Assessment of Bone Health in Adult Patients with Inflammatory Bowel Disease: A Single-Center Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Clinical Evaluation and Bone Assessment Techniques
2.3. Statistical Analysis
3. Results
3.1. Characteristics of the Study Populations
3.2. Correlations Between Bone Assessment Techniques
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IBD | inflammatory bowel disease |
| DXA | dual-energy X-ray absorptiometry |
| aBMD | areal bone mineral density |
| VFA | vertebral fracture assessment |
| TBS | trabecular bone score |
| QUS | calcaneal quantitative ultrasound |
| vBMD | volumetric bone mineral density |
| UC | ulcerative colitis |
| CD | Crohn’s disease |
| BUA | broadband ultrasound attenuation |
| TNF-α | tumor necrosis factor alpha |
| IL | interleukin |
| BMD | bone mineral density |
| BMI | body mass index |
| HF | hip fracture |
| MOF | major osteoporotic fractures |
| CV | coefficient of variation |
| vBMD | volumetric bone mineral density |
| LSC | least significant changes |
| SOS | speed of sound |
| BQI | bone quality index |
| iPTH | intact parathyroid hormone |
| ß-CTx | ß-isomer of carboxy-terminal telopeptide of type I collagen |
| P1NP | N-terminal propeptide of type I procollagen |
| SD | standard deviation |
| IQR | interquartile range |
References
- Silverberg, M.S.; Satsangi, J.; Ahmad, T.; Arnott, I.D.; Bernstein, C.N.; Brant, S.R.; Caprilli, R.; Colombel, J.F.; Gasche, C.; Geboes, K.; et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: Report of a working party of the 2005 Montreal world congress of gastroenterology. Can. J. Gastroenterol. 2005, 19 (Suppl. A), 5A–36A. [Google Scholar] [CrossRef] [PubMed]
- Szafors, P.; Che, H.; Barnetche, T.; Morel, J.; Gaujoux-Viala, C.; Combe, B.; Lukas, C. Risk of fracture and low bone mineral density in adults with inflammatory bowel diseases. A systematic literature review with meta-analysis. Osteoporos. Int. 2018, 29, 2389–2397. [Google Scholar] [CrossRef] [PubMed]
- Komaki, Y.; Komaki, F.; Micic, D.; Ido, A.; Sakuraba, A. Risk of fractures in inflammatory bowel diseases: A systematic review and meta-Analysis. J. Clin. Gastroenterol. 2019, 53, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Kärnsund, S.; Lo, B.; Bendtsen, F.; Holm, J.; Burisch, J. Systematic review of the prevalence and development of osteoporosis or low bone mineral density and its risk factors in patients with inflammatory bowel disease. World J. Gastroenterol. 2020, 26, 5362–5374. [Google Scholar] [CrossRef]
- Lo, B.; Holm, J.P.; Vester-Andersen, M.K.; Bendtsen, F.; Vind, I.; Burisch, J. Incidence, risk factors and evaluation of osteoporosis in patients with inflammatory bowel disease: A Danish population-based inception cohort with 10 years of follow-up. J. Crohns Colitis. 2020, 14, 904–914. [Google Scholar] [CrossRef]
- Van Bodegraven, A.A.; Bravenboer, N. Perspective on skeletal health in inflammatory bowel disease. Osteoporos. Int. 2020, 31, 637–646. [Google Scholar] [CrossRef]
