Trends and Risk Factors of Pediatric Venous Thromboembolism in Spain: A Nationwide Study from 2016 to 2023
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source and Patient Selection
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stein, P.D.; Kayali, F.; Olson, R.E. Incidence of venous thromboembolism in infants and children: Data from the National Hospital Discharge Survey. J. Pediatr. 2004, 145, 563–565. [Google Scholar] [CrossRef] [PubMed]
- Sabapathy, C.A.; Djouonang, T.N.; Kahn, S.R.; Platt, R.W.; Tagalakis, V. Incidence Trends and Mortality from Childhood Venous Thromboembolism: A Population-Based Cohort Study. J. Pediatr. 2016, 172, 175–180.e1. [Google Scholar] [CrossRef]
- van Ommen, C.H.; Heijboer, H.; Büller, H.R.; Hirasing, R.A.; Heijmans, H.S.; Peters, M. Venous thromboembolism in childhood: A prospective two-year registry in The Netherlands. J. Pediatr. 2001, 139, 676–681. [Google Scholar] [CrossRef]
- Krmpotic, K.; Ramsay, L.; McMullen, S.; Chan, A.K.C.; Plint, A.C.; Moorehead, P. Pediatric pulmonary thromboembolism: A 3-year Canadian Pediatric Surveillance Program study. J. Thromb. Haemost. 2024, 22, 1366–1371. [Google Scholar] [CrossRef] [PubMed]
- Biss, T.T.; Brandão, L.R.; Kahr, W.H.; Chan, A.K.; Williams, S. Clinical features and outcome of pulmonary embolism in children. Br. J. Haematol. 2008, 142, 808–818. [Google Scholar] [CrossRef]
- Andrew, M.; David, M.; Adams, M.; Ali, K.; Anderson, R.; Barnard, D.; Bernstein, M.; Brisson, L.; Cairney, B.; DeSai, D. Venous thromboembolic complications (VTE) in children: First analyses of the Canadian Registry of VTE. Blood 1994, 83, 1251–1257. [Google Scholar] [CrossRef]
- Raffini, L.; Huang, Y.-S.; Witmer, C.; Feudtner, C. Dramatic increase in venous thromboembolism in children’s hospitals in the United States from 2001 to 2007. Pediatrics 2009, 124, 1001–1008. [Google Scholar] [CrossRef]
