Comparative Analysis of Perceval and Conventional Bovine Bioprosthetic Valves in Aortic Valve Replacement: Hemodynamics, Reverse Remodeling, and Long-Term Outcomes
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Data Collection and Measurement
2.3. Valve Sizing and Implantation Technique
2.4. Endpoint Definitions and Classification
2.5. Statistical Analysis
3. Results
3.1. Baseline Demographic and Clinical Characteristics
3.2. Operative Characteristics and In-Hospital Adverse Events
3.3. Hemodynamic and Structural Outcomes Following AVR
3.4. Late Adverse Events and Clinical Outcomes
4. Discussion
4.1. Procedural Safety and In-Hospital Results
4.2. Aortic Stenosis Pathophysiology and Prosthetic Evolution for AVR
4.3. Reverse Remodeling and Mass Regression Following AVR
4.4. Late Adverse Events and Identification of Risk Predictors
4.5. Clinical Implication
4.6. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AS | Aortic Valve Stenosis |
| AVR | Aortic Valve Replacement |
| CABG | Coronary Artery Bypass Graft |
| CPB | Cardiopulmonary Bypass |
| EDD | End-Diastolic Dimension |
| EDV | End-Diastolic Volume |
| ESD | End-Systolic Dimension |
| ESV | End-Systolic Volume |
| EF | Ejection Fraction |
| FS | Fractional Shortening |
| IVST | Interventricular Septum Thickness |
| LVM | Left Ventricular Mass |
| LVMI | Indexed Left Ventricular Mass |
| MACE | Major Adverse Cardiac–Cerebral Event |
| PPI | Permanent Pacemaker Implantation |
| PPM | Patient–Prosthesis Mismatch |
| PVL | Paravalvular Leakage |
| RWT | Relative Wall Thickness |
| SPAP | Systolic Pulmonary Arterial Pressure |
| SV | Stroke Volume |
| TAVI | Transcatheter Aortic Valve Implantation |
| WS | Wall Stress |
| VIS | Vasoactive Inotropic Score |
| VTI | Velocity Time Integral |
References
- Evangelista, A. Aortic Stenosis in Bicuspid and Tricuspid Valves: A Different Spectrum of the Disease With Clinical Implications. JACC Cardiovasc. Imaging 2021, 14, 1127–1129. [Google Scholar] [CrossRef] [PubMed]
- Nkomo, V.T.; Gardin, J.M.; Skelton, T.N.; Gottdiener, J.S.; Scott, C.G.; Enriquez-Sarano, M. Burden of valvular heart diseases: A population-based study. Lancet 2006, 368, 1005–1011. [Google Scholar] [CrossRef]
- Dweck, M.R.; Boon, N.A.; Newby, D.E. Calcific aortic stenosis: A disease of the valve and the myocardium. J. Am. Coll. Cardiol. 2012, 60, 1854–1863. [Google Scholar] [CrossRef] [PubMed]
- Lindman, B.R.; Clavel, M.A.; Mathieu, P.; Iung, B.; Lancelloti, P.; Otto, C.M.; Pibarot, P. Calcific aortic stenosis. Nat. Rev. Dis. Primers 2016, 2, 16006. [Google Scholar] [CrossRef]