- Targownik, L.E.; Bernstein, C.N.; Leslie, W.D. Risk factors and management of osteoporosis in inflammatory bowel disease. Curr. Opin. Gastroenterol. 2014, 30, 168–174. [Google Scholar] [CrossRef]
- Briot, K.; Geusens, P.; Em Bultink, I.; Lems, W.F.; Roux, C. Inflammatory diseases and bone fragility. Osteoporos. Int. 2017, 28, 3301–3314. [Google Scholar] [CrossRef]
- Vestergaard, P.; Mosekilde, L. Fracture risk in patients with celiac disease, Crohn’s disease, and ulcerative colitis: A nationwide follow-up study of 16,416 patients in Denmark. Am. J. Epidemiol. 2002, 156, 1–10. [Google Scholar] [CrossRef]
- Ewid, M.; Mutiri, N.A.; Omar, K.A.; Shamsan, A.N.; Rathore, A.A.; Saquib, N.; Salaas, A.; Al Sarraj, O.; Nasri, Y.; Attal, A.; et al. Updated bone mineral density status in Saudi patients with inflammatory bowel disease. World J. Gastroenterol. 2020, 26, 5343–5353. [Google Scholar] [CrossRef]
- Jahnsen, J.; Falch, J.A.; Aadland, E.; Mowinckel, P. Bone mineral density is reduced in patients with Crohn’s disease but not in patients with ulcerative colitis: A population based study. Gut 1997, 40, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Rosen, H.N.; Vokes, T.J.; Malabanan, A.O.; Deal, C.L.; Alele, J.D.; Olenginski, T.P.; Schousboe, J.T. The official positions of the international society for clinical densitometry: Vertebral fracture assessment. J. Clin. Densitom. 2013, 16, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Beck, T.J.; Broy, S.B. Measurement of hip geometry—Technical background. J. Clin. Densitom. 2015, 18, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Humbert, L.; Martelli, Y.; Fonolla, R.; Steghofer, M.; Di Gregorio, S.; Malouf, J.; Romera, J.; Barquero, L.M. 3D-DXA: Assessing the femoral shape, the trabecular macrostructure and the cortex in 3D from DXA images. IEEE Trans. Med. Imaging. 2017, 36, 27–39. [Google Scholar] [CrossRef]
- Silva, B.C.; Leslie, W.D.; Resch, H.; Lamy, O.; Lesnyak, O.; Binkley, N.; McCloskey, E.V.; Kanis, J.A.; Bilezikian, J.P. Trabecular bone score: A noninvasive analytical method based upon the DXA image. J. Bone Miner. Res. 2014, 29, 518–530. [Google Scholar] [CrossRef]
- Marín, F.; González-Macías, J.; Díez-Pérez, A.; Palma, S.; Delgado-Rodríguez, M. Relationship between bone quantitative ultrasound and fractures: A meta-analysis. J. Bone Miner. Res. 2006, 21, 1126–1135. [Google Scholar] [CrossRef]
- Hans, D.; Goertzen, A.L.; Krieg, M.A.; Leslie, W.D. Bone microarchitecture assessed by TBS predicts osteoporotic fractures independent of bone density: The Manitoba study. J. Bone Miner. Res. 2011, 26, 2762–2769. [Google Scholar] [CrossRef]
- McCloskey, E.V.; Kanis, J.A.; Odén, A.; Harvey, N.C.; Bauer, D.; González-Macias, J.; Hans, D.; Kaptoge, S.; Krieg, M.A.; Kwok, T.; et al. Predictive ability of heel quantitative ultrasound for incident fractures: An individual-level meta-analysis. Osteoporos. Int. 2015, 26, 1979–1987. [Google Scholar] [CrossRef] [PubMed]