- Tuckuviene, R.; Christensen, A.L.; Helgestad, J.; Johnsen, S.P.; Kristensen, S.R. Pediatric venous and arterial noncerebral thromboembolism in Denmark: A nationwide population-based study. J. Pediatr. 2011, 159, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Takemoto, C.M.; Sohi, S.; Desai, K.; Bharaj, R.; Khanna, A.; McFarland, S.; Klaus, S.; Irshad, A.; Goldenberg, N.A.; Strouse, J.J.; et al. Hospital-associated venous thromboembolism in children: Incidence and clinical characteristics. J. Pediatr. 2014, 164, 332–338. [Google Scholar] [CrossRef]
- Rajpurkar, M.; Biss, T.; Amankwah, E.K.; Martinez, D.; Williams, S.; Van Ommen, C.H.; Goldenberg, N.A. Pulmonary embolism and in situ pulmonary artery thrombosis in paediatrics. A systematic review. Thromb. Haemost. 2017, 117, 1199–1207. [Google Scholar]
- Lassandro, G.; Palmieri, V.V.; Palladino, V.; Amoruso, A.; Faienza, M.F.; Giordano, P. Venous Thromboembolism in Children: From Diagnosis to Management. Int. J. Environ. Res. Public Health 2020, 17, 4993. [Google Scholar] [CrossRef] [PubMed]
- Santoro, N.; Colombini, A.; Silvestri, D.; Grassi, M.; Giordano, P.; Parasole, R.; Barisone, E.; Caruso, R.; Conter, V.; Valsecchi, M.G.; et al. Screening for coagulopathy and identification of children with acute lymphoblastic leukemia at a higher risk of symptomatic venous thrombosis: An AIEOP experience. J. Pediatr. Hematol. Oncol. 2013, 35, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Spentzouris, G.; Scriven, R.J.; Lee, T.K.; Labropoulos, N. Pediatric venous thromboembolism in relation to adults. J. Vasc. Surg. 2012, 55, 1785–1793. [Google Scholar] [CrossRef]
- Patocka, C.; Nemeth, J. Pulmonary Embolism in Pediatrics. J. Emerg. Med. 2012, 42, 105–116. [Google Scholar] [CrossRef]
- Witmer, C.; Raffini, L. Treatment of venous thromboembolism in pediatric patients. Blood 2020, 135, 335–343. [Google Scholar] [CrossRef]
