Antithrombotic Therapy in Acute Coronary Syndrome Patients with End-Stage Renal Disease: Navigating Efficacy and Safety
Abstract
1. Introduction
2. High Thrombotic Risk in ESRD
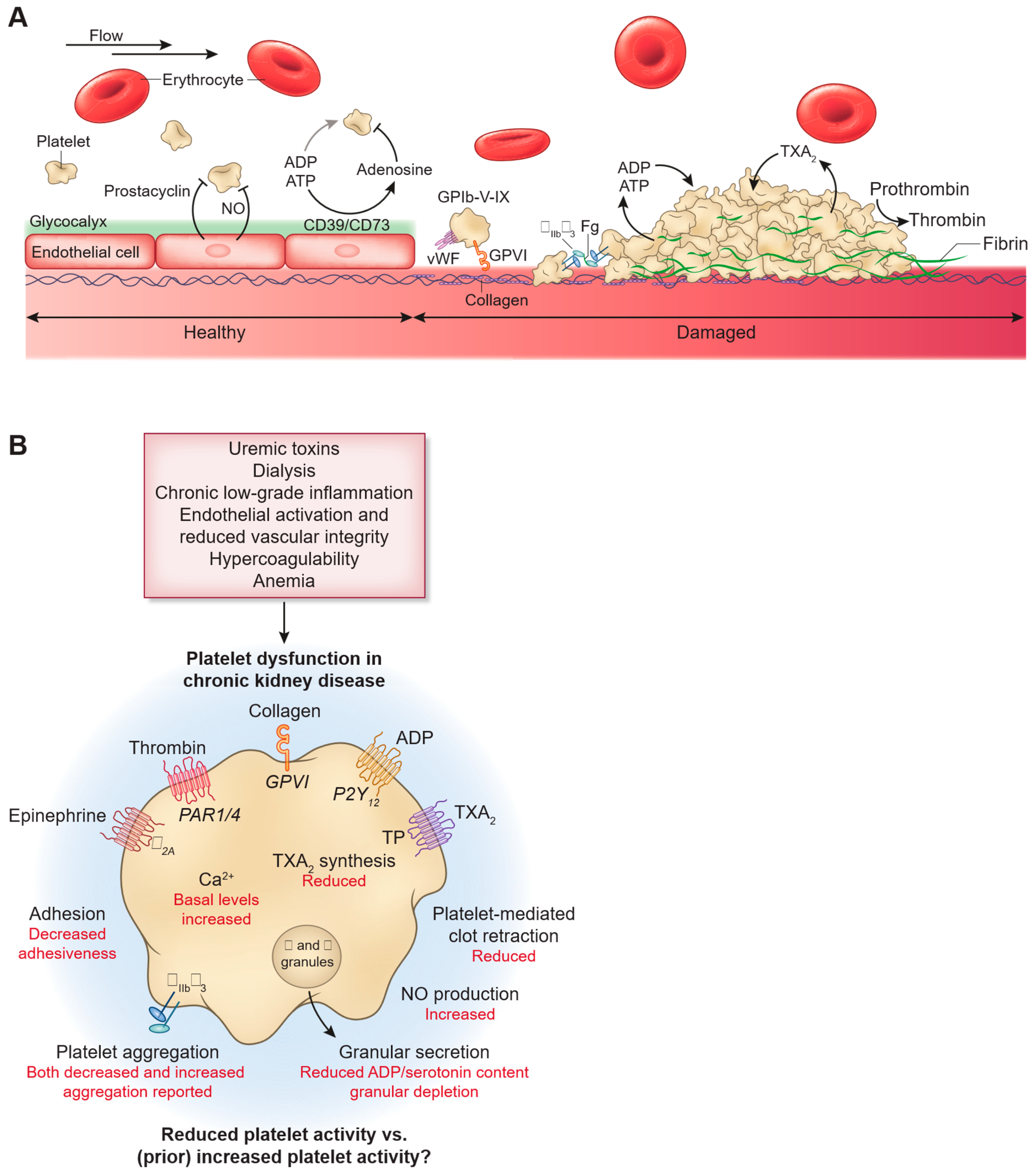
3. Mechanisms of Higher Bleeding Risk in ESRD Patients
4. Balancing the Thrombotic and Bleeding Risks, Navigating the Challenges
4.1. Acetylsalicylic Acid
4.2. Potent P2Y12 Inhibitors vs. Clopidogrel
4.3. The Duration of DAPT After PCI
4.4. Alternative Anti-Thrombotic Regimens
5. Laboratory Guided Precision Medicine Approaches
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Matsushita, K.; Ballew, S.H.; Wang, A.Y.; Kalyesubula, R.; Schaeffner, E.; Agarwal, R. Epidemiology and risk of cardiovascular disease in populations with chronic kidney disease. Nat. Rev. Nephrol. 2022, 18, 696–707. [Google Scholar] [CrossRef] [PubMed]
- Go, A.S.; Bansal, N.; Chandra, M.; Lathon, P.V.; Fortmann, S.P.; Iribarren, C.; Hsu, C.-Y.; Hlatky, M.A. Chronic kidney disease and risk for presenting with acute myocardial infarction versus stable exertional angina in adults with coronary heart disease. J. Am. Coll. Cardiol. 2011, 58, 1600–1607. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gao, L. Inflammation and Cardiovascular Disease Associated with Hemodialysis for End-Stage Renal Disease. Front Pharmacol. 2022, 13, 800950. [Google Scholar] [CrossRef] [PubMed]
- Echefu, G.; Stowe, I.; Burka, S.; Basu-Ray, I.; Kumbala, D. Pathophysiological concepts and screening of cardiovascular disease in dialysis patients. Front Nephrol. 2023, 3, 1198560. [Google Scholar] [CrossRef]
- Charytan, D.; Kuntz, R.E.; Mauri, L.; DeFilippi, C. Distribution of coronary artery disease and relation to mortality in asymptomatic hemodialysis patients. Am. J. Kidney Dis. 2007, 49, 409–416. [Google Scholar] [CrossRef]
- Byrne, R.; Asteggiano, R.; Marjeh, M.Y.B.; Rocca, B.; Zeppenfeld, K.; Geisler, T.; Dan, G.-A.; Ryödi, E.; Coughlan, J.J.; Wiseth, R.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef]
- Jakubiak, G.K.; Cieślar, G.; Stanek, A.; Pawlas, N. Pathogenesis and Clinical Significance of In-Stent Restenosis in Patients with Diabetes. Int. J. Environ. Res. Public. Health 2021, 18, 11970. [Google Scholar] [CrossRef]
- Charytan, D.; Kuntz, R.E. The exclusion of patients with chronic kidney disease from clinical trials in coronary artery disease. Kidney Int. 2006, 70, 2021–2030. [Google Scholar] [CrossRef]
- Fox, C.S.; Muntner, P.; Chen, A.Y.; Alexander, K.P.; Roe, M.T.; Cannon, C.P.; Saucedo, J.F.; Kontos, M.C.; Wiviott, S.D. Use of evidence-based therapies in short-term outcomes of ST-segment elevation myocardial infarction and non-ST-segment elevation myocardial infarction in patients with chronic kidney disease: A report from the National Cardiovascular Data Acute Coronary Treatment and Intervention Outcomes Network registry. Circulation 2010, 121, 357–365. [Google Scholar] [CrossRef]