- Krajcovicova, A.; Kuzma, M.; Hlavaty, T.; Hans, D.; Koller, T.; Jackuliak, P.; Leskova, Z.; Sturdik, I.; Killinger, Z.; Payer, J. Decrease of trabecular bone score reflects severity of Crohn’s disease: Results of a case–control study. Eur. J. Gastroenterol. Hepatol. 2018, 30, 101–106. [Google Scholar] [CrossRef]
- Soare, I.; Sirbu, A.; Martin, S.; Diculescu, M.; Mateescu, B.; Tieranu, C.; Fica, S. Assessment of bone quality with trabecular bone score in patients with inflammatory bowel disease. Sci. Rep. 2021, 11, 20345–20352. [Google Scholar] [CrossRef]
- Humbert, L.; Winzenrieth, R.; Di Gregorio, S.; Thomas, T.; Vico, L.; Malouf, J.; Del Río Barquero, L.M. 3D analysis of cortical and trabecular bone from hip DXA: Precision and trend assessment interval in postmenopausal women. J. Clin. Densitom. 2019, 22, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Hong, A.R.; Kim, J.H.; Kim, S.W.; Kim, S.Y.; Shin, C.S. Trabecular bone score as a skeletal fragility index in acromegaly patients. Osteoporos. Int. 2016, 27, 1123–1129. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Lee, H.Y.; Yoon, D.; Kim, A.; Shin, Y.S.; Park, H.S.; Ye, Y.M. Trabecular bone score is more sensitive to asthma severity and glucocorticoid treatment than bone mineral density in asthmatics. Allergy Asthma Immunol. Res. 2019, 11, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Kužma, M.; Vaňuga, P.; Binkley, N.; Ságová, I.; Pávai, D.; Blažíček, P.; Kužmová, Z.; Jackuliak, P.; Vaňuga, A.; Killinger, Z.; et al. High serum fractalkine is associated with lower trabecular bone score in premenopausal women with Graves’ disease. Horm. Metab. Res. 2018, 50, 609–614. [Google Scholar] [CrossRef]
- Catalano, A.; Gaudio, A.; Agostino, R.M.; Morabito, N.; Bellone, F.; Lasco, A. Trabecular bone score and quantitative ultrasound measurements in the assessment of bone health in breast cancer survivors assuming aromatase inhibitors. J. Endocrinol. Investig. 2019, 42, 1337–1343. [Google Scholar] [CrossRef]
- Marques, J.V.O.; Nalevaiko, J.Z.; Oliveira, M.F.; Raetsch, A.W.P.; Marques, G.L.; Petterle, R.R.; Moreira, C.A.; Borba, V.Z.C. Trabecular bone score (TBS) and bone mineral density in patients with long-term therapy with warfarin. Arch. Osteoporos. 2020, 15, 102–108. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kang, K.Y.; Shin, J.; Jun, Y.; Kim, S.I.; Kim, Y.R. Trabecular bone scores in young HIV-infected men: A matched case-control study. BMC Musculoskelet. Disord. 2020, 21, 94–101. [Google Scholar] [CrossRef]
- Stachowska, B.; Halupczok-Żyła, J.; Kuliczkowska-Płaksej, J.; Syrycka, J.; Bolanowski, M. Decreased trabecular bone score in patients with active endogenous Cushing’s syndrome. Front. Endocrinol. 2021, 11, 593173. [Google Scholar] [CrossRef]
- Banaszkiewicz, K.; Sikorska, K.; Panas, D.; Sworczak, K. The role of the trabecular bone score in the assessment of osteoarticular disorders in patients with HFE-hemochromatosis: A single-center study from Poland. Genes 2021, 12, 1304. [Google Scholar] [CrossRef]