- Branchford, B.R.; Mourani, P.; Bajaj, L.; Manco-Johnson, M.; Wang, M.; Goldenberg, N.A. Risk factors for in-hospital venous thromboembolism in children: A case-control study employing diagnostic validation. Haematologica 2012, 97, 509–515. [Google Scholar] [CrossRef]
- Barba, R.; Losa, J.E.; Guijarro, C.; Zapatero, A. Reliability of minimal basic data set in the diagnosis of thromboembolic disease. Med. Clin. 2006, 127, 255–257. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, K.; Joos, C.; Jones, A.E.; Johnson, S.A.; Witt, D.M. Assessing the accuracy of ICD-10 codes for identifying acute thromboembolic events among patients receiving anticoagulation therapy. J. Thromb. Thrombolysis 2019, 48, 181–186. [Google Scholar] [CrossRef]
- Bikdeli, B.; Khairani, C.D.; Bejjani, A.; Lo, Y.-C.; Mahajan, S.; Caraballo, C.; Jimenez, J.V.; Krishnathasan, D.; Zarghami, M.; Rashedi, S.; et al. Validating International Classification of Diseases Code 10th Revision algorithms for accurate identification of pulmonary embolism. J. Thromb. Haemost. 2025, 23, 556–564. [Google Scholar] [CrossRef]
- Feinstein, J.A.; Hall, M.; Davidson, A.; Feudtner, C. Pediatric Complex Chronic Condition System Version 3. JAMA Netw. Open 2024, 7, e2420579. [Google Scholar] [CrossRef]
- INE. Instituto Nacional de Estadística. Available online: https://www.ine.es/ (accessed on 31 March 2025).
- Le Garf, S.; Nègre, V.; Anty, R.; Gual, P. Metabolic Fatty Liver Disease in Children: A Growing Public Health Problem. Biomedicines 2021, 9, 1915. [Google Scholar] [CrossRef] [PubMed]
- Whitworth, H.; Sartain, S.E.; Kumar, R.; Armstrong, K.; Ballester, L.; Betensky, M.; Cohen, C.T.; Diaz, R.; Diorio, C.; Goldenberg, N.A.; et al. Rate of thrombosis in children and adolescents hospitalized with COVID-19 or MIS-C. Blood 2021, 138, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Monagle, P.; Adams, M.; Mahoney, M.; Ali, K.; Barnard, D.; Bernstein, M.; Brisson, L.; David, M.; Desai, S.; Scully, M.