- Urban, P.; Mehran, R.; Colleran, R.; Angiolillo, D.J.; Byrne, R.A.; Capodanno, D.; Cuisset, T.; Cutlip, D.; Eerdmans, P.; Eikelboom, J.; et al. Defining High Bleeding Risk in Patients Undergoing Percutaneous Coronary Intervention. Circulation 2019, 140, 240–261. [Google Scholar] [CrossRef]
- Chermiti, R.; Burtey, S.; Dou, L. Role of Uremic Toxins in Vascular Inflammation Associated with Chronic Kidney Disease. J. Clin. Med. 2024, 13, 7149. [Google Scholar] [CrossRef] [PubMed]
- Baaten, C.C.F.M.J.; Schröer, J.R.; Floege, J.; Marx, N.; Jankowski, J.; Berger, M.; Noels, H. Platelet Abnormalities in CKD and Their Implications for Antiplatelet Therapy. Clin. J. Am. Soc. Nephrol. 2022, 17, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lindholm, B. Cardiovascular Risk Prediction in Chronic Kidney Disease. Am. J. Nephrol. 2022, 53, 730–739. [Google Scholar] [CrossRef] [PubMed]
- Trimarchi, G.; Pizzino, F. An in-depth look at electrolytes in acute heart failure: The role of sodium-to-chloride ratio. Int. J. Cardiol. 2024, 417, 132585. [Google Scholar] [CrossRef]
- Bonello, L.; Angiolillo, D.J.; Aradi, D.; Sibbing, D. P2Y12-ADP Receptor Blockade in Chronic Kidney Disease Patients With Acute Coronary Syndromes. Circulation 2018, 138, 1582–1596. [Google Scholar] [CrossRef]
- Kadowaki, T.; Maegawa, H.; Yabe, D.; Wada, J.; Node, K.; Murohara, T.; Watada, H. Interconnection between cardiovascular, renal and metabolic disorders: A narrative review with a focus on Japan. Diabetes Obes. Metab. 2022, 24, 2283–2296. [Google Scholar] [CrossRef]
- Burlacu, A.; Genovesi, S.; Ortiz, A.; Kanbay, M.; Rossignol, P.; Banach, M.; Małyszko, J.; Goldsmith, D.; Covic, A. The quest for equilibrium: Exploring the thin red line between bleeding and ischaemic risks in the management of acute coronary syndromes in chronic kidney disease patients. Nephrol. Dial. Transplant. 2017, 32, 1967–1976. [Google Scholar] [CrossRef]
- Qiu, Z.; Pang, X.; Xiang, Q.; Cui, Y. The Crosstalk between Nephropathy and Coagulation Disorder: Pathogenesis, Treatment, and Dilemmas. J. Am. Soc. Nephrol. 2023, 34, 1793–1811. [Google Scholar] [CrossRef]
- Galbusera, M.; Remuzzi, G.; Boccardo, P. Treatment of bleeding in dialysis patients. Semin. Dial. 2009, 22, 279–286. [Google Scholar] [CrossRef]
- Boccardo, P.; Remuzzi, G.; Galbusera, M. Platelet dysfunction in renal failure. Semin. Thromb. Hemost. 2004, 30, 579–589. [Google Scholar] [CrossRef]
- Portolés, J.; Broseta, J.J.; Cases, A.; Martín, L. Anemia in Chronic Kidney Disease: From Pathophysiology and Current Treatments, to Future Agents. Front. Med. 2021, 8 (Suppl. S2), 642296. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-H.; Luo, J.-F.; Jiang, Y.; Ma, Y.-J.; Ji, Y.-Q.; Zhu, G.-L.; Zhou, C.; Chu, H.-W.; Zhang, H.-D. Red Blood Cell Lifespan Shortening in Patients with Early-Stage Chronic Kidney Disease. Kidney Blood Press. Res. 2019, 44, 1158–1165. [Google Scholar] [CrossRef] [PubMed]