- Yokomoto-Umakoshi, M.; Umakoshi, H.; Sakamoto, R.; Fukumoto, T.; Ogata, M.; Nakano, Y.; Iwahashi, N.; Kaneko, H.; Mizoguchi, N.; Hattori, A.; et al. Role of deteriorated bone quality in the development of osteoporosis in pheochromocytoma and paraganglioma. Bone 2021, 142, 115607–115612. [Google Scholar] [CrossRef]
- Olmos-Martínez, J.M.; Hernández, J.L.; Fábrega, E.; Olmos, J.M.; Crespo, J.; González-Macías, J. Bone mineral density and trabecular bone score in treatment-naïve patients with non-cirrhotic hepatitis C virus infection. Arch. Osteoporos. 2020, 15, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Calatayud, M.; Pérez-Olivares Martín, L.; Librizzi, S.; Lora Pablos, D.; González Méndez, V.; Aramendi Ramos, M.; Martínez Diaz-Guerra, G.; Hawkins, F. Trabecular bone score and bone mineral density in patients with long-term controlled acromegaly. Clin. Endocrinol. 2021, 95, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.H.; Jeon, Y.K.; Pak, K.; Kang, T.; Kim, K.E.; Kim, S.J.; Kim, I.J.; Kim, K. Effect of tamoxifen with or without gonadotropin-releasing hormone analog on DXA values in women with breast cancer. Sci. Rep. 2021, 11, 3407. [Google Scholar] [CrossRef]
- Levy-Shraga, Y.; Megnazi, O.; Modan-Moses, D.; Tripto-Shkolnik, L.; Gruber, N.; Haberman, Y.; Shouval, D.S.; Weiss, B. Trabecular bone score in children and adolescents with inflammatory bowel diseases. J. Clin. Densitom. 2021, 24, 243–251. [Google Scholar] [CrossRef]
- Pepe, J.; Zawadynski, S.; Herrmann, F.R.; Juillerat, P.; Michetti, P.; Ferrari-Lacraz, S.; Belli, D.; Ratib, O.; Rizzoli, R.; Chevalley, T.; et al. Structural basis of bone fragility in young subjects with inflammatory bowel disease: A high-resolution pQCT study of the SWISS IBD Cohort (SIBDC). Inflamm. Bowel Dis. 2017, 23, 1410–1417. [Google Scholar] [CrossRef]
- Robinson, R.J.; Carr, I.; Lqbal, S.J.; AI-Azzawi, F.; Abrams, K.; Mayberry, J.F. Screening for osteoporosis in Crohn’s disease. A detailed evaluation of calcaneal ultrasound. Eur. J. Gastroenterol. Hepatol. 1998, 10, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Fries, W.; Dinca, M.; Luisetto, G.; Peccolo, F.; Bottega, F.; Martin, A. Calcaneal ultrasound bone densitometry in inflammatory bowel disease--a comparison with double X-ray densitometry of the lumbar spine. Am. J. Gastroenterol. 1998, 93, 2339–2344. [Google Scholar] [CrossRef]
- Turk, N.; Kastelan, D.; Cukovic-Cavka, S.; Kraljevic, I.; Korsic, M.; Vucelic, B. Discriminatory ability of calcaneal quantitative ultrasound in the assessment of bone status in patients with inflammatory bowel disease. Ultrasound Med. Biol. 2007, 33, 863–869. [Google Scholar] [CrossRef][Green Version]
- Wear, K.A. Mechanisms of interaction of ultrasound with cancellous bone: A review. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2020, 67, 454–482. [Google Scholar] [CrossRef]