- Shen, M.; Tastet, L.; Capoulade, R.; Arsenault, M.; Bédard, É.; Clavel, M.-A.; Pibarot, P. Effect of bicuspid aortic valve phenotype on progression of aortic stenosis. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 727–734. [Google Scholar] [CrossRef]
- Díez, J.; González, A.; López, B.; Querejeta, R. Mechanisms of Disease: Pathologic structural remodeling is more than adaptive hypertrophy in hypertensive heart disease. Nat. Clin. Pract. Cardiovasc. Med. 2005, 2, 209–216. [Google Scholar] [CrossRef]
- Paulus, W.J.; Tschope, C. A novel paradigm for heart failure with preserved ejection fraction: Comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J. Am. Coll. Cardiol. 2013, 62, 263–271. [Google Scholar] [CrossRef]
- Chen, J.-S.; Huang, J.-H.; Chiu, K.-M.; Chiang, C.-Y. Extent of Left Ventricular Mass Regression and Impact of Global Left Ventricular Afterload on Cardiac Events and Mortality after Aortic Valve Replacement. J. Clin. Med. 2022, 11, 7482. [Google Scholar] [CrossRef]
- Schwarzl, M.; Ojeda, F.; Zeller, T.; Seiffert, M.; Becher, P.M.; Munzel, T.; Wild, P.S.; Blettner, M.; Lackner, K.J.; Pfeiffer, N.; et al. Risk factors for heart failure are associated with alterations of the LV end-diastolic pressure–volume relationship in non-heart failure individuals: Data from a large-scale, population-based cohort. Eur. Heart J. 2016, 37, 1807–1814. [Google Scholar] [CrossRef]
- Pibarot, P.; Dumesnil, J.G. Hemodynamic and clinical impact of prosthesis–patient mismatch in the aortic valve position and its prevention. J. Am. Coll. Cardiol. 2000, 36, 1131–1141. [Google Scholar] [CrossRef]
- Flameng, W.; Herregods, M.-C.; Vercalsteren, M.; Herijgers, P.; Bogaerts, K.; Meuris, B. Prosthesis-Patient Mismatch Predicts Structural Valve Degeneration in Bioprosthetic Heart Valves. Circulation 2010, 121, 2123–2129. [Google Scholar] [CrossRef] [PubMed]
- Manouguian, S.; Seybold-Epting, W. Patch enlargement of the aortic valve ring by extending the aortic incision into the anterior mitral leaflet. J. Thorac. Cardiovasc. Surg. 1979, 78, 402–412. [Google Scholar] [CrossRef]
- Nicks, R.; Cartmill, T.; Bernstein, L. Hypoplasia of the aortic root. The problem of aortic valve replacement. Thorax 1970, 25, 339–346. [Google Scholar] [CrossRef]
- Yang, B. Aortic annular enlargement with Y-incision/rectangular patch. Ann. Cardiothorac. Surg. 2024, 13, 294–302. [Google Scholar] [CrossRef]
- Said, S.M. The Ross-Konno procedure for congenital aortic stenosis. Ann. Cardiothorac. Surg. 2021, 10, 527–537. [Google Scholar] [CrossRef]
