Positive Surgical Margins in Clear Cell Renal Cell Carcinoma: Prognostic Impact and Implications for Risk Stratification and Adjuvant Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Patient Selection
2.2. Data Collected
2.3. Data Analysis
3. Results
3.1. Baseline Characteristics
3.2. Overall Prognostic Impact of PSM
3.3. Kaplan–Meier Survival Analysis by AJCC Stage
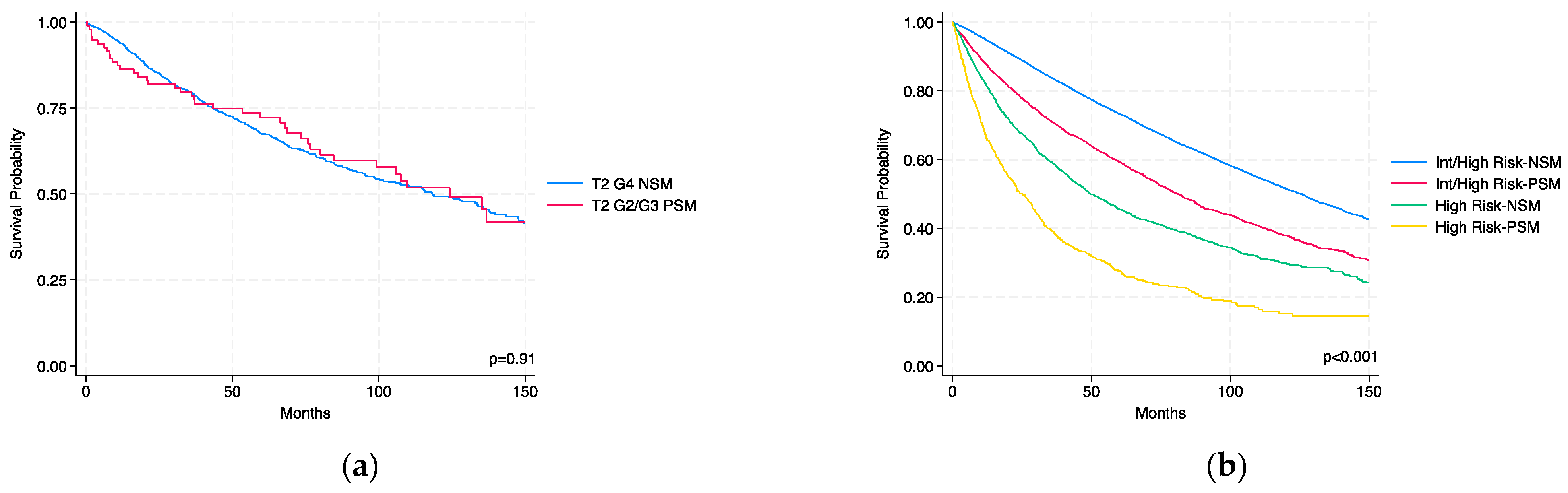
3.4. Comparison of T2 G2/G3 PSM vs. T2 G4 NSM
3.5. Survival Analysis Within KEYNOTE-564 Risk Categories
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pal, S.K.; Uzzo, R.; Karam, J.A.; Master, V.A.; Donskov, F.; Suarez, C.; Albiges, L.; Rini, B.; Tomita, Y.; Kann, A.G.; et al. Adjuvant Atezolizumab versus Placebo for Patients with Renal Cell Carcinoma at Increased Risk of Recurrence Following Resection (IMmotion010): A Multicentre, Randomised, Double-Blind, Phase 3 Trial. Lancet 2022, 400, 1103–1116. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Russo, P.; Grünwald, V.; Tomita, Y.; Zurawski, B.; Parikh, O.; Buti, S.; Barthélémy, P.; Goh, J.C.; Ye, D.; et al. Adjuvant Nivolumab plus Ipilimumab versus Placebo for Localised Renal Cell Carcinoma after Nephrectomy (CheckMate 914): A Double-Blind, Randomised, Phase 3 Trial. Lancet 2023, 401, 821–832. [Google Scholar] [CrossRef]
- Motzer, R.J.; Bex, A.; Russo, P.; Tomita, Y.; Cutuli, H.J.; Rojas, C.; Gross-Goupil, M.; Schinzari, G.; Melichar, B.; Barthélémy, P.; et al. Adjuvant Nivolumab for Localized Renal Cell Carcinoma at High Risk of Recurrence After Nephrectomy: Part B of the Randomized, Placebo-Controlled, Phase III CheckMate 914 Trial. J. Clin. Oncol. 2025, 43, 189–200. [Google Scholar] [CrossRef]
- Allaf, M.E.; Kim, S.-E.; Master, V.; McDermott, D.F.; Harshman, L.C.; Cole, S.M.; Drake, C.G.; Signoretti, S.; Akgul, M.; Baniak, N.; et al. Perioperative Nivolumab versus Observation in Patients with Renal Cell Carcinoma Undergoing Nephrectomy (PROSPER ECOG-ACRIN EA8143): An Open-Label, Randomised, Phase 3 Study. Lancet Oncol. 2024, 25, 1038–1052. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Wang, L.; Pandolfo, S.D.; Autorino, R. Re: Adjuvant Atezolizumab Versus Placebo for Patients with Renal Cell Carcinoma at Increased Risk of Recurrence Following Resection (IMmotion010): A Multicentre, Randomised, Double-blind, Phase 3 Trial. Eur. Urol. 2023, 83, 297–298. [Google Scholar] [CrossRef]
- Bedke, J.; Ghanem, Y.A.; Albiges, L.; Bonn, S.; Campi, R.; Capitanio, U.; Dabestani, S.; Hora, M.; Klatte, T.; Kuusk, T.; et al. Updated European Association of Urology Guidelines on the Use of Adjuvant Immune Checkpoint Inhibitors and Subsequent Therapy for Renal Cell Carcinoma. Eur. Urol. 2025, 87, 491–496. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Tomczak, P.; Park, S.H.; Venugopal, B.; Ferguson, T.; Chang, Y.-H.; Hajek, J.; Symeonides, S.N.; Lee, J.L.; Sarwar, N.; et al. Adjuvant Pembrolizumab after Nephrectomy in Renal-Cell Carcinoma. N. Engl. J. Med. 2021, 385, 683–694. [Google Scholar] [CrossRef] [PubMed]