- Weisel, J.W.; Litvinov, R.I. Red blood cells: The forgotten player in hemostasis and thrombosis. J. Thromb. Haemost. 2019, 17, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Gäckler, A.; Rohn, H.; Lisman, T.; Benkö, T.; Witzke, O.; Kribben, A.; Saner, F.H. Evaluation of hemostasis in patients with end-stage renal disease. PLoS ONE 2019, 14, e0212237. [Google Scholar] [CrossRef]
- Szummer, K.; Lundman, P.; Jacobson, S.H.; Schön, S.; Lindbäck, J.; Stenestrand, U.; Wallentin, L.; Jernberg, T.; Swedeheart. Relation between renal function, presentation, use of therapies and in-hospital complications in acute coronary syndrome: Data from the SWEDEHEART register. J. Intern. Med. 2010, 268, 40–49. [Google Scholar] [CrossRef]
- Melloni, C.; Cornel, J.H.; Hafley, G.; Neely, M.L.; Clemmensen, P.; Zamoryakhin, D.; Prabhakaran, D.; White, H.D.; Fox, K.A.; Ohman, E.M.; et al. Impact of chronic kidney disease on long-term ischemic and bleeding outcomes in medically managed patients with acute coronary syndromes: Insights from the TRILOGY ACS Trial. Eur. Heart J. Acute Cardiovasc. Care 2016, 5, 443–454. [Google Scholar] [CrossRef]
- McCullough, P.A.; Sandberg, K.R.; Borzak, S.; Hudson, M.P.; Garg, M.; Manley, H.J. Benefits of aspirin and beta-blockade after myocardial infarction in patients with chronic kidney disease. Am. Heart J. 2002, 144, 226–232. [Google Scholar] [CrossRef]
- Jain, N.; Hedayati, S.S.; Sarode, R.; Banerjee, S.; Reilly, R.F. Antiplatelet therapy in the management of cardiovascular disease in patients with CKD: What is the evidence? Clin. J. Am. Soc. Nephrol. 2013, 8, 665–674. [Google Scholar] [CrossRef]
- Berger, A.K.; Duval, S.; Krumholz, H.M. Aspirin, beta-blocker, and angiotensin-converting enzyme inhibitor therapy in patients with end-stage renal disease and an acute myocardial infarction. J. Am. Coll. Cardiol. 2003, 42, 201–208. [Google Scholar] [CrossRef]
- Baigent, C.; Landray, M.; Leaper, C.; Altmann, P.; Armitage, J.; Baxter, A.; Cairns, H.S.; Collins, R.; Foley, R.N.; Frighi, V.; et al. First United Kingdom Heart and Renal Protection (UK-HARP-I) study: Biochemical efficacy and safety of simvastatin and safety of low-dose aspirin in chronic kidney disease. Am. J. Kidney Dis. 2005, 45, 473–484. [Google Scholar] [CrossRef]
- Patrono, C.; García Rodríguez, L.A.; Landolfi, R.; Baigent, C. Low-dose aspirin for the prevention of atherothrombosis. N. Engl. J. Med. 2005, 353, 2373–2383. [Google Scholar] [CrossRef] [PubMed]
- James, S.; Budaj, A.; Aylward, P.; Buck, K.K.; Cannon, C.P.; Cornel, J.H.; Harrington, R.A.; Horrow, J.; Katus, H.; Keltai, M.; et al. Ticagrelor versus clopidogrel in acute coronary syndromes in relation to renal function: Results from the Platelet Inhibition and Patient Outcomes (PLATO) trial. Circulation 2010, 122, 1056–1067. [Google Scholar] [CrossRef] [PubMed]
