A Pragmatic Randomized Trial Comparing Suturing Techniques for Vesicourethral Anastomosis: One-Year Voiding Function Outcomes After Radical Prostatectomy
Abstract
1. Introduction
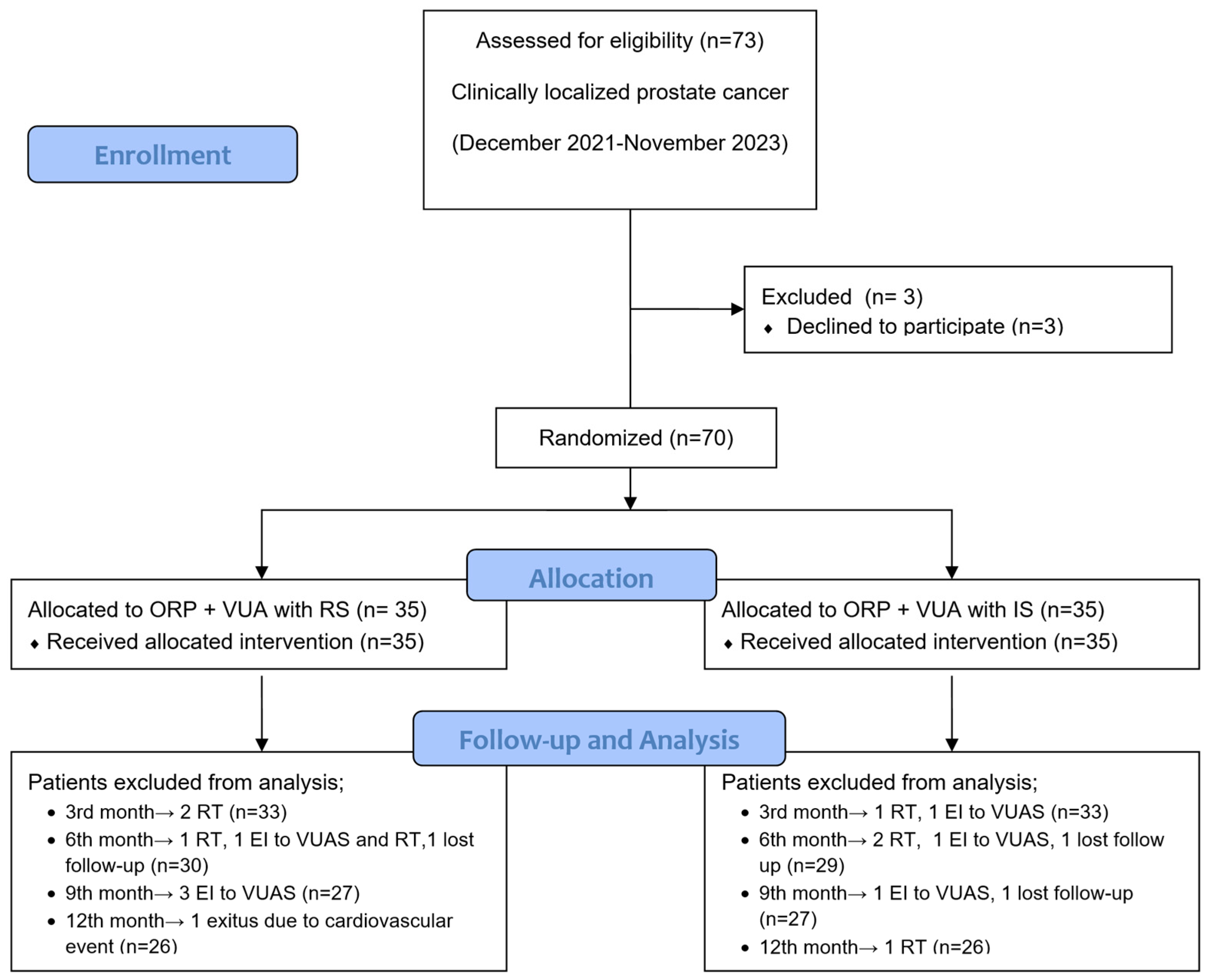
2. Materials and Methods
2.1. Study Population and Data Collection
2.2. Surgical Techniques
2.3. Outcome Assessment
2.4. Statistical Analysis
3. Results
3.1. Baseline Demographics
3.2. Perioperative Findings, Oncological Results and Complications
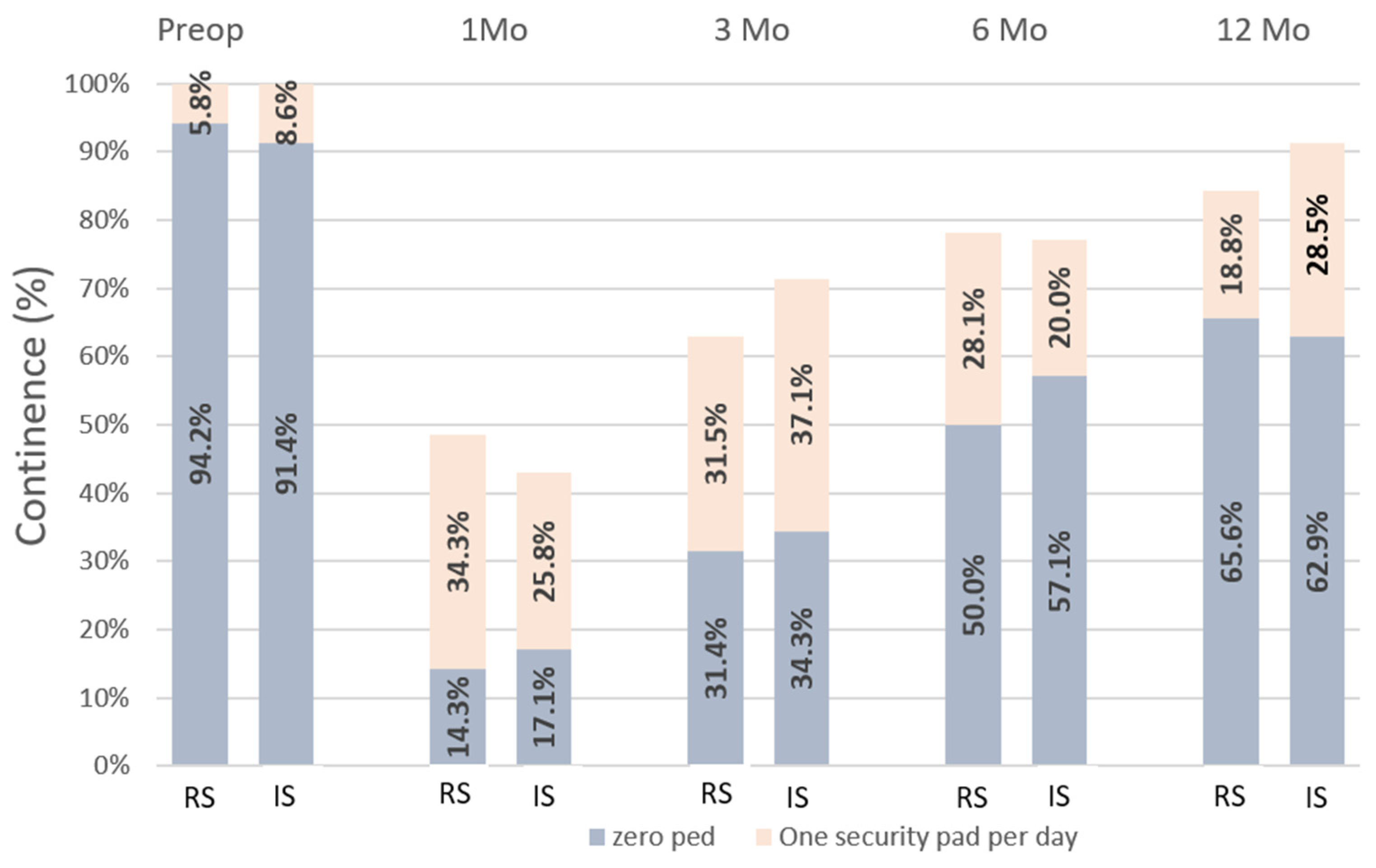
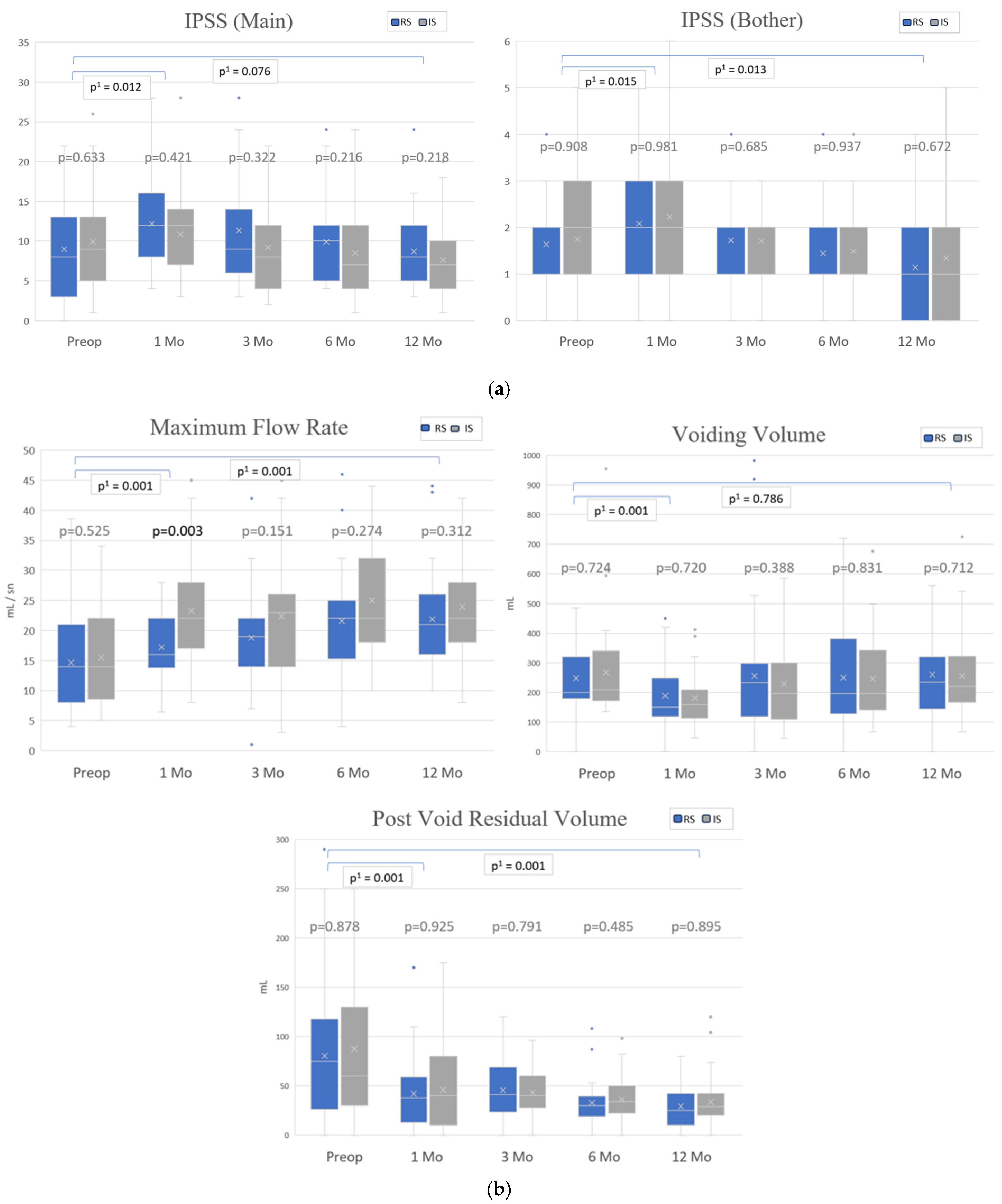
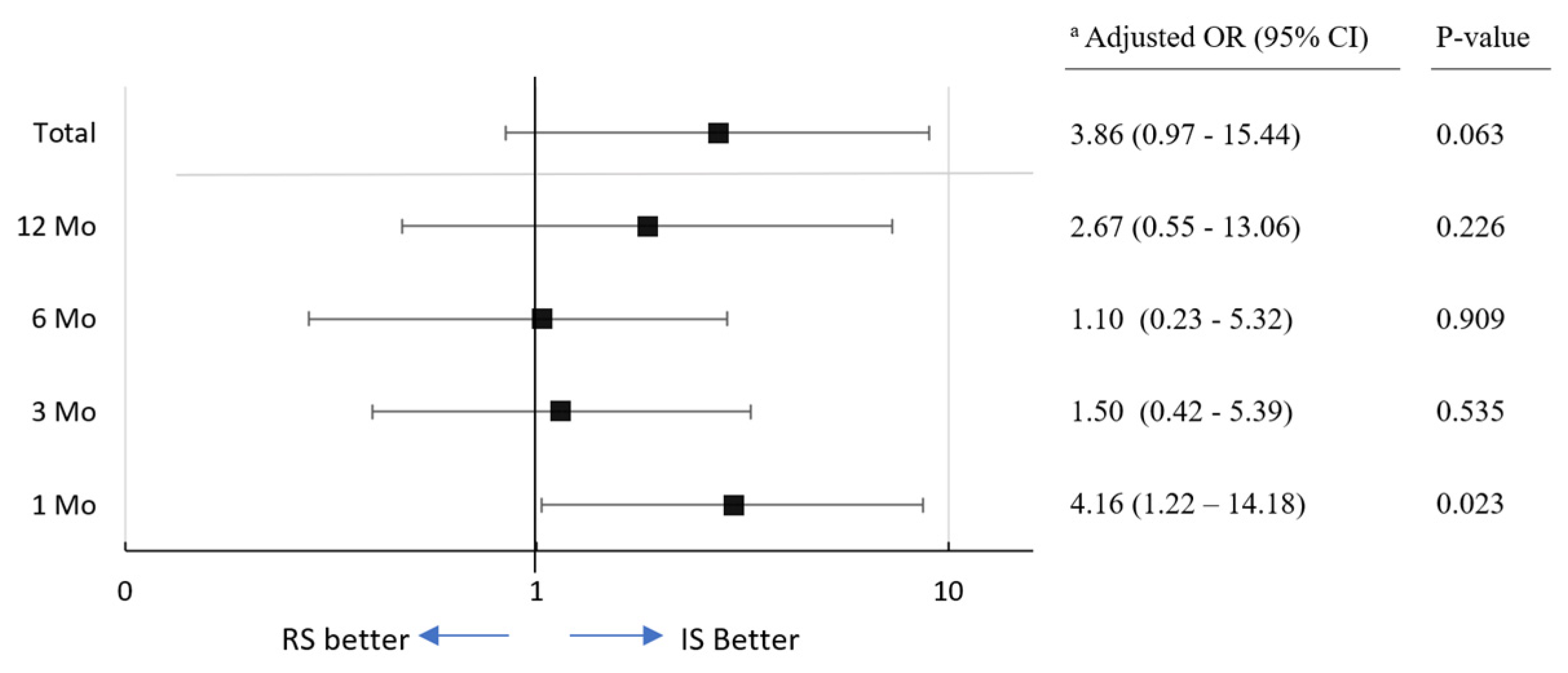
3.3. Continence Recovery, Uroflowmetry Parameters and International Prostate Symptom Scores
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Tewari, A.; Sooriakumaran, P.; Bloch, D.A.; Seshadri-Kreaden, U.; Hebert, A.E.; Wiklund, P. Positive surgical margin and perioperative complication rates of primary surgical treatments for prostate cancer: A systematic review and meta-analysis comparing retropubic, laparoscopic, and robotic prostatectomy. Eur. Urol. 2012, 62, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Yaxley, J.W.; Coughlin, G.D.; Chambers, S.K.; Occhipinti, S.; Samaratunga, H.; Zajdlewicz, L.; Dunglison, N.; Carter, R.; Williams, S.; Payton, D.J.; et al. Robot-assisted laparoscopic prostatectomy versus open radical retropubic prostatectomy: Early outcomes from a randomised controlled phase 3 study. Lancet 2016, 388, 1057–1066. [Google Scholar] [CrossRef] [PubMed]
- Su, L.M. Robot-assisted radical prostatectomy: Advances since 2005. Curr. Opin. Urol. 2010, 20, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Rassweiler-Seyfried, M.; Klein, J.; Stolzenburg, J.-U.; Rassweiler, J. The Past, Present and Future of Minimally Invasive Therapy in Urology: A Review and Speculative Outlook. J. Clin. Med. 2022, 11, 1056. [Google Scholar] [CrossRef]