-F.; et al. Outcome of pediatric thromboembolic disease: A report from the Canadian Childhood Thrombophilia Registry. Pediatr. Res. 2000, 47, 763–766. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.K.; Deveber, G.; Monagle, P.; Brooker, L.A.; Massicotte, P.M. Venous thrombosis in children. J. Thromb. Haemost. 2003, 1, 1443–1455. [Google Scholar] [CrossRef]
- Barba, R. Administrative data and clinical care. Med. Clin. 2021, 156, 447–448. [Google Scholar] [CrossRef]
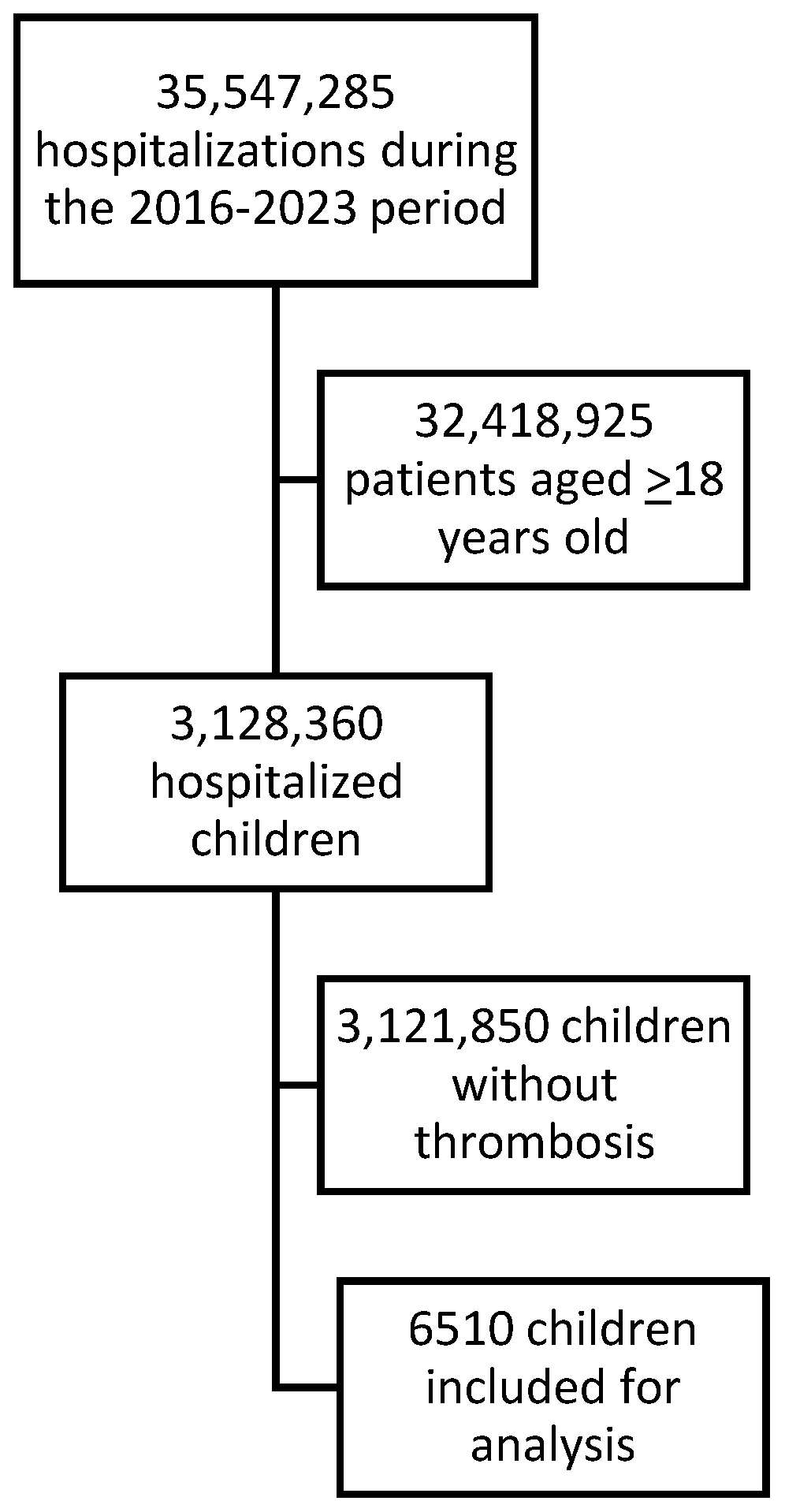
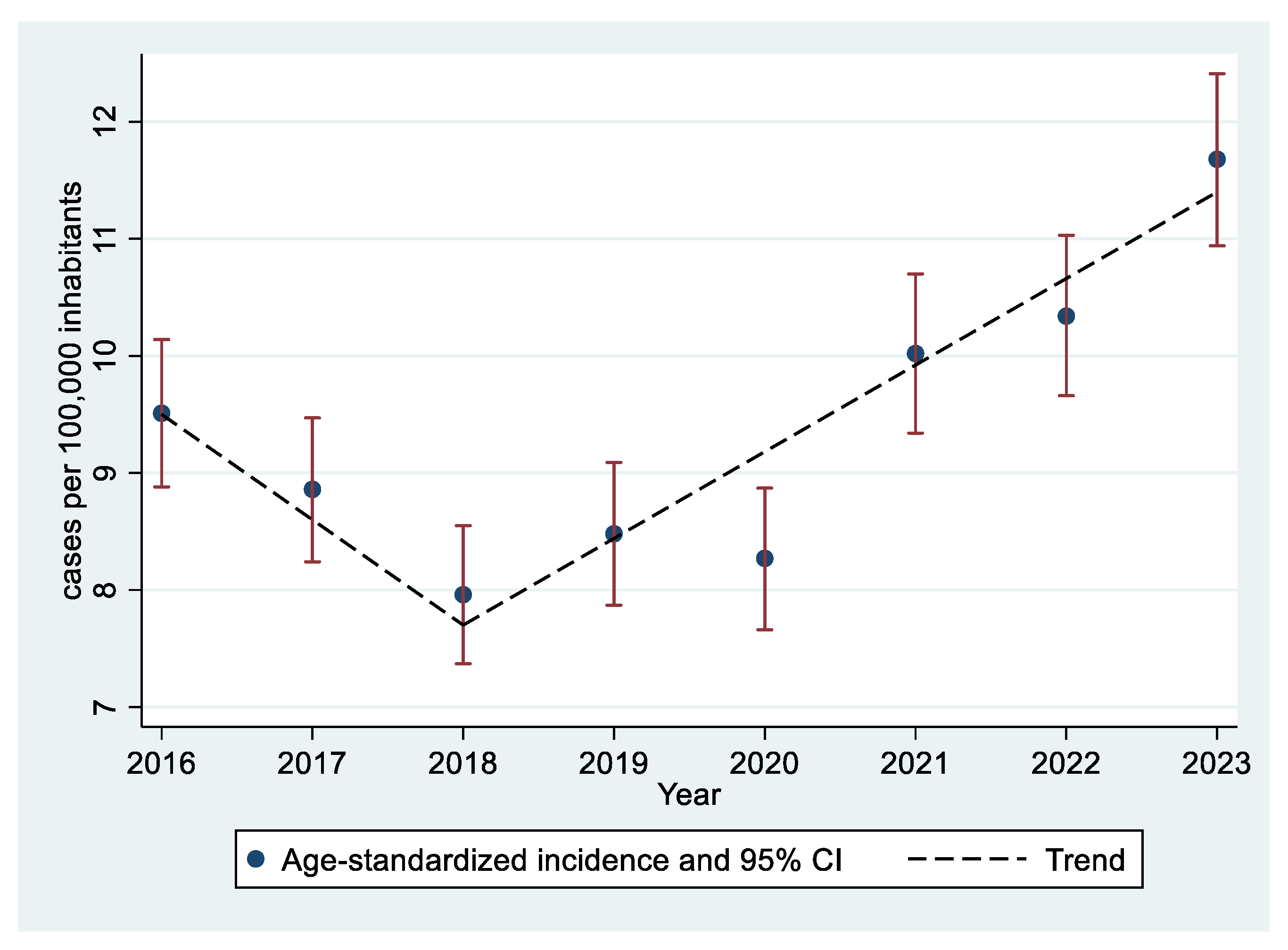
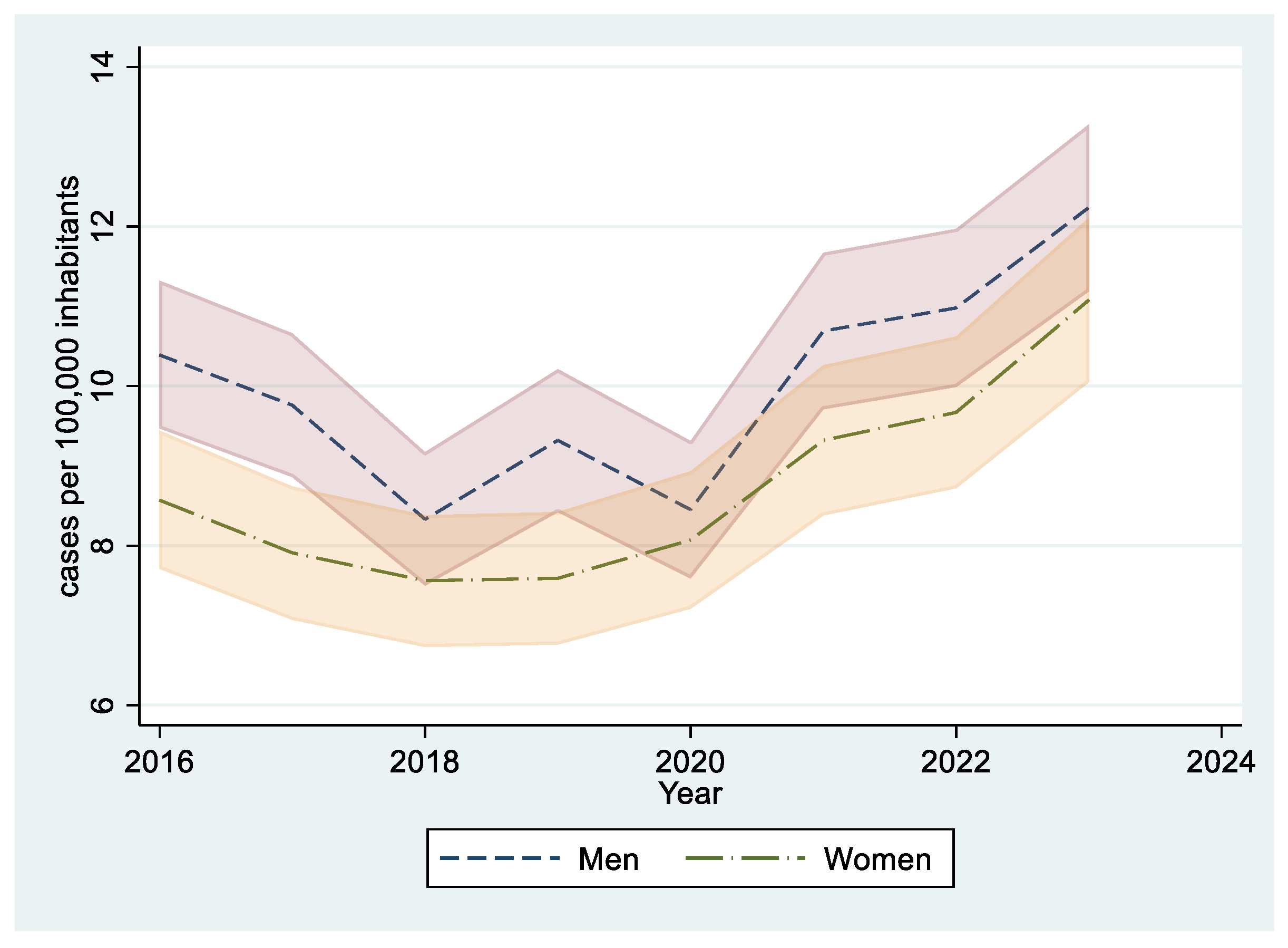
| Overall | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | 2023 | p (*) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Women | 2935 (45.1) | 381 (43.6) | 346 (43.3) | 323 (45.9) | 321 (43.4) | 338 (47.5) | 380 (45.5) | 398 (45.6) | 448 (45.9) | 0.174 |
| Age (years) | 3 (0–13) | 1 (0–11) | 3 (0–12) | 3 (0–13) | 2 (0–12) | 3 (0–14) | 4 (0–14) | 4 (0–14) | 7 (0–13) | 0.165 |
| Previous VTE | 65 (1.0) | 8 (0.9) | 3 (0.4) | 6 (0.9) | 11 (1.5) | 7 (1) | 6 (0.7) | 9 (1) | 15 (1.5) | 0.112 |
| Cancer | 674 (10.4) | 129 (14.8) | 102 (12.8) | 59 (8.4) | 49 (6.6) | 75 (10.5) | 85 (10.2) | 66 (7.6) | 109 (11.2) | 0.002 |
| COVID-19 | 95 (1.5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (0.3) | 26 (3.1) | 47 (5.4) | 20 (2) | <0.001 |
| Liver disease | 567 (8.7) | 44 (5) | 32 (4) | 42 (6) | 75 (10.1) | 66 (9.3) | 69 (8.3) | 91 (10.4) | 148 (15.2) | <0.001 |
| CKD | 155 (2.4) | 17 (1.9) | 31 (3.9) | 16 (2.3) | 14 (1.9) | 15 (2.1) | 16 (1.9) | 25 (2.9) | 21 (2.2) | 0.542 |
| Connective tissue disease | 50 (0.8) | 6 (0.7) | 7 (0.9) | 9 (1.3) | 5 (0.7) | 4 (0.6) | 5 (0.6) | 3 (0.3) | 11 (1.1) | 0.784 |
| Sepsis | 344 (5.3) | 41 (4.7) | 54 (6.8) | 37 (5.3) | 40 (5.4) | 40 (5.6) | 28 (3.3) | 51 (5.8) | 53 (5.4) | 0.683 |