- Choueiri, T.K.; Tomczak, P.; Park, S.H.; Venugopal, B.; Ferguson, T.; Symeonides, S.N.; Hajek, J.; Chang, Y.-H.; Lee, J.-L.; Sarwar, N.; et al. Overall Survival with Adjuvant Pembrolizumab in Renal-Cell Carcinoma. N. Engl. J. Med. 2024, 390, 1359–1371. [Google Scholar] [CrossRef]
- Ryan, S.T.; Patel, D.N.; Ghali, F.; Patel, S.H.; Sarkar, R.; Yim, K.; Eldefrawy, A.; Cotta, B.H.; Bradshaw, A.W.; Meagher, M.F.; et al. Impact of Positive Surgical Margins on Survival after Partial Nephrectomy in Localized Kidney Cancer: Analysis of the National Cancer Database. Minerva Urol. Nephrol. 2021, 73, 233–244. [Google Scholar] [CrossRef]
- Khalifeh, A.; Kaouk, J.H.; Bhayani, S.; Rogers, C.; Stifelman, M.; Tanagho, Y.S.; Kumar, R.; Gorin, M.A.; Sivarajan, G.; Samarasekera, D.; et al. Positive Surgical Margins in Robot-Assisted Partial Nephrectomy: A Multi-Institutional Analysis of Oncologic Outcomes (Leave No Tumor Behind). J. Urol. 2013, 190, 1674–1679. [Google Scholar] [CrossRef]
- Abu-Ghanem, Y.; Ramon, J.; Berger, R.; Kaver, I.; Fridman, E.; Leibowitz-Amit, R.; Dotan, Z.A. Positive Surgical Margin Following Radical Nephrectomy Is an Independent Predictor of Local Recurrence and Disease-Specific Survival. World J. Surg. Oncol. 2017, 15, 193. [Google Scholar] [CrossRef]
- Morris, L.K.; Altahan, A.; Gandhi, J.; Mays, J.; Giri, U.; Fleming, M.; Martin, M.G. Impact of Margin Status on Survival after Radical Nephrectomy for Renal Cell Carcinoma. J. Surg. Oncol. 2021, 123, 687–692. [Google Scholar] [CrossRef]
- Campbell, S.C.; Clark, P.E.; Chang, S.S.; Karam, J.A.; Souter, L.; Uzzo, R.G. Renal Mass and Localized Renal Cancer: Evaluation, Management, and Follow-Up: AUA Guideline: Part I. J. Urol. 2021, 206, 199–208. [Google Scholar] [CrossRef]
- Winchester, D.P.; Stewart, A.K.; Phillips, J.L.; Ward, E.E. Editorial: The National Cancer Data Base: Past, Present, and Future. Ann. Surg. Oncol. 2010, 17, 4–7. [Google Scholar] [CrossRef]
- Saitta, C.; Autorino, R.; Capitanio, U.; Lughezzani, G.; Meagher, M.F.; Yim, K.; Nguyen, M.V.; Mantovani, M.; Guer, M.; Amparore, D.; et al. Propensity Score-Matched Analysis of Radical and Partial Nephrectomy in PT3aN0M0 Renal Cell Carcinoma. Clin. Genitourin. Cancer 2025, 23, 102343. [Google Scholar] [CrossRef] [PubMed]
- Hung, A.J.; Cai, J.; Simmons, M.N.; Gill, I.S. “Trifecta” in Partial Nephrectomy. J. Urol. 2013, 189, 36–42. [Google Scholar] [CrossRef]