- Begg, C.B.; Riedel, E.R.; Bach, P.B.; Kattan, M.W.; Schrag, D.; Warren, J.L.; Scardino, P.T. Variations in morbidity after radical prostatectomy. N. Engl. J. Med. 2002, 346, 1138–1144. [Google Scholar] [CrossRef]
- Coelho, R.F.; Palmer, K.J.; Rocco, B.; Moniz, R.R.; Chauhan, S.; Orvieto, M.A.; Coughlin, G.; Patel, V.R. Early complication rates in a single-surgeon series of 2500 robotic-assisted radical prostatectomies: Report applying a standardized grading system. Eur. Urol. 2010, 57, 945–952. [Google Scholar] [CrossRef]
- Wang, R.; Wood, D.P.; Hollenbeck, B.K.; Li, A.Y.; He, C.; Montie, J.E.; Latini, J.M. Risk factors and quality of life for post-prostatectomy vesicourethral anastomotic stenoses. Urology 2012, 79, 449–457. [Google Scholar] [CrossRef]
- Surya, B.V.; Provet, J.; Johanson, K.E.; Brown, J. Anastomotic strictures following radical prostatectomy: Risk factors and management. J. Urol. 1990, 143, 755–758. [Google Scholar] [CrossRef]
- Van Velthoven, R.; Ahlering, T.E.; Skarecky, D.W.; Huynh, L.; Clayman, R.V. Technique for Laparoscopic Running Urethrovesical Anastomosis: The Single Knot Method. Urology 2020, 145, 331–332. [Google Scholar] [CrossRef]
- Miki, T.; Okihara, K.; Ukimura, O.; Usijima, S.; Yoneda, K.; Mizutani, Y.; Kawauchi, A.; Koga, M.; Takeyama, M. Running suture for vesicourethral anastomosis in minilaparotomy radical retropubic prostatectomy. Urology 2006, 67, 410–412. [Google Scholar] [CrossRef]
- Kowalewski, K.F.; Tapking, C.; Hetjens, S.; Nickel, F.; Mandel, P.; Ritter, M.; Kriegmair, M.C. Interrupted versus continuous suturing for vesicourethral anastomosis during radical prostatectomy: Protocol for a systematic review and meta-analysis. BMJ Open 2017, 7, e019823. [Google Scholar] [CrossRef] [PubMed]