| CHD | 978 (15.0) | 117 (13.4) | 136 (17) | 126 (17.9) | 111 (15) | 116 (16.3) | 126 (15.1) | 122 (14) | 124 (12.7) | 0.087 |
| Sickle cell disease | 41 (0.6) | 6 (0.7) | 2 (0.3) | 5 (0.7) | 4 (0.5) | 5 (0.7) | 5 (0.6) | 6 (0.7) | 8 (0.8) | 0.394 |
| Nephrotic syndrome | 44 (0.7) | 5 (0.6) | 10 (1.3) | 5 (0.7) | 5 (0.7) | 8 (1.1) | 1 (0.1) | 4 (0.5) | 6 (0.6) | 0.183 |
| Transplantation | 130 (2.0) | 22 (2.5) | 16 (2) | 9 (1.3) | 14 (1.9) | 18 (2.5) | 15 (1.8) | 14 (1.6) | 22 (2.3) | 0.750 |
| Thrombophilia | 154 (2.4) | 23 (2.6) | 22 (2.8) | 16 (2.3) | 13 (1.8) | 23 (3.2) | 15 (1.8) | 16 (1.8) | 26 (2.7) | 0.532 |
| Overweight or obesity | 112 (1.7) | 12 (1.4) | 12 (1.5) | 9 (1.3) | 10 (1.4) | 21 (2.9) | 12 (1.4) | 13 (1.5) | 23 (2.4) | 0.122 |
| Intravascular device | 2083 (32.0) | 362 (41.5) | 300 (37.5) | 230 (32.7) | 222 (30) | 227 (31.9) | 279 (33.4) | 239 (27.4) | 224 (23) | <0.001 |
| Contraceptive use | 34 (0.5) | 5 (0.6) | 5 (0.6) | 7 (1) | 2 (0.3) | 4 (0.6) | 1 (0.1) | 4 (0.5) | 6 (0.6) | 0.435 |
| CCC | 4625 (71.0) | 662 (75.8) | 597 (74.7) | 497 (70.7) | 537 (72.7) | 512 (71.9) | 579 (69.3) | 574 (65.8) | 667 (68.3) | <0.001 |
| Surgery | 71 (1.1) | 2 (0.2) | 4 (0.5) | 3 (0.4) | 8 (1.1) | 13 (1.8) | 9 (1.1) | 19 (2.2) | 13 (1.3) | <0.001 |
| Length of stay (days) | 10 (5–23) | 8 (4–22) | 10 (5–24) | 10 (5–22) | 11 (5–23) | 10 (5–26) | 9 (5–22) | 10 (5–26) | 9 (4–21) | 0.909 |
| <1 Year | 1–4 Years | 5–9 Years | 10–14 Years | 15–18 Years | p (*) | |
|---|---|---|---|---|---|---|
| Women | 1105 (43.6) | 411 (42.6) | 343 (45.6) | 420 (42.8) | 656 (51.3) | <0.001 |
| Previous VTE | 7 (0.3) | 21 (2.2) | 6 (0.8) | 7 (0.7) | 24 (1.9) | <0.001 |
| Cancer | 28 (1.1) | 187 (19.4) | 122 (16.2) | 189 (19.2) | 148 (11.6) | <0.001 |
| COVID-19 | 21 (0.8) | 15 (1.6) | 15 (2) | 9 (0.9) | 35 (2.7) | <0.001 |
| Liver disease | 37 (1.5) | 144 (14.9) | 114 (15.1) | 166 (16.9) | 106 (8.3) | <0.001 |