- Métrailler, A.; Hans, D.; Lamy, O.; Gonzalez Rodriguez, E.; Shevroja, E. Heel quantitative ultrasound (QUS) predicts incident fractures independently of trabecular bone score (TBS), bone mineral density (BMD), and FRAX: The OsteoLaus Study. Osteoporos. Int. 2023, 34, 1401–1409. [Google Scholar] [CrossRef]
- Olmos, J.M.; Hernández, J.L.; Pariente, E.; Martínez, J.; Valero, C.; González-Macías, J. Trabecular bone score and bone quantitative ultrasound in Spanish postmenopausal women. The Camargo Cohort Study. Maturitas 2020, 132, 24–29. [Google Scholar] [CrossRef]
- Jahnsen, J.; Falch, J.A.; Mowinck, P. Ultrasound measurements of calcaneus for estimation of skeletal status in patients with inflammatory bowel disease. Scand. J. Gastroenterol. 1999, 34, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Heijckmann, A.C.; Dumitrescu, B.; Nieuwenhuijzen Kruseman, A.C.; Geusens, P.; Wolffenbuttel, B.H.; De Vries, J.; Drent, M.; Huijberts, M.S. Quantitative ultrasound does not identify patients with an inflammatory disease at risk of vertebral deformities. BMC Musculoskelet. Disord. 2008, 9, 72. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, D.A.; Connolley, C.D.; Koyama, T.; Wise, P.E.; Herline, A.J. Calcaneal ultrasound bone densitometry is not a useful tool to screen patients with inflammatory bowel disease at high risk for metabolic bone disease. Inflamm. Bowel Dis. 2005, 11, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Montalbán, B.; Arias, Á.; Friginal-Ruiz, A.B.; Lucendo, A.J. The use of the fracture risk assessment (FRAX®) tool in predicting risk of fractures in patients with inflammatory bowel disease: A systematic review. J. Clin. Densitom. 2017, 20, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Vihinen, M.K.; Kolho, K.L.; Ashorn, M.; Verkasalo, M.; Raivio, T. Bone turnover and metabolism in paediatric patients with inflammatory bowel disease treated with systemic glucocorticoids. Eur. J. Endocrinol. 2008, 159, 693–698. [Google Scholar] [CrossRef]
- Tulewicz-Marti, E.; Szwarc, P.; Więcek, M.; Lewandowski, K.; Korcz, T.; Cicha, M.; Rydzewska, G. Effect of intravenous iron administration on bone mineral and iron homeostasis in patients with inflammatory bowel disease—Results of a prospective single-centre study. J. Pers. Med. 2023, 13, 458. [Google Scholar] [CrossRef]

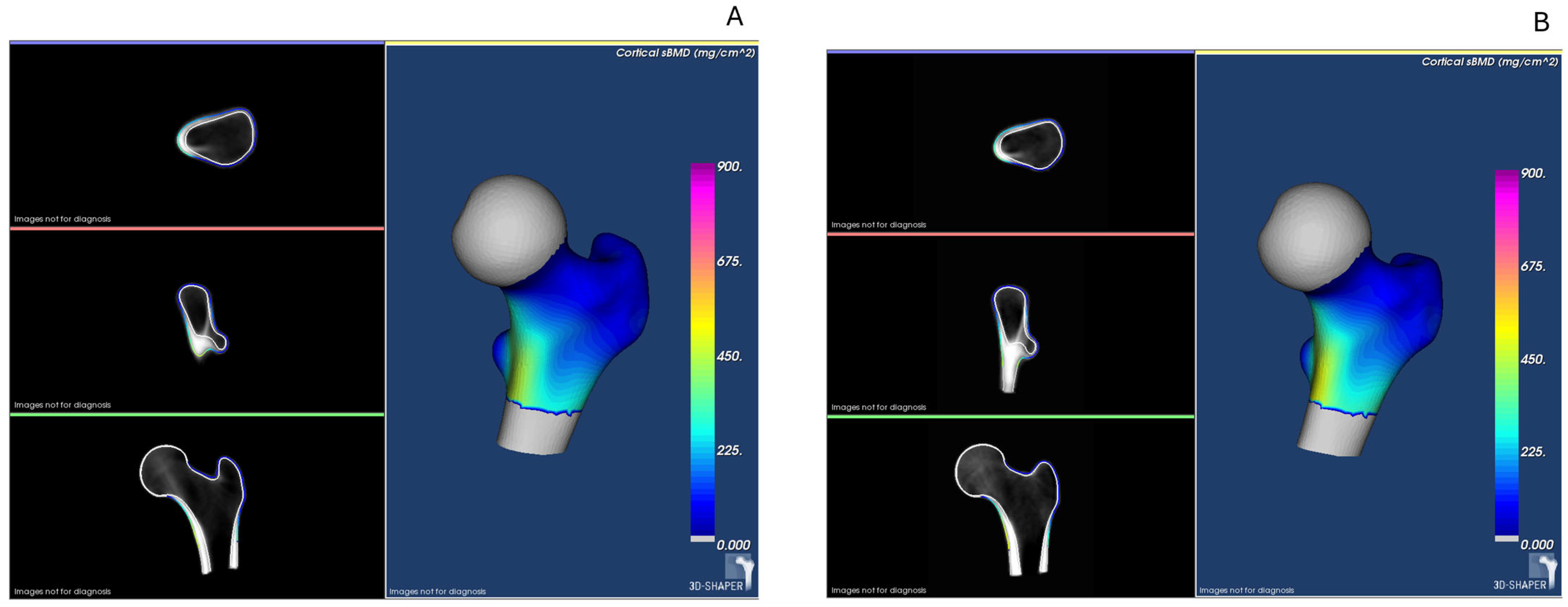
| Variables | IBD (n = 50) | UC (n = 29) | CD (n = 21) | Controls (n = 50) | p Value (IBD vs. Controls) | p Value Controls vs. UC vs. CD |
|---|---|---|---|---|---|---|
| Demographic Characteristics | ||||||
| Sex (male/female) | 20/30 | 12/17 | 8/13 | 20/30 | 1.000 | 0.973 |
| Age (years) | 52.0 ± 13.3 | 50.4 ± 11.9 | 54.3 ± 15.1 | 52.0 ± 14.2 | 0.988 | 0.608 |
| BMI (kg/m2) | 25.4 ± 4.1 | 25.6 ± 4.4 | 25.0 ± 3.8 | 26.1 ± 4.3 | 0.387 | 0.604 |
| Weight (kg) | 72.7 ± 16.4 | 74.2 ± 17.1 | 70.7 ± 15.4 | 74.0 ± 15.8 | 0.687 | 0.693 |
| Height (cm) | 168.6 ± 10.5 | 169.4 ± 9.9 | 167.5 ± 11.5 | 167.9 ± 9.5 | 0.721 | 0.756 |
| Risks Factors | ||||||
| Currently smokers | 9 (18) | 5 (17) | 4 (19) | 8 (16) | 0.790 | 0.099 |