- Kowalewski, K.F.; Tapking, C.; Hetjens, S.; Nickel, F.; Mandel, P.; Nuhn, P.; Ritter, M.; Moul, J.W.; Thüroff, J.W.; Kriegmair, M.C. Interrupted versus Continuous Suturing for Vesicourethral Anastomosis During Radical Prostatectomy: A Systematic Review and Meta-analysis. Eur. Urol. Focus 2019, 5, 980–991. [Google Scholar] [CrossRef] [PubMed]
- Walsch, P.C. Anatomic radical prostatectomy: Evolution of the surgical technique. J. Urol. 1998, 160 Pt 2, 2418–2424. [Google Scholar] [CrossRef]
- Rocco, B.; Gregori, A.; Stener, S.; Santoro, L.; Bozzola, A.; Galli, S.; Knez, R.; Scieri, F.; Scaburri, A.; Gaboardi, F. Posterior reconstruction of the rhabdosphincter allows a rapid recovery of continence after transperitoneal videolaparoscopic radical prostatectomy. Eur. Urol. 2007, 51, 996–1003. [Google Scholar] [CrossRef]
- Calvert, M.; Blazeby, J.; Altman, D.G.; Revicki, D.A.; Moher, D.; Brundage, M.D. Reporting of patient-reported outcomes in randomized trials: The CONSORT PRO extension. JAMA 2013, 309, 814–822. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- De Barros, R.S.M.; Leal, R.A.; Teixeira, R.K.C.; Yamaki, V.N.; Feijó, D.H.; Gouveia, E.H.H.; Valente, A.L.; Feitosa-Junior, D.J.S.; De Carvalho, L.T.F. Continuous versus interrupted suture technique in microvascular anastomosis in rats. Acta Cir. Bras. 2017, 32, 691–696. [Google Scholar] [CrossRef]
- Brady, J.S.; Desai, S.V.; Crippen, M.M.; Eloy, J.A.; Gubenko, Y.; Baredes, S.; Park, R.C.W. Association of Anesthesia Duration with Complications After Microvascular Reconstruction of the Head and Neck. JAMA Facial Plast. Surg. 2018, 20, 188–195. [Google Scholar] [CrossRef]
- Groeben, C.; Koch, R.; Baunacke, M.; Wirth, M.P.; Huber, J. High volume is the key for improving in-hospital outcomes after radical prostatectomy: A total population analysis in Germany from 2006 to 2013. World J. Urol. 2017, 35, 1045–1053. [Google Scholar] [CrossRef]