- Flameng, W.; Herregods, M.-C.; Hermans, H.; Van der Mieren, G.; Vercalsteren, M.; Poortmans, G.; Van Hemelrijck, J.; Meuris, B. Effect of sutureless implantation of the Perceval S aortic valve bioprosthesis on intraoperative and early postoperative outcomes. J. Thorac. Cardiovasc. Surg. 2011, 142, 1453–1457. [Google Scholar] [CrossRef]
- Fischlein, T.; Meuris, B.; Hakim-Meibodi, K.; Misfeld, M.; Carrel, T.; Zembala, M.; Gaggianesi, S.; Madonna, F.; Laborde, F.; Asch, F.; et al. The sutureless aortic valve at 1 year: A large multicenter cohort study. J. Thorac. Cardiovasc. Surg. 2016, 151, 1617–1626.e4. [Google Scholar] [CrossRef]
- Shrestha, M.; Fischlein, T.; Meuris, B.; Flameng, W.; Carrel, T.; Madonna, F.; Misfeld, M.; Folliguet, T.; Haverich, A.; Laborde, F. European multicentre experience with the sutureless Perceval valve: Clinical and haemodynamic outcomes up to 5 years in over 700 patients. Eur. J. Cardio-Thorac. Surg. 2016, 49, 234–241. [Google Scholar] [CrossRef]
- Vogt, F.; Pfeiffer, S.; Dell’Aquila, A.A.; Fischlein, T.; Santarpino, G. Sutureless aortic valve replacement with Perceval bioprosthesis: Are there predicting factors for postoperative pacemaker implantation? Interact. Cardiovasc. Thorac. Surg. 2016, 22, 253–258. [Google Scholar] [CrossRef]
- Grube, E.; Laborde, J.C.; Zickmann, B.; Gerckens, U.; Felderhoff, T.; Sauren, B.; Bootsveld, A.; Buellesfeld, L.; Iversen, S. First report on a human percutaneous transluminal implantation of a self-expanding valve prosthesis for interventional treatment of aortic valve stenosis. Catheter. Cardiovasc. Interv. 2005, 66, 465–469. [Google Scholar] [CrossRef]
- Zahn, R.; Gerckens, U.; Grube, E.; Linke, A.; Sievert, H.; Eggebrecht, H.; Hambrecht, R.; Sack, S.; Hauptmann, K.E.; Richardt, G.; et al. Transcatheter aortic valve implantation: First results from a multi-centre real-world registry. Eur. Heart J. 2011, 32, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Zamorano, J.L.; Badano, L.P.; Bruce, C.; Chan, K.-L.; Gonçalves, A.; Hahn, R.T.; Keane, M.G.; La Canna, G.; Monaghan, M.J.; Nihoyannopoulos, P.; et al. EAE/ASE recommendations for the use of echocardiography in new transcatheter interventions for valvular heart disease. Eur. Heart J. 2011, 32, 2189–2214. [Google Scholar] [CrossRef]
- Genereux, P.; Head, S.J.; Hahn, R.; Daneault, B.; Kodali, S.; Williams, M.R.; van Mieghem, N.M.; Alu, M.C.; Serruys, P.W.; Kappetein, A.P.; et al. Paravalvular leak after transcatheter aortic valve replacement: The new Achilles’ heel? A comprehensive review of the literature. J. Am. Coll. Cardiol. 2013, 61, 1125–1136. [Google Scholar] [CrossRef]