| Alcohol ≥3 units/day | 0 (0) | 0 (0) | 0(0) | 3 (6) | 0.242 | 1.000 |
| Menopause | 13 (43) | 7 (41) | 6 (46) | 13 (43) | 1.000 | 0.964 |
| Previous fracture | 3 (6) | 1 (3) | 2 (10) | 0 (0) | 0.242 | 0.098 |
| Hip fracture history in parents | 3 (6) | 2 (7) | 1 (5) | 5 (10) | 0.715 | 1.000 |
| Use of calcium supplement | 5 (10) | 2 (7) | 3 (14) | 3 (6) | 0.715 | 1.000 |
| Use of vitamin D supplement | 19 (38) | 9 (31) | 10 (48) | 21 (42) | 0.683 | 0.458 |
| Calcium intake (mg/day) | 837.9 ± 357.1 | 762.6 ± 223.2 | 941.8 ± 472.4 | 854.2 ± 277.8 | 0.799 | 0.141 |
| Laboratory findings | ||||||
| Albumin (g/dL) | 4.60 (4.40; 4.70) | 4.60 (4.40; 4.70) | 4.60 (4.50; 4.70) | 4.80 (4.60; 4.90) | < 0.001 | 0.003 |
| Albumin corrected calcium (mg/dL) | 9.19 ± 0.40 | 9.23 ± 0.32 | 9.14 ± 0.50 | 9.00 ± 0.33 | 0.012 | 0.029 |
| Phosphorus (mg/dL) | 3.6 ± 0.5 | 3.6 ± 0.5 | 3.4 ± 0.5 | 3.4 ± 0.4 | 0.220 | 0.121 |
| Magnesium (mg/dL) | 2.0 (1.9; 2.2) | 2.0 (1.9; 2.1) | 2.1 (1.9; 2.2) | 2.1 (2.0; 2.2) | 0.273 | 0.230 |
| GFR (mL/min/1.73 m2) | 94.5 ± 17.9 | 97.3 ± 14.2 | 90.7 ± 21.9 | 91.1 ± 16.0 | 0.313 | 0.234 |
| iPTH (pg/mL) | 52.8 ± 20.7 | 49.1 ± 19.6 | 57.8 ± 21.5 | 52.2 ± 15.1 | 0.887 | 0.219 |
| ß-CTX (ng/mL) | 0.340 (0.219; 0.444) | 0.340 (0.230; 0.420) | 0.350 (0.210; 0.440) | 0.335 (0.250; 0.460) | 0.634 | 0.870 |
| P1NP (ng/mL) | 43.7 (35.8; 69.5) | 43.6 (37.3; 70.6) | 44.4 (30.8; 57.3) | 36.9 (33.5; 54.3) | 0.104 | 0.207 |
| 25-OH-VitaminD (ng/mL) | 24.0 (21.0; 32.0) | 24.5 (21.0; 33.5) | 24.0 (20.0; 32.0) | 29.0 (20.0; 37.0) | 0.314 | 0.518 |
| Alkaline phosphatase (UI/L) | 63.5 (53.0; 83.0) | 59.0 (54.0; 82.0) | 67.0 (48.0; 83.0) | 64.0 (55.0; 73.0) | 0.809 | 0.972 |
| Urinary calcium/creatinine (mg/g) | 0.11 (0.05; 0.20) | 0.13 (0.08; 0.24) | 0.07 (0.05; 0.16) | 0.14 (0.10; 0.21) | 0.378 | 0.218 |
| FRAX * | IBD (n = 41) | UC (n = 24) | CD (n = 17) | Controls (n = 40) | ||
| Hip fracture (%) | 0.8 (0.1; 1.1) | 0.3 (0.1; 0.6) | 0.8 (0.4; 1.3) | 0.4 (0.1; 0.7) | 0.328 | 0.045 |
| MOF (%) | 2.2 (1.6; 4.2) | 1.8 (1.4; 3.1) | 3.1 (2.2; 4.3) | 2.2 (1.4; 3.5) | 0.499 | 0.050 |
| TBS Hip Fracture (%) | 0.3 (0.1; 1.1) | 0.3 (0.1; 0.5) | 0.9 (0.2; 1.8) | 0.4 (0.1; 0.8) | 0.559 | 0.066 |
| TBS MOF (%) | 2.7 (1.7; 4.7) | 2.4 (1.5; 4.0) | 4.2 (2.1; 5.7) | 2.6 (1.6; 4.3) | 0.441 | 0.128 |
| Variables | IBD (n = 50) | UC (n = 29) | CD (n = 21) |
|---|---|---|---|
| Disease duration (years) | 10.6 ± 11.7 | 11.3 ± 11.3 | 9.6 (12.4) |
| Therapies at baseline n (%) | |||
| Mesalamine | 29 (58) | 23 (79) | 6 (29) |
| Biologic therapies | 11 (22) | 3 (10) | 8 (38) |
| Thiopurines | 8 (16) | 2 (7) | 5 (24) |
| Non-systemic glucocorticoids | 10 (20) | 4 (14) | 6 (29) |
| Systemic glucocorticoids | 6 (12) | 2 (7) | 4 (19) |
| Prior surgery n (%) | 4 (8) | 0 (0) | 4 (19) |
| Non-systemic corticosteroid (past users) n (%) | 10 (20) | 4 (14) | 6 (29) |
| Systemic corticosteroid (past users) n (%) | 27 (54) | 15 (52) | 12 (57) |