- Ozu, C.; Hagiuda, J.; Nakagami, Y.; Hamada, R.; Horiguchi, Y.; Yoshioka, K.; Nakashima, J.; Hatano, T.; Tachibana, M. Radical retropubic prostatectomy with running vesicourethral anastomosis and early catheter removal: Our experience. Int. J. Urol. Off. J. Jpn. Urol. Assoc. 2009, 16, 487–492. [Google Scholar] [CrossRef]
- Lim, J.H.; Park, C.M.; Kim, H.K.; Park, J.Y. Comparison of perioperative outcomes between running versus interrupted vesicourethral anastomosis in open radical prostatectomy: A single-surgeon experience. Korean J. Urol. 2015, 56, 443–448. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Teber, D.; Erdogru, T.; Cresswell, J.; Gözen, A.S.; Frede, T.; Rassweiler, J.J. Analysis of three different vesicourethral anastomotic techniques in laparoscopic radical prostatectomy. World J. Urol. 2008, 26, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Massoud, W.; Thanigasalam, R.; El Hajj, A.; Girard, F.; Théveniaud, P.E.; Chatellier, G.; Baumert, H. Does the use of a barbed polyglyconate absorbable suture have an impact on urethral anastomosis time, urethral stenosis rates, and cost effectiveness during robot-assisted radical prostatectomy? Urology 2013, 82, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Han, D.H.; Lee, K.S.; Jeon, S.S. Effect of Continuous Urethro-Vesical Anastomosis Technique in Incontinence After Radical Retropubic Prostatectomy, 1:1 Matching Study. Int. Neurourol. J. 2015, 19, 113–119. [Google Scholar] [CrossRef]
- Britton, M.T.; Mehta, M.; Liss, M.A.; Pearce, S.M.; O’Neil, B.; Skolarus, T.A. Long-term outcomes of vesicourethral anastomotic stricture after radical prostatectomy: A national analysis of 18,000 men. J. Urol. 2023, 210, 312–322. [Google Scholar] [CrossRef]