- Warraich, N.; Brown, J.A.; Ashwat, E.; Kliner, D.; Serna-Gallegos, D.; Toma, C.; West, D.; Makani, A.; Wang, Y.; Sultan, I. Paravalvular Leak After Transcatheter Aortic Valve Implantation: Results From 3600 Patients. Ann. Thorac. Surg. 2025, 119, 1037–1044. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–271. [Google Scholar] [CrossRef]
- Grossman, W.; Jones, D.; McLaurin, L.P. Wall stress and patterns of hypertrophy in the human left ventricle. J. Clin. Investig. 1975, 56, 56–64. [Google Scholar] [CrossRef]
- Giannini, C.; Petronio, A.S.; De Carlo, M.; Guarracino, F.; Benedetti, G.; Donne, M.G.D.; Dini, F.L.; Marzilli, M.; Di Bello, V. The Incremental Value of Valvuloarterial Impedance in Evaluating the Results of Transcatheter Aortic Valve Implantation in Symptomatic Aortic Stenosis. J. Am. Soc. Echocardiogr. 2012, 25, 444–453. [Google Scholar] [CrossRef]
- Meuris, B.; Flameng, W.J.; Laborde, F.; Folliguet, T.A.; Haverich, A.; Shrestha, M. Five-year results of the pilot trial of a sutureless valve. J. Thorac. Cardiovasc. Surg. 2015, 150, 84–88. [Google Scholar] [CrossRef]
- VARC-3 WRITING COMMITTEE; Généreux, P.; Piazza, N.; Alu, M.C.; Nazif, T.; Hahn, R.; Pibarot, P.; Bax, J.J.; Leipsic, J.A.; Blanke, P.; et al. Valve Academic Research Consortium 3: Updated Endpoint Definitions for Aortic Valve Clinical Research. J. Am.Coll. Cardiol. 2021, 77, 2717–2746. [Google Scholar] [CrossRef]
- Moscarelli, M.; Santarpino, G.; Athanasiou, T.; Mastroroberto, P.; Fattouch, K.; Nasso, G.; Speziale, G. A pooled analysis of pacemaker implantation after Perceval sutureless aortic valve replacement. Interact. Cardiovasc. Thorac. Surg. 2021, 33, 501–509. [Google Scholar] [CrossRef]
- Vilalta, V.; Cediel, G.; Mohammadi, S.; López, H.; Kalavrouziotis, D.; Resta, H.; Dumont, E.; Voisine, P.; Philippon, F.; Escabia, C.; et al. Incidence, predictors and prognostic value of permanent pacemaker implantation following sutureless valve implantation in low-risk aortic stenosis patients. Eur. J. Cardio-Thorac. Surg. 2022, 62, ezac307. [Google Scholar] [CrossRef] [PubMed]
- Garcia, D.; Camici, P.G.; Durand, L.-G.; Rajappan, K.; Gaillard, E.; Rimoldi, O.E.; Pibarot, P. Impairment of coronary flow reserve in aortic stenosis. J. Appl. Physiol. 2009, 106, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Rajappan, K.; Rimoldi, O.E.; Dutka, D.P.; Ariff, B.; Pennell, D.J.; Sheridan, D.J.; Camici, P.G. Mechanisms of Coronary Microcirculatory Dysfunction in Patients With Aortic Stenosis and Angiographically Normal Coronary Arteries. Circulation 2002, 105, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.; Marcos-Alberca, P.; Almeria, C.; Feltes, G.; Rodríguez, E.; Hernández-Antolín, R.A.; Garcia, E.; Maroto, L.; Perez, C.F.; Cardoso, J.C.S.; et al. Acute left ventricle diastolic function improvement after transcatheter aortic valve implantation. Eur. J. Echocardiogr. 2011, 12, 790–797. [Google Scholar] [CrossRef]