| CKD | 14 (0.6) | 42 (4.4) | 33 (4.4) | 25 (2.5) | 41 (3.2) | <0.001 |
| Connective tissue disease | 5 (0.2) | 6 (0.6) | 8 (1.1) | 13 (1.3) | 18 (1.4) | <0.001 |
| Sepsis | 144 (5.7) | 50 (5.2) | 30 (4) | 51 (5.2) | 69 (5.4) | 0.538 |
| CHD | 744 (29.4) | 85 (8.8) | 67 (8.9) | 41 (4.2) | 41 (3.2) | <0.001 |
| Sickle cell disease | 0 (0) | 4 (0.4) | 14 (1.9) | 8 (0.8) | 15 (1.2) | <0.001 |
| Thrombophilia | 20 (0.8) | 19 (2) | 23 (3.1) | 34 (3.5) | 58 (4.5) | <0.001 |
| Nephrotic syndrome | 6 (0.2) | 21 (2.2) | 6 (0.8) | 3 (0.3) | 8 (0.6) | 0.788 |
| Transplantation | 1 (0) | 34 (3.5) | 24 (3.2) | 26 (2.6) | 45 (3.5) | <0.001 |
| Surgery | 43 (1.7) | 4 (0.4) | 5 (0.7) | 10 (1) | 9 (0.7) | 0.007 |
| Overweight or obesity | 1 (0) | 1 (0.1) | 5 (0.7) | 36 (3.7) | 69 (5.4) | <0.001 |
| Intravascular device | 1431 (56.5) | 194 (20.1) | 121 (16.1) | 145 (14.8) | 192 (15) | <0.001 |
| Contraceptive use | 0 (0) | 0 (0) | 0 (0) | 2 (0.2) | 32 (2.5) | <0.001 |
| CCC | 2089 (82.5) | 673 (69.8) | 496 (65.9) | 650 (66.2) | 717 (56.1) | <0.001 |
| Overall | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | 2023 | |
|---|---|---|---|---|---|---|---|---|---|
| Lower limb DVT | 3460 | 501 | 421 | 405 | 444 | 390 | 433 | 423 | 443 |
| PE | 784 | 62 | 81 | 75 | 89 | 99 | 112 | 136 | 130 |
| Upper limb DVT | 189 | 45 | 36 | 10 | 8 | 14 | 25 | 24 | 27 |
| Superficial | 104 | 14 | 20 | 6 | 5 | 14 | 11 | 16 | 18 |
| Renal | 187 | 34 | 35 | 29 | 14 | 12 | 20 | 17 | 26 |
| Portal | 1014 | 107 | 97 | 86 | 104 | 113 | 129 | 169 | 209 |
| Other | 772 | 110 | 109 | 92 | 75 | 70 | 106 | 87 | 123 |
| Year | Population | Cases | Crude Rate (CI 95%) | <1 Year | 1–4 Years | 5–9 Years | 10–14 Years | 15–18 Years | Age-Standardized Rate (CI 95%) |
|---|---|---|---|---|---|---|---|---|---|
| 2016 | 8,736,116 | 873 | 9.99 (9.34 a 10.68) | 102.46 | 7.26 | 3.38 | 4.37 | 8 | 9.51 (8.88–10.14) |