- Buffi, N.; Lista, G.; Larcher, A.; Lughezzani, G.; Ficarra, V.; Cestari, A.; Lazzeri, M.; Guazzoni, G. Margin, Ischemia, and Complications (MIC) Score in Partial Nephrectomy: A New System for Evaluating Achievement of Optimal Outcomes in Nephron-Sparing Surgery. Eur. Urol. 2012, 62, 617–618. [Google Scholar] [CrossRef] [PubMed]
- Ficarra, V.; Crestani, A.; Inferrera, A.; Novara, G.; Rossanese, M.; Subba, E.; Giannarini, G. Positive Surgical Margins After Partial Nephrectomy: A Systematic Review and Meta-Analysis of Comparative Studies. Kidney Cancer 2018, 2, 133–145. [Google Scholar] [CrossRef]
- Hakam, N.; Abou Heidar, N.; Khabsa, J.; Hneiny, L.; Akl, E.A.; Khauli, R. Does a Positive Surgical Margin After Nephron Sparing Surgery Affect Oncological Outcome in Renal Cell Carcinoma? A Systematic Review and Meta-Analysis. Urology 2021, 156, e30–e39. [Google Scholar] [CrossRef]
- Shah, P.H.; Moreira, D.M.; Okhunov, Z.; Patel, V.R.; Chopra, S.; Razmaria, A.A.; Alom, M.; George, A.K.; Yaskiv, O.; Schwartz, M.J.; et al. Positive Surgical Margins Increase Risk of Recurrence after Partial Nephrectomy for High Risk Renal Tumors. J. Urol. 2016, 196, 327–334. [Google Scholar] [CrossRef]
- Kang, H.W.; Lee, S.K.; Kim, W.T.; Yun, S.J.; Lee, S.; Kim, W.; Hwang, E.C.; Kang, S.H.; Hong, S.; Chung, J.; et al. Surgical Margin Does Not Influence Recurrence Rate in PT1 Clear Cell Renal Cell Carcinoma after Partial Nephrectomy: A Multicenter Study. J. Surg. Oncol. 2016, 114, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Hulin, M.; Audigé, V.; Baghli, A.; Larré, S.; Eschwege, P.; Bensalah, K.; Khene, Z. Long-Term Consequences of Positive Surgical Margin after Partial Nephrectomy for Renal Cell Carcinoma: Multi-institutional Analysis. Int. J. Clin. Oncol. 2024, 29, 1509–1515. [Google Scholar] [CrossRef] [PubMed]
- Ficarra, V.; Novara, G.; Secco, S.; Macchi, V.; Porzionato, A.; De Caro, R.; Artibani, W. Preoperative Aspects and Dimensions Used for an Anatomical (PADUA) Classification of Renal Tumours in Patients Who Are Candidates for Nephron-Sparing Surgery. Eur. Urol. 2009, 56, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Kutikov, A.; Uzzo, R.G. The RENAL Nephrometry Score: A Comprehensive Standardized System for Quantitating Renal Tumor Size, Location and Depth. J. Urol. 2009, 182, 844–853. [Google Scholar] [CrossRef]


| Variable | Overall (n = 171,151) | PSM (n = 10,127) | NSM (n = 161,024) | p-Value | Test |
|---|---|---|---|---|---|
| Age, years: median (IQR) | 61 (52–69) | 63 (55–71) | 61 (52–69) | <0.001 | # |
| Gender, n (%) | <0.001 | * | |||
| Male | 105,196 (61.46) | 6518 (64.36) | 98,678 (61.28) | ||
| Female | 65,955 (38.54) | 3609 (35.64) | 62,346 (38.72) | ||
| Race, n (%) | <0.001 | * | |||