- Brookes, J.D.; Mathew, M.; Brookes, E.M.; Jaya, J.S.; Almeida, A.A.; Smith, J.A. Predictors of Pacemaker Insertion Post-Sutureless (Perceval) Aortic Valve Implantation. Heart Lung Circ. 2021, 30, 917–921. [Google Scholar] [CrossRef]
- Chiang, C.-Y.; Lin, S.-C.; Hsu, J.-C.; Chen, J.-S.; Huang, J.-H.; Chiu, K.-M. Reducing Left Ventricular Wall Stress through Aortic Valve Enlargement via Transcatheter Aortic Valve Implantation in Severe Aortic Stenosis. J. Clin. Med. 2024, 13, 3777. [Google Scholar] [CrossRef]
- Lin, S.-C.; Chiang, C.-Y.; Hsu, J.-C.; Huang, J.-H.; Chen, J.-S.; Chiu, K.-M. Acute Effect in Mechanical Efficiency by Pressure-Volume Loop Analysis after Transcatheter Aortic Valve Implantation. Acta Cardiol. Sin. 2024, 40, 242–252. [Google Scholar]
- Paolisso, P.; Belmonte, M.; Gallinoro, E.; Scarsini, R.; Bergamaschi, L.; Portolan, L.; Armillotta, M.; Esposito, G.; Moscarella, M.; Benfari, G. SGLT2-inhibitors in diabetic patients with severe aortic stenosis and cardiac damage undergoing transcatheter aortic valve implantation (TAVI). Cardiovasc. Diabetol. 2024, 23, 420. [Google Scholar] [CrossRef]
| Total N = 115 | Perceval N = 44 | Conventional N = 71 | p | |
|---|---|---|---|---|
| Age | 65.0 ± 9.7 | 65.8 ± 7.6 | 64.5 ± 10.9 | 0.46 |
| Male | 67 (58) | 28 (64) | 39 (55) | 0.44 |
| Comorbidity | ||||
| Coronary artery disease | 41 (36) | 20 (45) | 21 (30) | 0.11 |
| Hypertension | 72 (63) | 28 (64) | 44 (62) | 1.00 |
| Diabetic | 39 (34) | 15 (34) | 24 (34) | 0.18 |
| Dyslipidemia | 85 (74) | 34 (77) | 51 (72) | 0.66 |
| Chronic renal disease | 29 (25) | 12 (27) | 17 (24) | 0.83 |
| Hemodialysis | 8 (7) | 2 (5) | 6 (8) | 0.71 |
| Cerebral infarct | 9 (8) | 3 (7) | 6 (8) | 1.00 |
| Infective endocarditis | 4 (3) | 0 (0) | 4 (6) | 0.16 |
| Atrial fibrillation | 11 (10) | 1 (2) | 10 (14) | 0.05 |
| Left bundle branch block | 17 (15) | 5 (11) | 12 (17) | 0.59 |
| Previous pacemaker implant | 3 (3) | 1 (2) | 2 (3) | 1.00 |
| Bicuspid aortic valve | 54 (47) | 19 (43) | 35 (49) | 0.57 |
| Aortic valve pathology | ||||
| Annulus diameter | 21.3 ± 2.3 | 21.1 ± 2.1 | 21.4 ± 2.4 | 0.58 |
| Annulus diameter 21 mm | 61 (53) | 23 (52) | 38 (54) | 1.00 |
| Effective orifice area index 0.65, 0.85 cm2m−2 | 10 (9) | 3 (7) | 7 (10) | 0.74 |
| Effective orifice area index 0.65 cm2m−2 | 101 (88) | 39 (89) | 62 (87) | 1.00 |