| 2017 | 8,736,111 | 799 | 9.15 (8.52 a 9.8) | 84.3 | 7.06 | 4.86 | 3.62 | 7.81 | 8.86 (8.24–9.47) |
| 2018 | 8,736,859 | 703 | 8.05 (7.46 a 8.66) | 78.13 | 5.56 | 3.62 | 3.3 | 8.06 | 7.96 (7.37–8.55) |
| 2019 | 8,733,132 | 739 | 8.46 (7.86 a 9.09) | 90.58 | 6.27 | 2.53 | 4.32 | 7.49 | 8.48 (7.87–9.09) |
| 2020 | 8,699,072 | 712 | 8.18 (7.59 a 8.81) | 80.34 | 6.05 | 3.09 | 4.2 | 8.1 | 8.27 (7.66–8.87) |
| 2021 | 8,593,001 | 836 | 9.73 (9.08 a 10.41) | 100.47 | 7.89 | 3.11 | 5.13 | 9.53 | 10.02 (9.34–10.7) |
| 2022 | 8,584,406 | 872 | 10.16 (9.49 a 10.86) | 88.23 | 9.58 | 4.84 | 5.5 | 9.27 | 10.34 (9.66–11.03) |
| 2023 | 8,562,979 | 976 | 11.4 (10.69 a 12.14) | 77.48 | 11.04 | 7.15 | 9.14 | 8.59 | 11.68 (10.94–12.41) |
| Year | Population | Cases | Crude Rate (CI 95%) | <1 Year | 1–4 Years | 5–9 Years | 10–14 Years | 15–18 Years | Age-Standardized Rate (CI 95%) |
|---|---|---|---|---|---|---|---|---|---|
| 2016 | 4,498,422 | 492 | 10.94 (9.99 a 11.95) | 113.07 | 8.09 | 3.79 | 4.62 | 8.48 | 10.39 (9.47–11.32) |
| 2017 | 4,497,516 | 453 | 10.07 (9.17 a 11.04) | 87.15 | 9.18 | 5.59 | 4.21 | 7.91 | 9.76 (8.86–10.66) |
| 2018 | 4,497,683 | 380 | 8.45 (7.62 a 9.34) | 84.18 | 6.78 | 3.75 | 3.4 | 7.31 | 8.33 (7.5–9.17) |
| 2019 | 4,496,846 | 418 | 9.3 (8.43 a 10.23) | 97.15 | 8.32 | 3 | 4.66 | 7.32 | 9.32 (8.42–10.21) |
| 2020 | 4,478,756 | 374 | 8.35 (7.53 a 9.24) | 87.68 | 5.82 | 3.04 | 4 | 8.07 | 8.45 (7.59–9.31) |
| 2021 | 4,424,081 | 456 | 10.31 (9.38 a 11.3) | 121.2 | 8.25 | 2.67 | 5.71 | 8.11 | 10.69 (9.71–11.67) |
| 2022 | 4,419,345 | 474 | 10.73 (9.78 a 11.74) | 101.96 | 10.44 | 5.75 | 5.37 | 7.89 | 10.98 (9.99–11.97) |
| 2023 | 4,409,571 | 528 | 11.97 (10.97 a 13.04) | 78.22 | 10.41 | 6.77 | 12.01 | 8.28 | 12.24 (11.19–13.28) |
| Year | Population | Cases | Crude Rate (CI 95%) | <1 Year | 1–4 Years | 5–9 Years | 10–14 Years | 15–18 Years | Age-Standardized Rate (CI 95%) |
|---|---|---|---|---|---|---|---|---|---|