| White | 150,827 (88.13) | 9037 (89.24) | 141,790 (88.06) | ||
| Black | 11,585 (6.77) | 588 (5.81) | 10,997 (6.83) | ||
| Native American | 1170 (0.68) | 82 (0.81) | 1088 (0.68) | ||
| Asian/Pacific Islander | 4001 (2.34) | 224 (2.21) | 3777 (2.35) | ||
| Other/Unknown | 3568 (2.08) | 196 (1.94) | 3372 (2.09) | ||
| CCI | <0.001 | * | |||
| 0 | 114,708 (67.02) | 6561 (64.79) | 108,147 (67.16) | ||
| 1 | 37,185 (21.73) | 2309 (22.80) | 34,876 (21.66) | ||
| 2 | 11,763 (6.87) | 752 (7.43) | 11,011 (6.84) | ||
| ≥3 | 7495 (4.38) | 505 (4.99) | 6990 (4.34) | ||
| Tumor size, mm: median (IQR) | 42 (27–65) | 50 (29–91) | 42 (27–65) | <0.001 | # |
| Surgery type | <0.001 | * | |||
| Partial nephrectomy | 72,507 (42.36) | 5072 (50.08) | 67,435 (41.88) | ||
| Radical nephrectomy | 98,644 (57.64) | 5055 (49.92) | 93,589 (58.12) | ||
| Pathological T stage, n (%) | <0.001 | * | |||
| T1 | 116,317 (67.96) | 4582 (45.25) | 111,735 (69.39) | ||
| T2 | 15,267 (8.92) | 168 (1.66) | 15,099 (9.38) | ||
| T3 | 38,411 (22.44) | 4905 (48.43) | 33,506 (20.81) | ||
| T4 | 1156 (0.68) | 472 (4.66) | 684 (0.42) | ||
| Pathological N stage, n (%) | <0.001 | * | |||
| N0/Nx | 167,903 (98.10) | 9261 (91.45) | 158,642 (98.52) | ||
| N1 | 3248 (1.90) | 866 (8.55) | 2382 (1.48) | ||
| Metastatic status, n (%) | <0.001 | * | |||
| M0 | 164,594 (96.17) | 8943 (88.31) | 155,651 (96.66) | ||
| M1 | 6557 (3.83) | 1184 (11.69) | 5373 (3.34) | ||
| AJCC stage, n (%) | <0.001 | * | |||
| I | 115,167 (67.29) | 4523 (44.66) | 110,644 (68.71) | ||
| II | 14,119 (8.25) | 122 (1.20) | 13,997 (8.69) | ||
| III | 34,575 (20.20) | 4026 (39.76) | 30,549 (18.97) | ||
| IV | 7290 (4.26) | 1456 (14.38) | 5834 (3.62) | ||
| Grade, n (%) | <0.001 | * | |||
| G1 | 19,588 (11.44) | 782 (7.72) | 18,806 (11.68) | ||
| G2 | 91,399 (53.40) | 4130 (40.78) | 87,269 (54.20) | ||
| G3 | 48,236 (28.18) | 3418 (33.75) | 44,818 (27.83) | ||
| G4 | 11,928 (6.97) | 1797 (17.74) | 10,131 (6.29) |
| (a) | |||
| Variable | HR | p-Value | 95% CI |
| Surgical margin | |||
| NSM | Reference | ||
| PSM | 1.43 | <0.001 | 1.38–1.49 |
| Age | 1.046 | <0.001 | 1.045–1.047 |
| CCI | |||
| 0 | Reference | ||
| 1 | 1.30 | <0.001 | 1.28–1.34 |
| 2 | 1.78 | <0.001 | 1.72–1.84 |
| ≥3 | 2.27 | <0.001 | 2.16–2.37 |
| Tumor size (mm) | 1.001 | <0.001 | 1.0009–1.0011 |
| Surgery type | |||
| Partial nephrectomy | Reference | ||
| Radical nephrectomy | 1.47 | <0.001 | 1.43–1.51 |
| AJCC stage | |||
| I | Reference | ||
| II | 1.25 | <0.001 | 1.21–1.30 |
| III | 1.66 | <0.001 | 1.61–1.70 |
| IV | 4.74 | <0.001 | 4.57–4.92 |
| Grade | |||
| G1 | Reference | ||
| G2 | 1.02 | 0.41 | 0.98–1.06 |
| G3 | 1.31 | <0.001 | 1.26–1.36 |
| G4 | 2.04 | <0.001 | 1.94–2.14 |