- Ficarra, V.; Novara, G.; Rosen, R.C.; Artibani, W.; Carroll, P.R.; Costello, A.; Menon, M.; Montorsi, F.; Patel, V.R.; Stolzenburg, J.-U.; et al. Systematic review and meta-analysis of studies reporting urinary continence recovery after robot-assisted radical prostatectomy. Eur. Urol. 2012, 62, 405–417. [Google Scholar] [CrossRef]
- Matsuyama, H.; Matsumoto, H.; Nagao, K.; Harada, N.; Hara, T.; Sakano, S. Running suture versus interrupted suture for vesicourethral anastomosis in retropubic radical prostatectomy: A randomized study. Int. J. Urol. Off. J. Jpn. Urol. Assoc. 2015, 22, 271–277. [Google Scholar] [CrossRef]
- Coughlin, G.D.; Yaxley, J.W.; Chambers, S.K.; Occhipinti, S.; Samaratunga, H.; Zajdlewicz, L.; Teloken, P.; Dunglison, N.; Williams, S.; Lavin, M.F.; et al. Robot-assisted laparoscopic prostatectomy versus open radical retropubic prostatectomy: 24-month outcomes from a randomised controlled study. Lancet Oncol. 2018, 19, 1051–1060. [Google Scholar] [CrossRef]
- Masters, J.G.; Rice, M.L. Improvement in urinary symptoms after radical prostatectomy: A prospective evaluation of flow rates and symptom scores. BJU Int. 2003, 91, 795–797. [Google Scholar] [CrossRef]
- Shandall, A.; Lowndes, R.; Young, H.L. Colonic anastomotic healing and oxygen tension. Br. J. Surg. 1985, 72, 606–609. [Google Scholar] [CrossRef]
- Bayoud, Y.; de la Taille, A.; Ouzzane, A.; Ploussard, G.; Allory, Y.; Yiou, R.; Vordos, D.; Hoznek, A.; Salomon, L. International Prostate Symptom Score is a predictive factor of lower urinary tract symptoms after radical prostatectomy. Int. J. Urol. Off. J. Jpn. Urol. Assoc. 2015, 22, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Walker, N.F.; Canagasingham, A.; Van Diepen, D.; Pirpiris, A.; Tse, V.; Leslie, S.; Thanigasalam, R.; Chan, L. Lower Urinary Tract Functional Assessment of Men Undergoing Radical Prostatectomy: Correlation of Preoperative