- Edfors, R.; Sahlén, A.; Szummer, K.; Renlund, H.; Evans, M.; Carrero, J.-J.; Spaak, J.; James, S.K.; Lagerqvist, B.; Varenhorst, C.; et al. Outcomes in patients treated with ticagrelor versus clopidogrel after acute myocardial infarction stratified by renal function. Heart 2018, 104, 1575–1582. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Choi, Y.J.; Kang, J.E.; Kim, M.G.; Geum, M.J.; Kim, S.D.; Rhie, S.J. P2Y12 Antiplatelet Choice for Patients with Chronic Kidney Disease and Acute Coronary Syndrome: A Systematic Review and Meta-Analysis. J. Pers. Med. 2021, 11, 222. [Google Scholar] [CrossRef]
- Mullangi, R.; Srinivas, N.R. Clopidogrel: Review of bioanalytical methods, pharmacokinetics/pharmacodynamics, and update on recent trends in drug-drug interaction studies. Biomed. Chromatogr. 2009, 23, 26–41. [Google Scholar] [CrossRef]
- Gimbel, M.; Qaderdan, K.; Willemsen, L.; Hermanides, R.; Bergmeijer, T.; de Vrey, E.; Heestermans, T.; Gin, M.T.J.; Waalewijn, R.; Hofma, S.; et al. Clopidogrel versus ticagrelor or prasugrel in patients aged 70 years or older with non-ST-elevation acute coronary syndrome (POPular AGE): The randomised, open-label, non-inferiority trial. Lancet 2020, 395, 1374–1381. [Google Scholar] [CrossRef]
- Shah, R.P.; Shafiq, A.; Hamza, M.; Maniya, M.T.; Duhan, S.; Keisham, B.; Patel, B.; Alamzaib, S.M.; Yashi, K.; Uppal, D.; et al. Ticagrelor Versus Prasugrel in Patients With Acute Coronary Syndrome: A Systematic Review and Meta-Analysis. Am J Cardiol. 2023, 207, 206–214. [Google Scholar] [CrossRef]
- Kamran, H.; Jneid, H.; Kayani, W.T.; Virani, S.S.; Levine, G.N.; Nambi, V.; Khalid, U. Oral Antiplatelet Therapy After Acute Coronary Syndrome: A Review. JAMA 2021, 325, 1545–1555. [Google Scholar] [CrossRef]
- De Filippo, O.; D’Ascenzo, F.; Raposeiras-Roubin, S.; Abu-Assi, E.; Peyracchia, M.; Bocchino, P.P.; Kinnaird, T.; Ariza-Solé, A.; Liebetrau, C.; Manzano-Fernández, S.; et al. P2Y12 inhibitors in acute coronary syndrome patients with renal dysfunction: An analysis from the RENAMI and BleeMACS projects. Eur. Heart J. Cardiovasc. Pharmacother. 2020, 6, 31–42. [Google Scholar] [CrossRef]
- Müller, I.; Besta, F.; Schulz, C.; Massberg, S.; Schönig, A.; Gawaz, M. Prevalence of clopidogrel non-responders among patients with stable angina pectoris scheduled for elective coronary stent placement. Thromb. Haemost. 2003, 89, 783–787. [Google Scholar] [CrossRef]
- Woo, J.S.; Kim, W.; Lee, S.R.; Jung, K.H.; Kim, W.S.; Lew, J.H.; Lee, T.W.; Lim, C.K. Platelet reactivity in patients with chronic kidney disease receiving adjunctive cilostazol compared with a high-maintenance dose of clopidogrel: Results of the effect of platelet inhibition according to clopidogrel dose in patients with chronic kidney disease (PIANO-2 CKD) randomized study. Am. Heart J. 2011, 162, 1018–1025. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Wang, Q.; Ullah, I.; Lin, Q.; Wu, T.; Yang, M.; Fan, Y.; Dong, Z.; Wang, T.; Teng, J.; et al. Impact of hemodialysis on efficacies of the antiplatelet agents in coronary artery disease patients complicated with end-stage renal disease. J. Thromb. Thrombolysis 2024, 57, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Jeong, K.H.; Cho, J.H.; Woo, J.S.; Kim, J.B.; Kim, W.