| (b) | |||
| Variable | HR | p-Value | 95% CI |
| T2 subgroups | |||
| T2 G4 NSM | Reference | ||
| T2 G2/G3 PSM | 1.08 | 0.69 | 0.74–1.57 |
| Age | 1.03 | <0.001 | 1.03–1.04 |
| CCI | |||
| 0 | Reference | ||
| 1 | 1.06 | 0.63 | 0.84–1.32 |
| 2 | 1.04 | 0.83 | 0.70–1.56 |
| ≥3 | 2.15 | <0.001 | 1.42–3.23 |
| Tumor size (mm) | 0.99 | 0.90 | 0.98–1.00 |
| Surgery type | |||
| Partial nephrectomy | Reference | ||
| Radical nephrectomy | 1.35 | 0.11 | 0.94–1.95 |
| (c) | |||
| Variable | |||
| Keynote group | |||
| Intermediate-to-High Risk | Reference | ||
| High Risk | 2.40 | <0.001 | 2.28–2.53 |
| Surgical margin | |||
| NSM | Reference | ||
| PSM | 1.62 | <0.001 | 1.55–1.70 |
| Age | 1.034 | <0.001 | 1.032–1.036 |
| CCI | |||
| 0 | Reference | ||
| 1 | 1.25 | <0.001 | 1.20–1.30 |
| 2 | 1.43 | <0.001 | 1.34–1.52 |
| ≥3 | 1.75 | <0.001 | 1.62–1.88 |
| Tumor size (mm) | 1.001 | <0.001 | 1.0009–1.0011 |
| Surgery type | |||
| Partial nephrectomy | Reference | ||
| Radical nephrectomy | 1.61 | <0.001 | 1.50–1.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garofano, G.; Saitta, C.; Musso, G.; Meagher, M.F.; Capitanio, U.; Dabbas, M.; Birouty, N.; Karamcheti, S.; Kim, B.; Yuen, K.L.; et al. Positive Surgical Margins in Clear Cell Renal Cell Carcinoma: Prognostic Impact and Implications for Risk Stratification and Adjuvant Therapy. J. Clin. Med. 2025, 14, 3908. https://doi.org/10.3390/jcm14113908
Garofano G, Saitta C, Musso G, Meagher MF, Capitanio U, Dabbas M, Birouty N, Karamcheti S, Kim B, Yuen KL, et al. Positive Surgical Margins in Clear Cell Renal Cell Carcinoma: Prognostic Impact and Implications for Risk Stratification and Adjuvant Therapy. Journal of Clinical Medicine. 2025; 14(11):3908. https://doi.org/10.3390/jcm14113908
Chicago/Turabian StyleGarofano, Giuseppe, Cesare Saitta, Giacomo Musso, Margaret F. Meagher, Umberto Capitanio, Mai Dabbas, Natalie Birouty, Sanjana Karamcheti, Breanna Kim, Kit L. Yuen, and et al. 2025. "Positive Surgical Margins in Clear Cell Renal Cell Carcinoma: Prognostic Impact and Implications for Risk Stratification and Adjuvant Therapy" Journal of Clinical Medicine 14, no. 11: 3908. https://doi.org/10.3390/jcm14113908
APA StyleGarofano, G., Saitta, C., Musso, G., Meagher, M. F., Capitanio, U., Dabbas, M., Birouty, N., Karamcheti, S., Kim, B., Yuen, K. L., Larcher, A., Baker, B., Autorino, R., Pandolfo, S. D., Montorsi, F., Saita, A., Lazzeri, M., Lughezzani, G., Casale, P., ... Derweesh, I. H. (2025). Positive Surgical Margins in Clear Cell Renal Cell Carcinoma: Prognostic Impact and Implications for Risk Stratification and Adjuvant Therapy. Journal of Clinical Medicine, 14(11), 3908. https://doi.org/10.3390/jcm14113908