Clinical and Urodynamic Parameters. Int. Neurourol. J. 2021, 25, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, V.; Taksler, G.B.; Sivarajan, G.; Laze, J.; Makarov, D.V.; Lepor, H. Radical prostatectomy improves and prevents age dependent progression of lower urinary tract symptoms. J. Urol. 2014, 191, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Flammia, R.S.; Tuderti, G.; Brassetti, A.; Anceschi, U.; Lugnani, F.; Ferriero, M.; Gallucci, M.; Simone, G. A Step-by-Step Approach for Vesicourethral Anastomosis in Robot-Assisted Radical Prostatectomy: A Single Knot Single Running Suture Technique. J. Pers. Med. 2023, 13, 1072. [Google Scholar] [CrossRef]
- Cabello, J.M.; Benway, B.M.; Bhayani, S.B. Reducing the number of sutures for vesicourethral anastomosis in radical retropubic prostatectomy. Int. Braz. J. Urol. 2009, 35, 158–163. [Google Scholar] [CrossRef]
- Ko, Y.H.; Kang, S.G.; Lim, J.H.; Lee, S.H. Application of barbed suture for vesicourethral anastomosis during robot-assisted radical prostatectomy. J. Endourol. 2021, 35, 223–229. [Google Scholar]
| Running | Interrupted | p Value | ||
|---|---|---|---|---|
| No.pts | 35 | 35 | ||
| Demographics and clinical findings | ||||
| Age (yr), mean (SD) | 63.4 ± 6.9 | 65.3 ± 6.5 | 0.218 | |
| BMI (kg/m2), mean (SD) | 26.3 ± 3.7 | 26 ± 3 | 0.538 | |
| PSA (ng/dL), median (IQR) | 8.2 (11.6) | 7.8 (5.1) | 0.378 | |
| CCI score, n (%) | 0.780 | |||
| ≤3 | 27 (77) | 26 (74) | ||
| >3 | 8 (23) | 9 (26) | ||
| Biopsy ISUP, n (%) | 1 | |||
| ≤2 | 29 (83) | 29 (83) | ||
| >2 | 6 (17) | 6 (17) | ||
| Number of positive biopsy cores, n (%) | 0.626 | |||
| ≤3 | 20 (57) | 22 (63) | ||
| >3 | 15 (43) | 13 (37) | ||
| D’amico risk classification, n (%) | 0.328 | |||
| Low | 15 (43) | 11 (31) | ||
| Intermediate | 16 (46) | 22 (6) | ||
| High | 4 (11) | 2 (6) | ||
