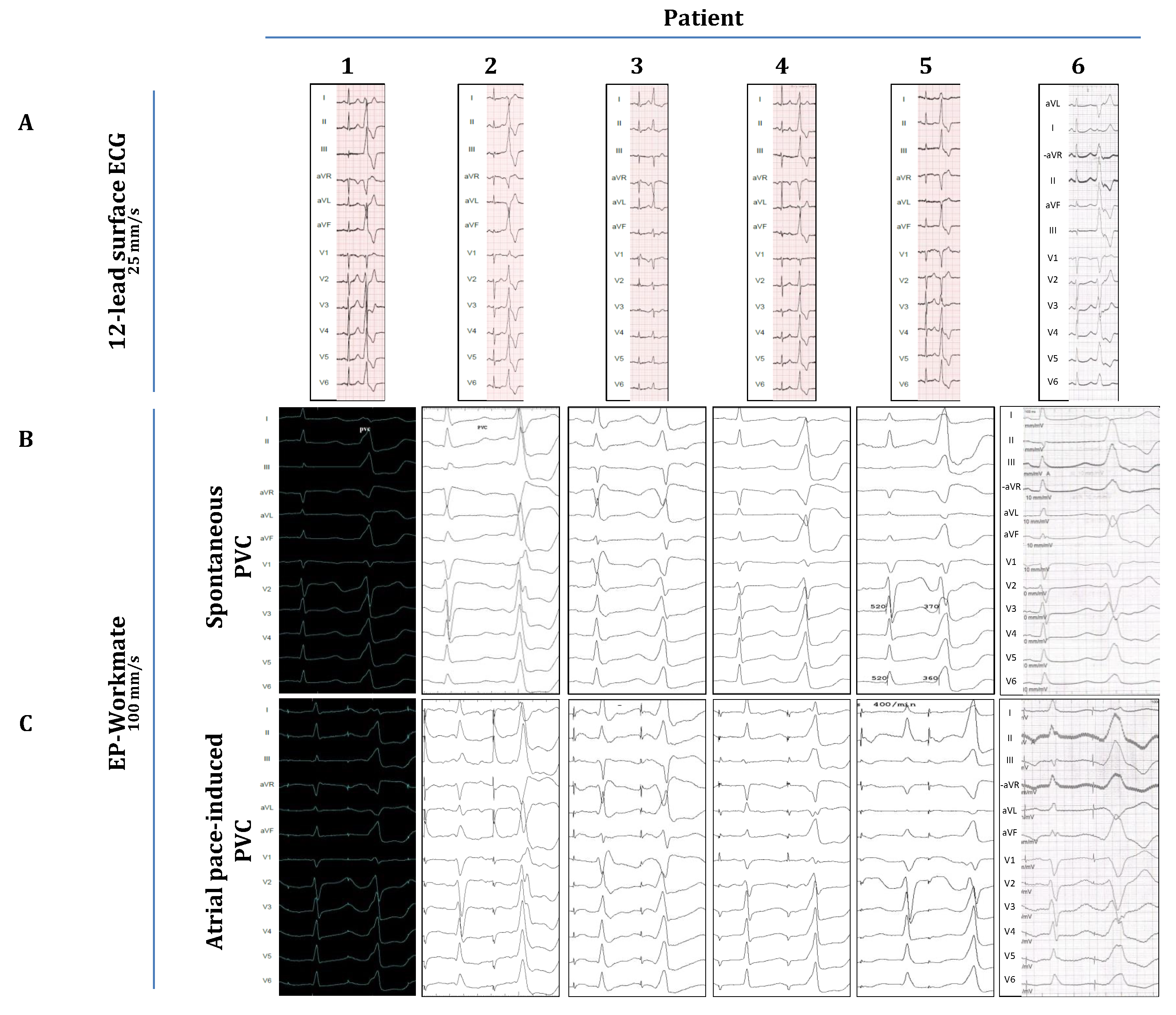

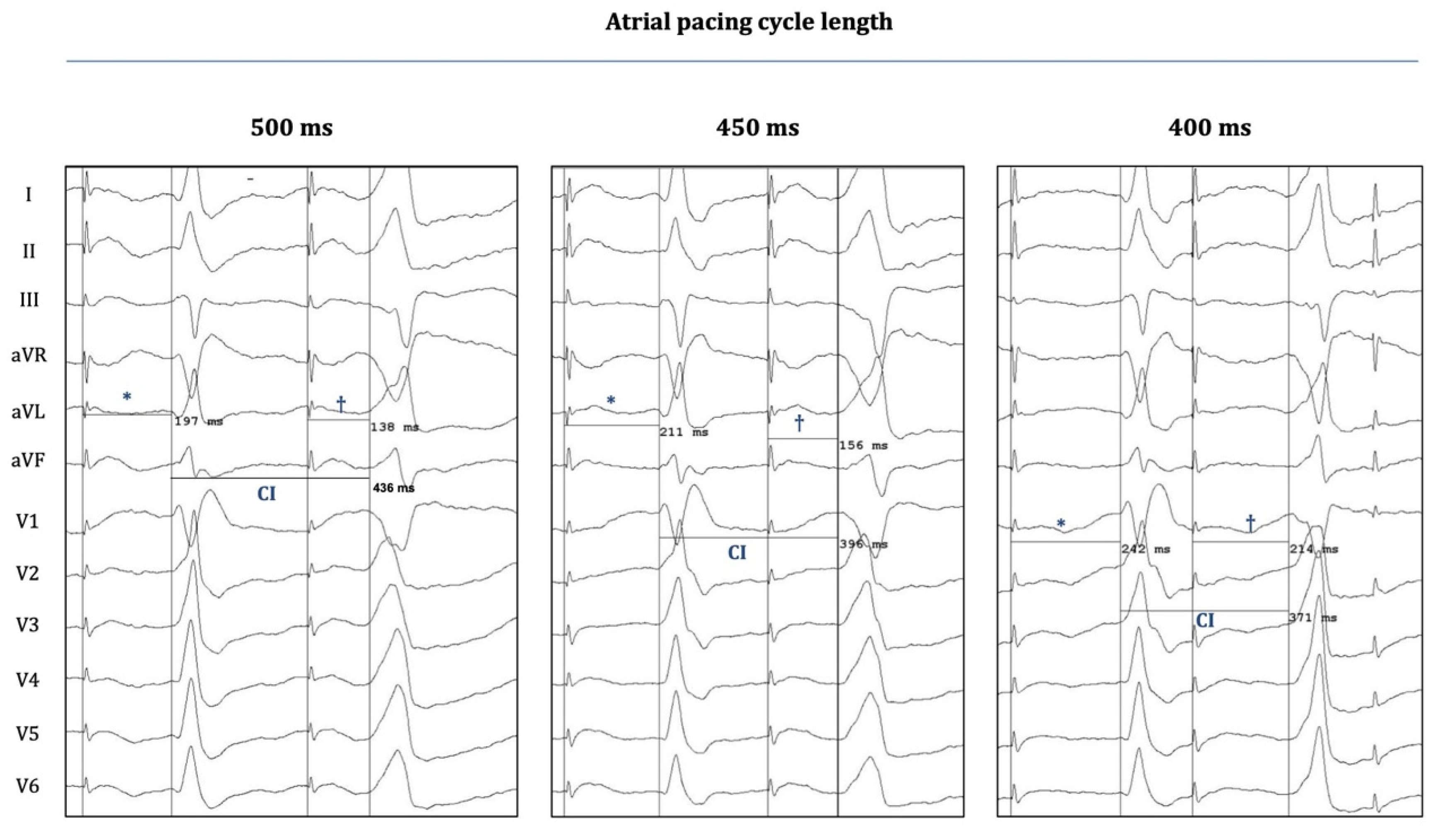
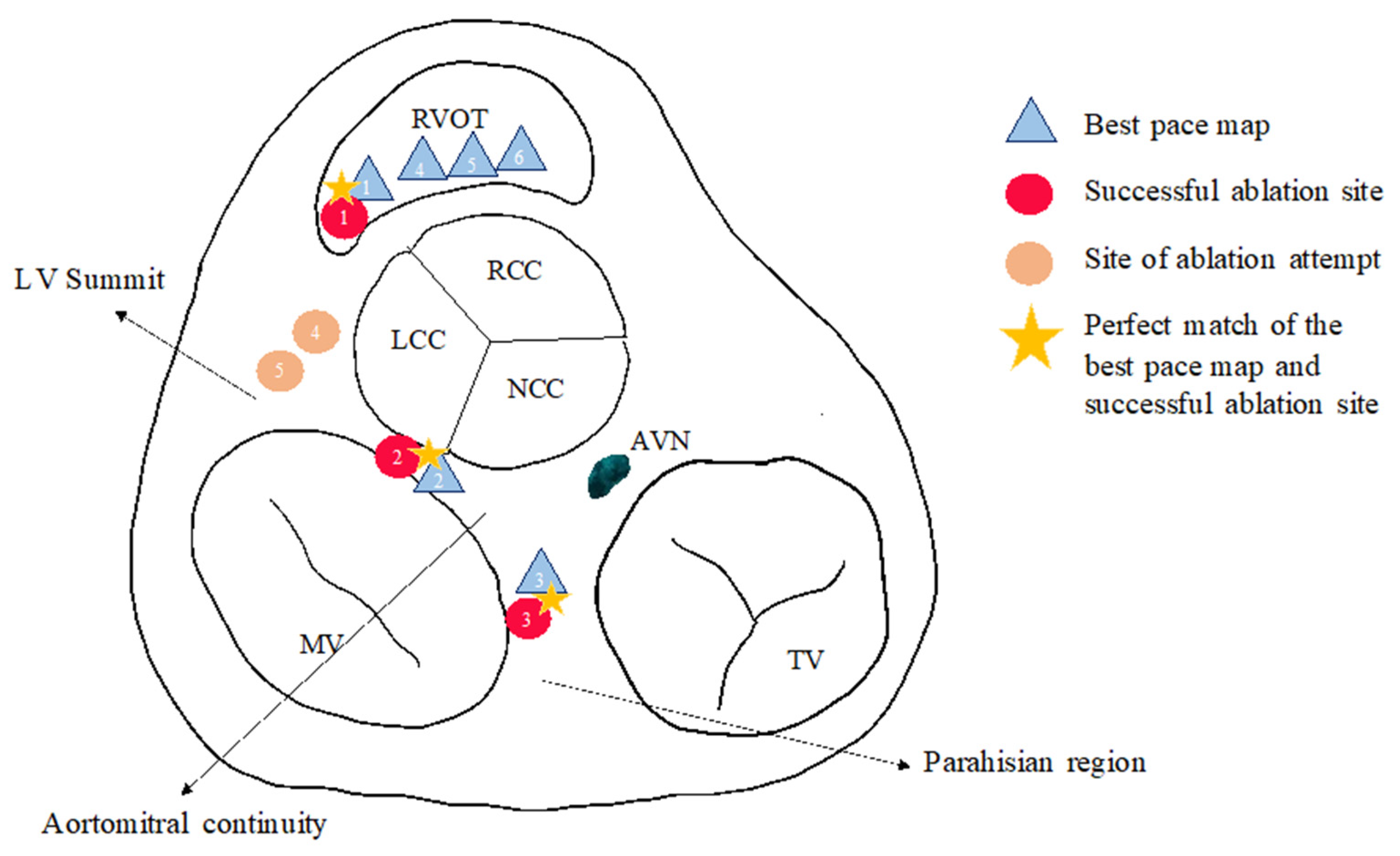
| Age | Sex (m/f) | LVEF (%) | Structural Heart Disease | Antiarrhythmic Medication | |
|---|---|---|---|---|---|
| Case 1 | 52 | m | 45 † | None * | Beta blocker |
| Case 2 | 57 | f | 45 † | None * | Beta blocker |
| Case 3 | 58 | f | 60 | None | Calcium channel blocker |
| Case 4 | 57 | f | 64 | None | Calcium channel blocker |
| Case 5 | 71 | m | 36 † | None * | Beta blocker |
| Case 6 | 43 | f | 67 | None | Beta blocker |
| PVC Burden | PVC Morphology | Possible PVC Origin | ||||
|---|---|---|---|---|---|---|
| Percentage | >10.000 /24 h | BBB Pattern | Horizontal Axis | Precordial Transition Zone | ||
| Case 1 | 17% | yes | LBBB | Left inferior | V2–V3 | outflow tract |