| 2016 | 4,237,694 | 381 | 8.99 (8.11 a 9.94) | 91.21 | 6.36 | 2.94 | 4.1 | 7.49 | 8.57 (7.71–9.44) |
| 2017 | 4,238,595 | 346 | 8.16 (7.33 a 9.07) | 81.28 | 4.81 | 4.08 | 2.99 | 7.7 | 7.91 (7.07–8.74) |
| 2018 | 4,239,176 | 323 | 7.62 (6.81 a 8.5) | 71.72 | 4.27 | 3.47 | 3.18 | 8.87 | 7.56 (6.73–8.38) |
| 2019 | 4,236,286 | 321 | 7.58 (6.77 a 8.45) | 83.64 | 4.1 | 2.04 | 3.95 | 7.67 | 7.59 (6.76–8.42) |
| 2020 | 4,220,316 | 338 | 8.01 (7.18 a 8.91) | 72.59 | 6.29 | 3.14 | 4.41 | 8.14 | 8.07 (7.21–8.93) |
| 2021 | 4,168,920 | 380 | 9.12 (8.22 a 10.08) | 78.55 | 7.51 | 3.57 | 4.51 | 11.03 | 9.32 (8.38–10.26) |
| 2022 | 4,165,061 | 398 | 9.56 (8.64 a 10.54) | 73.61 | 8.66 | 3.88 | 5.63 | 10.74 | 9.67 (8.72–10.62) |
| 2023 | 4,153,408 | 448 | 10.79 (9.81 a 11.83) | 76.71 | 11.71 | 7.55 | 6.09 | 8.93 | 11.08 (10.05–12.11) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rueda-Camino, J.A.; Sabrido-Bermúdez, G.; Barba-Martín, R. Trends and Risk Factors of Pediatric Venous Thromboembolism in Spain: A Nationwide Study from 2016 to 2023. J. Clin. Med. 2025, 14, 3950. https://doi.org/10.3390/jcm14113950
Rueda-Camino JA, Sabrido-Bermúdez G, Barba-Martín R. Trends and Risk Factors of Pediatric Venous Thromboembolism in Spain: A Nationwide Study from 2016 to 2023. Journal of Clinical Medicine. 2025; 14(11):3950. https://doi.org/10.3390/jcm14113950
Chicago/Turabian StyleRueda-Camino, José Antonio, Gema Sabrido-Bermúdez, and Raquel Barba-Martín. 2025. "Trends and Risk Factors of Pediatric Venous Thromboembolism in Spain: A Nationwide Study from 2016 to 2023" Journal of Clinical Medicine 14, no. 11: 3950. https://doi.org/10.3390/jcm14113950
APA StyleRueda-Camino, J. A., Sabrido-Bermúdez, G., & Barba-Martín, R. (2025). Trends and Risk Factors of Pediatric Venous Thromboembolism in Spain: A Nationwide Study from 2016 to 2023. Journal of Clinical Medicine, 14(11), 3950. https://doi.org/10.3390/jcm14113950