| Intraoperative findings | ||||
| Amount of bleeding (cc), mean (SD) | 664 ± 316 | 631 ± 267 | 0.731 | |
| Operative time (min), mean (SD) | 167 ± 28 | 187 ± 35 | 0.003 | |
| Anastomosis time (min), median (IQR) | 21 (6) | 33 (8) | 0.001 | |
| PLND, n (%) | 24 (69) | 27 (77) | 0.420 | |
| Nerve-sparing surgery, n | 0.513 | |||
| None | 7 | 11 | ||
| Unilateral | 8 | 8 | ||
| Bilateral | 20 | 16 | ||
| Postoperative findings | ||||
| Total amount of drainage (mL), median (IQR) | 300 (140) | 205 (561) | 0.106 | |
| Hospital stay (days), median (IQR) | 4 (1) | 4 (2) | 0.345 | |
| Catheterization time (days), median (IQR) | 5 (1) | 5 (5) | 0.860 | |
| No extravasation on the first cystogram, n (%) | 32 (91) | 27 (77) | 0.101 | |
| Total follow-up period (months), median (IQR) | 16 (8) | 17 (8) | 0.946 | |
| Pathological results and BCR | ||||
| Prostate specimen weight (g), mean (SD) | 47 ± 19 | 58 ± 20 | 0.463 | |
| Pathological stage, n (%) | 0.020 | |||
| T2 | 25 (71) | 16 (46) | ||
| T3a | 5 (14) | 18 (51) | ||
| T3b | 5 (14) | 1 (3) | ||
| Positive surgical margin, n (%) | 9 (26) | 14 (40) | 0.203 | |
| No.pts with one-year biochemical recurrence-free, n (%) | 31 (89) | 32 (91) | 0.690 | |
| No.pts receiving radiotherapy (Salvage or adjuvant), n (%) | 4 (11) | 4 (11) | 1 | |
| Running n = 35 | Interrupted n = 35 | p Value | Intervention | ||
|---|---|---|---|---|---|
| Clavien grade/Complication Intraoperative | |||||
| II | Bleeding | 6 (17%) | 4 (12%) | 0.721 | Blood Transfusion |
| Postoperative short period (≤6 weeks) | |||||
| I | Wound infection | 2 (6%) | 2 (6%) | 1 | Antibiotherapy, bedside intervention |
| Prolonged drainage | 0 (0%) | 1 (3%) | 0.314 | Long-time urethral catheterization | |
| Acute urinary retention | 2 (6%) | 0 (0%) | 0.151 | Urethral catheterization | |