| Ventricular dysfunction | ||||
| LV ejection fraction < 55% | 15 (13) | 5 (11) | 10 (14) | 0.78 |
| Systolic pulmonary artery pressure > 35 mm Hg | 25 (22) | 12 (27) | 13 (18) | 0.25 |
| Remodel mode | ||||
| Concentric hypertrophy | 87 (76) | 33 (75) | 54 (76) | 1.00 |
| Echocardiographic parameters | ||||
| Interventricular septum thickness, mm | 13.8 ± 3.0 | 13.4 ± 2.9 | 14.1 ± 3.1 | 0.197 |
| Posterior wall thickness, mm | 12.8 ± 2.8 | 12.8 ± 2.7 | 12.8 ± 2.8 | 0.918 |
| LV end-systolic dimension, mm | 30.8 ± 7.7 | 31.4 ± 7.7 | 30.5 ± 7.8 | 0.509 |
| LV end-diastolic dimension, mm | 50.1 ± 7.4 | 50.9 ± 6.4 | 49.5 ± 8.0 | 0.336 |
| LV end-systolic volume, mL | 41.3 ± 27.9 | 43.0 ± 29.9 | 40.2 ± 26.7 | 0.596 |
| LV end-diastolic volume, mL | 122.3 ± 43.8 | 126.0 ± 37.6 | 119.9 ± 47.3 | 0.473 |
| Indexed LV mass, g | 166.1 ± 62.3 | 163.7 ± 66.9 | 167.5 ± 59.8 | 0.747 |
| Indexed effective orifice area, | 0.48 ± 0.19 | 0.48 ± 0.16 | 0.48 ± 0.21 | 0.958 |
| Mean pressure gradient, mm Hg | 44.8 ± 19.1 | 46.1 ± 20.1 | 43.9 ± 18.5 | 0.559 |
| Velocity–time integral ratio, % | 0.25 ± 0.09 | 0.25 ± 0.10 | 0.24 ± 0.09 | 0.743 |
| Wall stress, Kdynecm−2 | 98.1 ± 35.1 | 102.4 ± 34.6 | 96.2 ± 41.3 | 0.411 |
| Total N = 115 | Perceval N = 44 | Conventional N = 71 | p | |
|---|---|---|---|---|
| Operative Characteristics | ||||
| Cardiopulmonary bypass time, min | 100 (93 to 105) | 93 (88 to102) | 105 (96 to 113) | 0.02 |
| Aortic clamp time, min | 53 (53 to 55) | 49 (47 to 54) | 56 (51 to 62) | 0.02 |
| Ministernotomy | 88 (77) | 37 (84) | 51 (72) | 0.18 |
| Previous aortic valve replacement | 6 (5) | 1 (2) | 5 (7) | 1.00 |
| Coronary artery bypass graft | 18 (16) | 6 (14) | 12 (17) | 0.79 |
| Root augmentation | 6 (5) | 0 (0) | 6 (8) | 0.08 |
| Pulmonary vein ablation | 7 (6) | 1 (2) | 6 (8) | 0.25 |
| Hospital Outcomes | ||||
| Re-exploration for bleeding | 4 (3) | 1 (2) | 3 (4) | 1.00 |
| Transient low cardiac output | 23 (20) | 3 (7) | 20 (28) | <0.01 |
| Intra-aortic ballon pump | 1 (1) | 0 (0) | 1 (1) | 1.00 |
| Extracorporeal life support | 3 (3) | 1 (2) | 2 (3) | 1.00 |
| Acute kidney injury | 11 (10) | 4 (10) | 7 (10) | 1.00 |
| Renal replacement therapy | 10 (9) | 3 (7) | 7 (10) | 0.74 |
| Cerebral infarct | 2 (2) | 1 (2) | 1 (1) | 1.00 |
| Ventilator support > 48 hr | 7 (6) | 2 (5) | 5 (7) | 0.71 |
| Pulmonary complication | 9 (8) | 2 (5) | 7 (10) | 0.48 |
| Paravalvular leakage | 4 (3) | 3 (7) | 1 (1) | 0.16 |
| New atrial fibrillation | 18 (16) | 5 (11) | 13 (18) | 0.43 |
| New left bundle branch block | 24 (21) | 11 (25) | 13 (18) | 0.35 |