| Case 2 | 12% | yes | RBBB | Right inferior | none | aortomitral continuity/superior mitral annulus |
| Case 3 | 16% | yes | LBBB | Horizontal | V3–V4 | parahisian region |
| Case 4 | 16% | yes | LBBB | Left inferior | V1–V2 | outflow tract |
| Case 5 | 19% | yes | LBBB | Left inferior | V2–V3 | outflow tract |
| Case 6 | 18% | yes | LBBB | Inferior | V2–V3 | outflow tract |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geczy, T.; Gagyi, R.B.; Nemes, A.; Szili-Torok, T. Novel Putative Effectors Identified in the Arrhythmogenesis of Idiopathic Outflow Tract Ventricular Arrhythmias: A Novel Concept Beyond Triggered Activity. J. Clin. Med. 2025, 14, 3957. https://doi.org/10.3390/jcm14113957
Geczy T, Gagyi RB, Nemes A, Szili-Torok T. Novel Putative Effectors Identified in the Arrhythmogenesis of Idiopathic Outflow Tract Ventricular Arrhythmias: A Novel Concept Beyond Triggered Activity. Journal of Clinical Medicine. 2025; 14(11):3957. https://doi.org/10.3390/jcm14113957
Chicago/Turabian StyleGeczy, Tamas, Rita B. Gagyi, Attila Nemes, and Tamas Szili-Torok. 2025. "Novel Putative Effectors Identified in the Arrhythmogenesis of Idiopathic Outflow Tract Ventricular Arrhythmias: A Novel Concept Beyond Triggered Activity" Journal of Clinical Medicine 14, no. 11: 3957. https://doi.org/10.3390/jcm14113957
APA StyleGeczy, T., Gagyi, R. B., Nemes, A., & Szili-Torok, T. (2025). Novel Putative Effectors Identified in the Arrhythmogenesis of Idiopathic Outflow Tract Ventricular Arrhythmias: A Novel Concept Beyond Triggered Activity. Journal of Clinical Medicine, 14(11), 3957. https://doi.org/10.3390/jcm14113957