| Cumulative prednisone-equivalent dose (g) | 3.28 ± 2.09 | 3.46 ± 2.55 | 3.07 ± 1.41 |
| UC Montreal classification n (%) | |||
| E1 | 11 (38) | ||
| E2 | 9 (31) | ||
| E3 | 9 (31) | ||
| S0 | 21 (72) | ||
| S1 | 3 (10) | ||
| S2 | 4 (14) | ||
| S3 | 1 (3) | ||
| CD Montreal classification n (%) | |||
| A1 | 1 (5) | ||
| A2 | 7 (33) | ||
| A3 | 13 (62) | ||
| L1 | 13 (62) | ||
| L2 | 2 (10) | ||
| L3 | 6 (29) | ||
| L4 | 0 (0) | ||
| B1 | 15 (71) | ||
| B2 | 2 (10) | ||
| B3 | 4 (19) |
| Variables | IBD (n = 50) | UC (n = 29) | CD (n = 21) | Controls (n = 50) | p Value (IBD vs. controls) | p Value Controls vs. UC vs. CD |
|---|---|---|---|---|---|---|
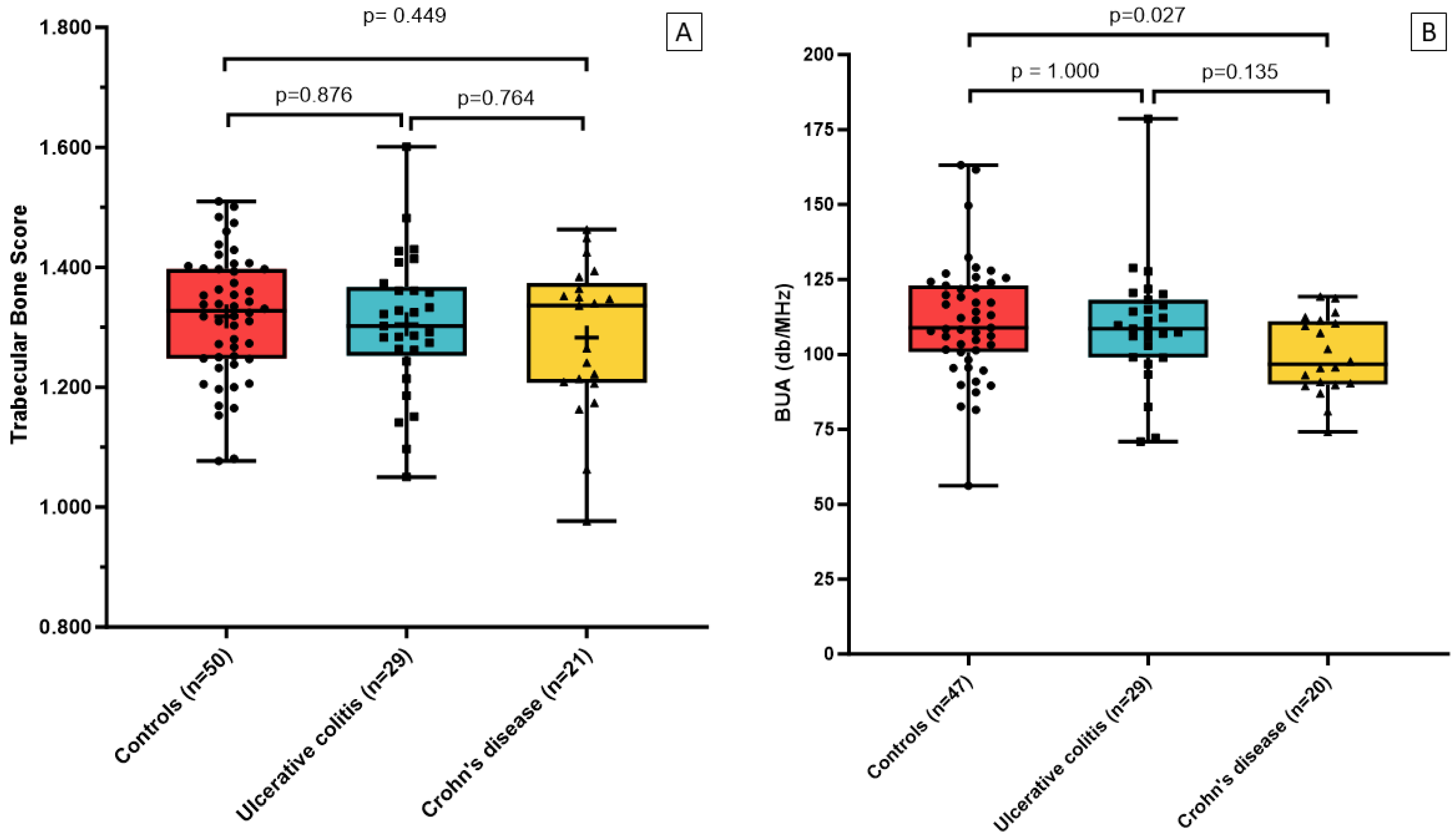
| TBS (unitless) | 1.296 ± 0.120 | 1.305 ± 0.116 | 1.283 ± 0.126 | 1.318 ± 0.103 | 0.322 | 0.481 |
| aBMD (DXA) | ||||||
| L2-L4 lat (g/cm2) | 0.720 ± 0.237 | 0.741 ± 0.263 | 0.691 ± 0.194 | 0.776 ± 0.242 | 0.254 | 0.409 |
| L1-L4 (g/cm2) | 1.120 ± 0.160 | 1.135 ± 0.174 | 1.100 ± 0.141 | 1.138 ± 0.171 | 0.603 | 0.633 |
| Femoral Neck (g/cm2) | 0.898 ± 0.149 | 0.910 ± 0.136 | 0.881 ± 0.167 | 0.922 ± 0. 136 | 0.409 | 0.557 |
| Total Hip (g/cm2) | 0.941 ± 0.151 | 0.964 ± 0.131 | 0.908 ± 0.173 | 0.972 ± 0.145 | 0.296 | 0.246 |
| vBMD (3D-DXA) | ||||||
| Integral total (g/cm3) | 313.17 (283.64; 335.01) | 318.60 (294.97; 327.04) | 296.82 (270.97; 335.01) | 313.79 (284.61; 360.47) | 0.397 | 0.484 |
| Cortical total (g/cm3) | 818.07 ± 71.33 | 829.17 ± 62.74 | 802.73 ± 80.81 | 827.23 ± 80.26 | 0.549 | 0.400 |
| Trabecular total (g/cm3) | 162.78 ± 38.98 | 165.80 ± 35.09 | 158.60 ± 44.34 | 173.53 ± 43.24 | 0.197 | 0.363 |
| Cortical thickness (mm) | 1.926 ± 0.177 | 1.938 ± 0.148 | 1.910 ± 0.214 | 1.948 ± 0.153 | 0.520 | 0.686 |
| QUS | ||||||
| BUA (db/Mhz) | 107.2 (93.3; 114.3) | 108.6 (99.0; 118.2) | 96.7 (90.1; 110.9) | 108.9 (100.8; 123.0) | 0.083 | 0.030 |
| BUA (db/Mhz) w/o outliers * | 103.6 ± 14.3 | 106.8 ± 14.7 | 99.5 ± 12.8 | 111.3 ± 19.5 | 0.033 | 0.030 |
| SOS (m/s) | 1512.9 (1501.9; 1522.5) | 1519.1 (1502.6; 1525.9) | 1509.7 (1497.9; 1514.3) | 1513.5 (1501.6; 1527.5) | 0.501 | 0.142 |
| BQI (%) | 86.9 ± 14.9 | 91.4 ± 14.3 | 80.7 ± 13.7 | 92.2 ± 20.3 | 0.151 | 0.041 |
| T-Score and Z-Score DXA ** | ||||||
| T-score L1-L4 | −0.93 ± 1.30 | −0.92 ± 1.47 | −0.93 ± 1.10 | −0.68 ± 1.47 | 0.475 | 0.777 |
| Z-score L1-L4 | −0.07 ± 1.24 | 0.08 ± 1.34 | −0.35 ± 1.07 | −0.25 ± 1.23 | 0.659 | 0.722 |
| T-score Femoral Neck | −1.50 (−2.00; −0.20) | −1.50 (−2.00; −0.50) | −1.50 (−2.00; −0.10) | −1.1 (−1.70; −0.70) | 0.423 | 0.835 |
| Z-score Femoral Neck | −0.38 ± 0.96 | −0.13 ± 0.98 | −0.88 ± 0.77 | 0.01 ± 1.29 | 0.321 | 0.254 |
| T-score Total Hip | −0.98 ± 1.21 | −0.89 ± 1.11 | −1.07 ± 1.34 | −0.78 ± 1.04 | 0.471 | 0.699 |
| Z-score Total Hip | −0.58 ± 0.87 | −0.38 ± 0.76 | 0.97 ± 1.03 | −0.05 ± 1.18 | 0.145 | 0.187 |
| Osteoporosis n (%) | 10 (20) | 5 (17) | 5 (24) | 3 (6) | 0.037 | 0.091 |
| Variables | Pearson Correlation (r) | p Value | Pearson Correlation (r) (w/o Outliers) | p Value (w/o Outliers) |