-S.; Lee, T.W.; Kim, K.S.; Ihm, C.G.; Kim, W. Platelet reactivity after receiving clopidogrel compared with ticagrelor in patients with kidney failure treated with hemodialysis: A randomized crossover study. Am. J. Kidney Dis. 2015, 65, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Morel, O.; El Ghannudi, S.; Jesel, L.; Radulescu, B.; Meyer, N.; Wiesel, M.-L.; Caillard, S.; Campia, U.; Moulin, B.; Gachet, C.; et al. Cardiovascular mortality in chronic kidney disease patients undergoing percutaneous coronary intervention is mainly related to impaired P2Y12 inhibition by clopidogrel. J. Am. Coll. Cardiol. 2011, 57, 399–408. [Google Scholar] [CrossRef]
- Rubin, G.A.; Kirtane, A.J.; Chen, S.; Redfors, B.; Weisz, G.; Baber, U.; Zhang, Y.; Stuckey, T.D.; Witzenbichler, B.; Rinaldi, M.J.; et al. Impact of high on-treatment platelet reactivity on outcomes following PCI in patients on hemodialysis: An ADAPT-DES substudy. Catheter. Cardiovasc. Interv. 2020, 96, 793–801. [Google Scholar] [CrossRef]
- Alexopoulos, D.; Xanthopoulou, I.; Plakomyti, T.E.; Goudas, P.; Koutroulia, E.; Goumenos, D. Ticagrelor in clopidogrel-resistant patients undergoing maintenance hemodialysis. Am. J. Kidney Dis. 2012, 60, 332–333. [Google Scholar] [CrossRef]
- Jain, N.; Phadnis, M.A.; Hunt, S.L.; Dai, J.; Shireman, T.I.; Davis, C.L.; Mehta, J.L.; Rasu, R.S.; Hedayati, S.S. Comparative Effectiveness and Safety of Oral P2Y12 Inhibitors in Patients on Chronic Dialysis. Kidney Int. Rep. 2021, 6, 2381–2391. [Google Scholar] [CrossRef]
- Park, S.; Kim, Y.; Jo, H.A.; Lee, S.; Kim, M.-S.; Yang, B.R.; Lee, J.; Han, S.S.; Lee, H.; Lee, J.P.; et al. Clinical outcomes of prolonged dual antiplatelet therapy after coronary drug-eluting stent implantation in dialysis patients. Clin. Kidney J. 2020, 13, 803–812. [Google Scholar] [CrossRef]
- Alagna, G.; Trimarchi, G.; Cascone, A.; Villari, A.; Cavolina, G.; Campanella, F.; Micari, A.; Taverna, G.; Andò, G. Effectiveness and Safety of Ticagrelor Monotherapy After Short-Duration Dual Antiplatelet Therapy in PCI Patients: A Systematic Review and Meta-Analysis. Am. J. Cardiol. 2025, 241, 69–74. [Google Scholar] [CrossRef]
- Pepe, M.; Carella, M.C.; Nestola, P.L.; Napoli, G.; Giordano, S.; Cirillo, P.; Giordano, A.; Carulli, E.; Bartolomucci, F.; Tritto, R.; et al. Comparative effectiveness of Cangrelor in patients with acute coronary syndrome undergoing percutaneous coronary intervention: An observational investigation from the M.O.Ca. registry. Sci. Rep. 2023, 13, 10685. [Google Scholar] [CrossRef]