| Id | Anastomotic leakage on the first cystourethrogram | 3 (9%) | 8 (23%) | 0.101 | Long-time urethral catheterization |
| II | Urinary infection | 2 (6%) | 2 (6%) | 1 | Antibiotherapy |
| Hematuria | 4 (12%) | 5 (14%) | 0.721 | Conservative approach | |
| Lymphorrhoea | 1 (3%) | 0 (0%) | 0.314 | Suction drainage | |
| Postoperative long period (>6 weeks) | |||||
| IIIa | Anastomotic stricture | 4 (12%) | 3 (9%) | 0.690 | Endoscopic bladder neck incision |
| Total (At least one complication) | 21 (60%) | 20 (57%) | 0.808 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Can, U.; Dinçer, E.; Coşkun, A.; Mert, M.S.; Çanakçı, C.; Göktaş, C. A Pragmatic Randomized Trial Comparing Suturing Techniques for Vesicourethral Anastomosis: One-Year Voiding Function Outcomes After Radical Prostatectomy. J. Clin. Med. 2025, 14, 3934. https://doi.org/10.3390/jcm14113934
Can U, Dinçer E, Coşkun A, Mert MS, Çanakçı C, Göktaş C. A Pragmatic Randomized Trial Comparing Suturing Techniques for Vesicourethral Anastomosis: One-Year Voiding Function Outcomes After Radical Prostatectomy. Journal of Clinical Medicine. 2025; 14(11):3934. https://doi.org/10.3390/jcm14113934
Chicago/Turabian StyleCan, Utku, Erdinç Dinçer, Alper Coşkun, Mahmut Selman Mert, Cengiz Çanakçı, and Cemal Göktaş. 2025. "A Pragmatic Randomized Trial Comparing Suturing Techniques for Vesicourethral Anastomosis: One-Year Voiding Function Outcomes After Radical Prostatectomy" Journal of Clinical Medicine 14, no. 11: 3934. https://doi.org/10.3390/jcm14113934
APA StyleCan, U., Dinçer, E., Coşkun, A., Mert, M. S., Çanakçı, C., & Göktaş, C. (2025). A Pragmatic Randomized Trial Comparing Suturing Techniques for Vesicourethral Anastomosis: One-Year Voiding Function Outcomes After Radical Prostatectomy. Journal of Clinical Medicine, 14(11), 3934. https://doi.org/10.3390/jcm14113934