|---|---|---|---|---|
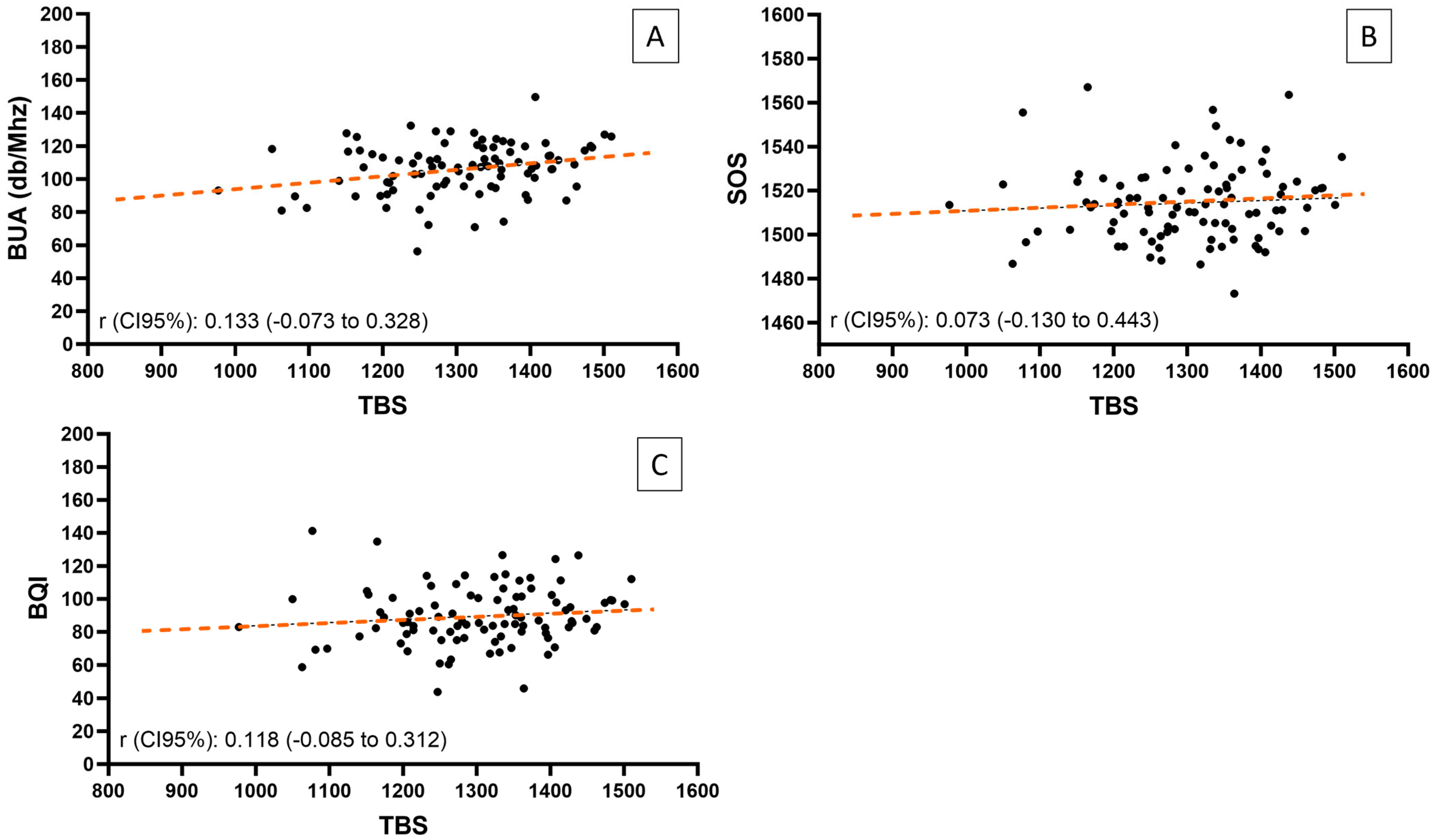
| TBS vs. SOS | 0.073 | 0.480 | ||
| TBS vs. BUA | 0.205 a | 0.047 | 0.133 | 0.205 |
| TBS vs. BQI | 0.118 | 0.254 | ||
| TBS vs. L1-L4 aBMD | 0.118 | 0.240 | ||
| TBS vs. L2-L4 lat aBMD | −0.006 | 0.955 | ||
| TBS vs. Femoral Neck aBMD | 0.174 | 0.083 | ||
| TBS vs. Total Hip aBMD | 0.060 | 0.553 | ||
| TBS vs. vBMD integral | 0.069 | 0.497 | ||
| TBS vs. vBMD cortical | 0.063 | 0.537 | ||
| TBS vs. vBMD trabecular | 0.146 | 0.148 | ||
| TBS vs. Cortical Thickness | −0.097 | 0.338 | ||
| SOS vs. vBMD integral | 0.528 | <0.001 | ||
| SOS vs. vBMD cortical | 0.507 | <0.001 | ||
| SOS vs. vBMD trabecular | 0.558 | <0.001 | ||
| SOS vs. Cortical Thickness | 0.474 | <0.001 | ||
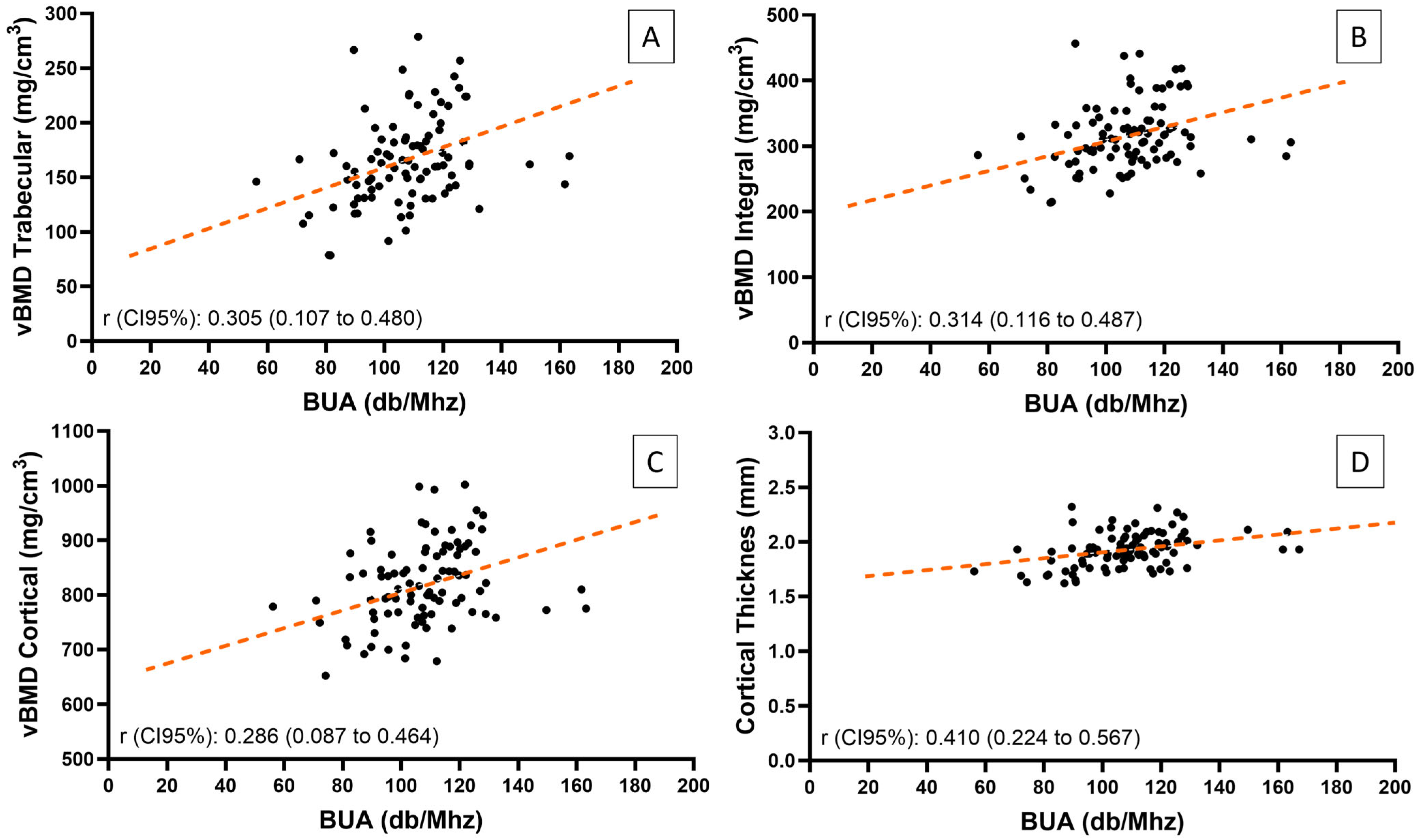
| BUA vs. vBMD integral | 0.293 | 0.004 | 0.314 a | 0.002 |
| BUA vs. vBMD cortical | 0.192 | 0.065 | 0.286 | 0.006 |
| BUA vs. vBMD trabecular | 0.271 | 0.009 | 0.305 | 0.003 |
| BUA vs. Cortical Thickness | 0.335 | 0.001 | 0.410 | <0.001 |
| BQI vs. vBMD integral | 0.491 | <0.001 | ||
| BQI vs. vBMD cortical | 0.449 | <0.001 | ||
| BQI vs. vBMD trabecular | 0.524 | <0.001 | ||
| BQI vs. Cortical Thickness | 0.488 | <0.001 | ||
| SOS vs. L1-L4 aBMD | 0.506 | <0.001 | ||
| SOS vs. L2-L4 lat aBMD | 0.515 | <0.001 | ||
| SOS vs. Femoral Neck aBMD | 0.513 | <0.001 | ||
| SOS vs. Total Hip aBMD | 0.592 | <0.001 | ||
| BUA vs. L1-L4 aBMD | 0.194 | 0.061 | 0.268 | 0.009 |
| BUA vs. L2-L4 lat aBMD | 0.333 | 0.001 | 0.427 | <0.001 |
| BUA vs. Femoral Neck aBMD | 0.343a | <0.001 | 0.365 a | <0.001 |
| BUA vs. Total Hip aBMD | 0.326 | 0.001 | 0.403 | <0.001 |
| BQI vs. L1-L4 aBMD | 0.450 | <0.001 | ||
| BQI vs. L2-L4 lat aBMD | 0.515 | <0.001 | ||
| BQI vs. Femoral Neck aBMD | 0.501 | <0.001 | ||
| BQI vs. Total Hipt aBMD | 0.569 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cortés-Berdonces, M.; Arberas, B.; de la Fuente, M.; Thuissard, I.J.; Marín, F. Assessment of Bone Health in Adult Patients with Inflammatory Bowel Disease: A Single-Center Cohort Study. J. Clin. Med. 2025, 14, 3933. https://doi.org/10.3390/jcm14113933
Cortés-Berdonces M, Arberas B, de la Fuente M, Thuissard IJ, Marín F. Assessment of Bone Health in Adult Patients with Inflammatory Bowel Disease: A Single-Center Cohort Study. Journal of Clinical Medicine. 2025; 14(11):3933. https://doi.org/10.3390/jcm14113933
Chicago/Turabian StyleCortés-Berdonces, María, Beatriz Arberas, Marina de la Fuente, Israel J. Thuissard, and Fernando Marín. 2025. "Assessment of Bone Health in Adult Patients with Inflammatory Bowel Disease: A Single-Center Cohort Study" Journal of Clinical Medicine 14, no. 11: 3933. https://doi.org/10.3390/jcm14113933
APA StyleCortés-Berdonces, M., Arberas, B., de la Fuente, M., Thuissard, I. J., & Marín, F. (2025). Assessment of Bone Health in Adult Patients with Inflammatory Bowel Disease: A Single-Center Cohort Study. Journal of Clinical Medicine, 14(11), 3933. https://doi.org/10.3390/jcm14113933