- Mega, J.L.; Verheugt, F.W.A.; Plotnikov, A.N.; Burton, P.; Braunwald, E.; Bode, C.; Bassand, J.-P.; Sun, X.; Bhatt, D.L.; Cook-Bruns, N.; et al. Rivaroxaban in Patients with a Recent Acute Coronary Syndrome. New Engl. J. Med. 2012, 366, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Aradi, D.; Sibbing, D.; Gross, L. Platelet Function Testing in Patients on Antiplatelet Medications. Semin. Thromb. Hemost. 2016, 42, 306–320. [Google Scholar] [CrossRef] [PubMed]
- Gremmel, T.; Panzer, S.; Seidinger, D.; Kopp, C.W.; Steiner, S.; Muller, M.; Koppensteiner, R. Chronic kidney disease is associated with increased platelet activation and poor response to antiplatelet therapy. Nephrol. Dial. Transplant. 2013, 28, 2116–2122. [Google Scholar] [CrossRef]
- El Abdallaoui, O.E.A.; Szabó, D.; Lukács, R.; Komócsi, A.; Tornyos, D. Individualized or Uniform De-Escalation Strategies for Antiplatelet Therapy in Acute Coronary Syndrome: A Review of Clinical Trials with Platelet Function Testing and Genetic Testing-Based Protocols. Int. J. Mol. Sci. 2023, 24, 9071. [Google Scholar] [CrossRef] [PubMed]
- Angiolillo, D.J.; Berg, J.T.; Gibson, C.M.; Waksman, R.; Cavallari, L.H.; Siller-Matula, J.M.; Capodanno, D.; Alexopoulos, D.; Franchi, F.; Bonello, L.; et al. International Consensus Statement on Platelet Function and Genetic Testing in Percutaneous Coronary Intervention: 2024 Update. JACC Cardiovasc. Interv. 2024, 17, 2639–2663. [Google Scholar] [CrossRef]
- Sibbing, D.; Zweiker, R.; Kääb, S.; Kovács, A.; Komócsi, A.; Aradi, D.; Müller, K.; Ungi, I.; Mügge, A.; Ili, R.; et al. Guided de-escalation of antiplatelet treatment in patients with acute coronary syndrome undergoing percutaneous coronary intervention (TROPICAL-ACS): A randomised, open-label, multicentre trial. Lancet 2017, 390, 1747–1757. [Google Scholar] [CrossRef]
- Pereira, N.L.; Gordon, P.; Weinshilboum, R.; Jeong, M.H.; Hasan, A.; Lennon, R.; Farkouh, M.E.; Bailey, K.; Bae, J.; So, D.; et al. Effect of Genotype-Guided Oral P2Y12 Inhibitor Selection vs. Conventional Clopidogrel Therapy on Ischemic Outcomes After Percutaneous Coronary Intervention. JAMA 2020, 324, 761. [Google Scholar] [CrossRef]
| Trial | Study Population | MACE Outcome (CV Death/MI/Stroke) | Bleeding |
|---|---|---|---|
| McCullough 2002 [27] | 1724 STEMI patients (registry); Aspirin + β-blocker vs. none, stratified by CrCl | Marked benefit in terms of mortality. In-hospital MACE (driven by death) was much lower with ASA + BB across all CKD strata. Mortality RR reduction was ~64–80% in CKD patients on ASA + BB (vs. no therapy) | Bleeding not reported (acute registry; no significant excess noted in-hospital). |
| UK-HARP-I 2005 [30] | 448 CKD patients (predialysis, dialysis, transplant—RCT of aspirin 100 mg vs. placebo (1 yr)) | Not powered for MACE (no significant difference observed) | Major bleeding: no increase with aspirin (2% vs. 3%, NS). Minor bleeding: 3-fold higher with aspirin (15% vs. 5%, p < 0.001) |
| DOPPS 2007 [31] | 28,320 hemodialysis patients (observational; Aspirin vs. no Aspirin) | No net CV benefit. Aspirin did not lower composite cardiac events | No increase in major GI bleeding noted with aspirin (no significant hemorrhagic risk observed) |