| Transient pacing | 22 (19) | 11 (25) | 11 (15) | 0.23 |
| Permanent pacemaker implantation | 5 (4) | 2 (5) | 3 (4) | 1.00 |
| Death | 2 (2) | 0 (0) | 2 (3) | 0.52 |
| Total N = 115 | Perceval N = 44 | Conventional N = 71 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Follow-Up | p | Baseline | Follow-Up | p | Baseline | Follow-Up | p | * p | † p | |
| Geometry | |||||||||||
| IVST, mm | 13.8 3.0 | 12.3 3.1 | <0.01 | 13.4 2.9 | 12.4 3.1 | 0.09 | 14.1 3.1 | 12.2 3.1 | <0.01 | 0.12 | 0.78 |
| PWT, mm | 12.8 2.8 | 11.5 2.4 | <001 | 12.8 2.7 | 11.6 2.4 | <0.01 | 12.8 2.8 | 11.4 2.3 | <0.01 | 0.92 | 0.64 |
| LVESD, mm | 30.8 7.7 | 27.1 6.1 | <0.01 | 31.4 7.7 | 26.7 5.8 | <0.01 | 30.5 7.8 | 27.4 6.3 | <0.01 | 0.51 | 0.58 |
| LVEDD, mm | 50.1 7.4 | 45.5 6.2 | <0.01 | 50.9 6.4 | 45.6 6.0 | <0.01 | 49.5 8.0 | 45.5 6.4 | <0.01 | 0.34 | 0.93 |
| LVESV, mL | 41.3 27.9 | 27.1 20.8 | <0.01 | 43.0 29.9 | 27.8 16.4 | <0.01 | 40.2 26.7 | 26.6 23.2 | <0.01 | 0.60 | 0.76 |
| LVEDV, mL | 122.3 43.8 | 89.1 41.3 | <0.01 | 126.0 37.6 | 95.8 34.0 | <0.01 | 119.9 47.3 | 85.1 44.9 | <0.01 | 0.47 | 0.18 |
| LVM, g | 281.3 108.6 | 187.49 1.4 | <0.01 | 282.8 117.6 | 205.0 80.1 | <0.01 | 280.4 103.5 | 176.7 96.5 | <0.01 | 0.91 | 0.11 |
| Indexed LVM, gm−2 | 166.1 62.3 | 110.3 51.6 | <0.01 | 163.7 66.9 | 118.1 44.6 | <0.01 | 167.5 59.8 | 105.6 55.2 | <0.01 | 0.75 | 0.21 |
| Hemodynamics | |||||||||||
| EOA, cm2 | 0.81 0.32 | 1.79 0.53 | <0.01 | 0.82 0.24 | 2.14 0.48 | <0.01 | 0.81 0.36 | 1.55 0.42 | <0.01 | 0.85 | <0.01 |
| Indexed EOA, cm2m−2 | 0.48 0.19 | 0.97 0.39 | <0.01 | 0.48 0.16 | 1.2 10.33 | <0.01 | 0.48 0.21 | 0.82 0.35 | <0.01 | 0.96 | <0.01 |
| MPG, mm Hg | 44.81 9.1 | 11.8 6.5 | <0.01 | 46.1 20.1 | 10.5 4.8 | <0.01 | 43.9 18.5 | 12.7 7.3 | <0.01 | 0.56 | 0.06 |
| VTI ratio, % | 0.25 0.09 | 0.53 0.16 | <0.01 | 0.25 0.10 | 0.61 0.02 | <0.01 | 0.24 0.09 | 0.48 0.13 | <0.01 | 0.74 | <0.01 |
| Function | |||||||||||
| SV, mL | 81.6 24.3 | 62.0 27.2 | <0.01 | 84.3 22.7 | 68.0 23.2 | <0.01 | 79.92 5.2 | 58.4 28.9 | <0.01 | 0.34 | <0.01 |
| FS, % | 38.9 8.4 | 40.7 7.6 | 0.01 | 38.8 8.7 | 41.7 8.0 | 0.03 | 39.0 8.3 | 40.1 7.3 | 0.14 | 0.88 | 0.28 |
| EF, % | 68.2 11.2 | 70.9 9.7 | <0.01 | 67.9 11.9 | 71.9 9.8 | 0.02 | 68.4 10.8 | 70.1 9.7 | 0.09 | 0.82 | 0.36 |
| Wall stress, Kdynecm−2 | 98.1 35.1 | 80.1 31.3 | <0.01 | 102.4 34.6 | 78.3 35.8 | <0.01 | 96.2 41.3 | 81.4 28.0 | 0.03 | 0.41 | 0.62 |
| E/e′ ratio | 16.2 8.3 | 14.7 | 0.06 | 16.4 6.7 | 15.0 6.8 | 0.14 | 15.9 9.6 | 14.4 7.8 | 0.21 | 0.79 | 0.96 |
| Systolic PAP, mm Hg | 24.9 10.5 | 24.0 | 0.48 | 25.4 11.0 | 21.8 7.9 | 0.05 | 24.5 10.2 | 25.5 13.3 | 0.54 | 0.26 | <0.01 |