| PLATO 2010 [32] | CKD subgroup = CrCl <60 mL/min (n = 3237) | Significant MACE reduction. Ticagrelor vs. clopidogrel lowered 12 month CV death/MI/stroke in CKD (17.3% vs. 22.0%; HR 0.77, 95% CI 0.65–0.90), an absolute risk reduction of ~4.7%. | Major bleeding: no significant difference (15.1% vs. 14.3%, HR 1.07, p = NS) in CKD. No increase in fatal bleeds; slight, non-significant ↑ in non-CABG major bleeds |
| TRILOGY-ACS 2012 [26] | Patients with NSTE-ACS managed medically without revascularization; CKD subgroup included | No significant difference in MACE between prasugrel and clopidogrel in CKD patients (13.9% versus 16.0%; p = 0.20) | Bleeding rates similar between prasugrel and clopidogrel in CKD subgroup (TIMI major 2.1% versus 1.5%; p = 0.27) |
| Edfors et al., 2018 [33] | 45,206 post-MI patients on DAPT (ticagrelor vs. clopidogrel), stratified by eGFR | Lower MACE with ticagrelor in moderate CKD. One-year death/MI/stroke rate was lower for ticagrelor vs. clopidogrel in eGFR 30–60 (adj. HR 0.82, 95% CI 0.70–0.97). In severe CKD (eGFR < 30), no significant benefit (HR 0.95, 95% CI 0.69–1.29) | Major bleeding (requiring hospitalization): no significant difference in moderate CKD (HR 1.13, 95% CI 0.84–1.51) but ↑ trend in severe CKD on ticagrelor (HR 1.79, 95% CI 1.00–3.21) |
| Meta-analysis (2020)—P2Y12 Inhibitors in CKD [34] | Pooled ACS trial data in CKD patients (prasugrel or ticagrelor vs. clopidogrel) | Improved outcomes. Potent P2Y12 inhibitors associated with lower MACE (especially reduced MI and mortality) in CKD | Bleeding: No significant increase in major bleeding with prasugrel or ticagrelor in CKD (vs. clopidogrel) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdeldayem, T.; Jeyalan, V.; Hayat, A.; Memon, S.; Farag, M.; Egred, M. Antithrombotic Therapy in Acute Coronary Syndrome Patients with End-Stage Renal Disease: Navigating Efficacy and Safety. J. Clin. Med. 2025, 14, 3956. https://doi.org/10.3390/jcm14113956
Abdeldayem T, Jeyalan V, Hayat A, Memon S, Farag M, Egred M. Antithrombotic Therapy in Acute Coronary Syndrome Patients with End-Stage Renal Disease: Navigating Efficacy and Safety. Journal of Clinical Medicine. 2025; 14(11):3956. https://doi.org/10.3390/jcm14113956
Chicago/Turabian StyleAbdeldayem, Tarek, Visvesh Jeyalan, Afzal Hayat, Saif Memon, Mohamed Farag, and Mohaned Egred. 2025. "Antithrombotic Therapy in Acute Coronary Syndrome Patients with End-Stage Renal Disease: Navigating Efficacy and Safety" Journal of Clinical Medicine 14, no. 11: 3956. https://doi.org/10.3390/jcm14113956
APA StyleAbdeldayem, T., Jeyalan, V., Hayat, A., Memon, S., Farag, M., & Egred, M. (2025). Antithrombotic Therapy in Acute Coronary Syndrome Patients with End-Stage Renal Disease: Navigating Efficacy and Safety. Journal of Clinical Medicine, 14(11), 3956. https://doi.org/10.3390/jcm14113956