| Total N = 115 | Perceval N = 44 | Conventional N = 71 | p | |
|---|---|---|---|---|
| Coronary-related intervention | 8 (7) | 3 (7) | 5 (7) | 1.00 |
| Stroke | 11 (10) | 7 (16) | 4 (6) | 0.10 |
| HF-related rehospitalization | 20 (17) | 5 (11) | 15 (21) | 0.21 |
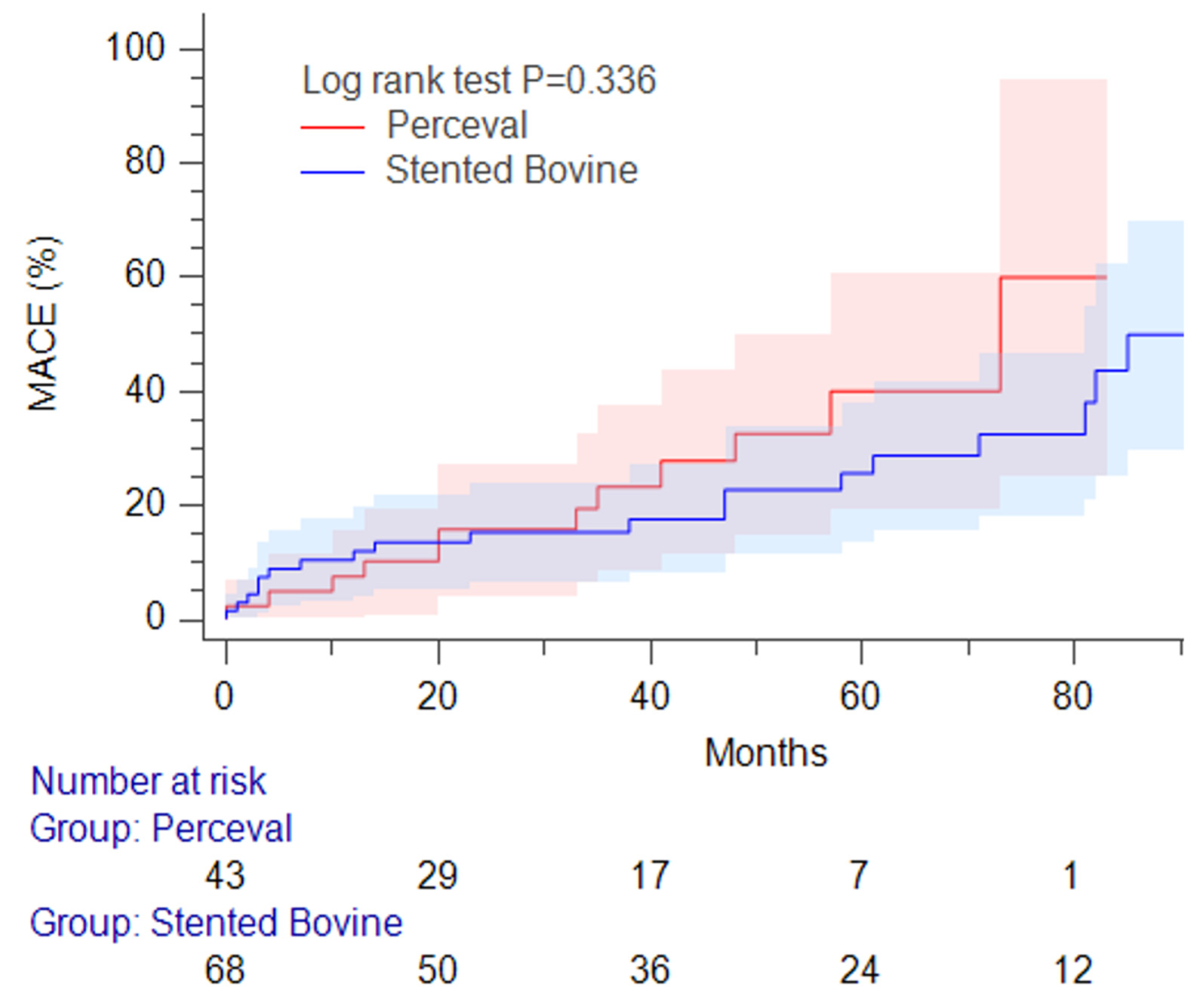
| Major cardio-cerebral events | 31 (27) | 12 (27) | 19 (27) | 1.00 |
| Paravalvular leakage | 6 (5) | 3 (7) | 3 (4) | 0.67 |
| Permanent pacemaker implantation | 4 (3) | 2 (5) | 2 (3) | 0.64 |
| Patient–prosthesis mismatch | 30 (26) | 3 (7) | 27 (38) | <0.01 |
| Infective endocarditis | 1 (1) | 0 (0) | 1 (1) | 1.00 |
| Redo AVR | 6 (5) | 2 (5) | 4 (6) | 1.00 |
| Cardiac-related mortality | 11 (10) | 2 (5) | 9 (13) | 0.20 |
| All-cause mortality | 12 (11) | 2 (5) | 10 (14) | 0.13 |
| Univariate | Multivariate * | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |
| Preoperative | ||||||
| E/e′ ratio 15 | 1.05 | 1.02 to 1.09 | <0.01 | |||
| Systolic PAP 5 mm Hg | 2.11 | 0.96 to 4.66 | 0.06 | |||
| Follow-up | ||||||
| indexed LV mass | 1.02 | 1.01 to 1.03 | <0.01 | 1.02 | 1.01 to 1.03 | <0.01 |
| E/e′ ratio 15 | 1.08 | 1.03 to 1.12 | <0.01 | |||
| Systolic PAP 35 mm Hg | 2.59 | 1.26 to 5.31 | <0.01 | |||
| PPI | 1.98 | 0.47 to 8.36 | 0.36 | |||
| Paravalvular leakage | 2.46 | 0.92 to 6.55 | 0.07 | |||
| Redo AVR | 2.17 | 0.87 to 5.41 | 0.09 | |||
| Perceval valve | 1.93 | 0.88 to 4.22 | 0.10 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, S.-C.; Chen, J.-S.; Huang, J.-H.; Chiu, K.-M.; Chiang, C.-Y. Comparative Analysis of Perceval and Conventional Bovine Bioprosthetic Valves in Aortic Valve Replacement: Hemodynamics, Reverse Remodeling, and Long-Term Outcomes. J. Clin. Med. 2025, 14, 3899. https://doi.org/10.3390/jcm14113899
Lin S-C, Chen J-S, Huang J-H, Chiu K-M, Chiang C-Y. Comparative Analysis of Perceval and Conventional Bovine Bioprosthetic Valves in Aortic Valve Replacement: Hemodynamics, Reverse Remodeling, and Long-Term Outcomes. Journal of Clinical Medicine. 2025; 14(11):3899. https://doi.org/10.3390/jcm14113899
Chicago/Turabian StyleLin, Shen-Che, Jer-Shen Chen, Jih-Hsin Huang, Kuan-Ming Chiu, and Chih-Yao Chiang. 2025. "Comparative Analysis of Perceval and Conventional Bovine Bioprosthetic Valves in Aortic Valve Replacement: Hemodynamics, Reverse Remodeling, and Long-Term Outcomes" Journal of Clinical Medicine 14, no. 11: 3899. https://doi.org/10.3390/jcm14113899
APA StyleLin, S.-C., Chen, J.-S., Huang, J.-H., Chiu, K.-M., & Chiang, C.-Y. (2025). Comparative Analysis of Perceval and Conventional Bovine Bioprosthetic Valves in Aortic Valve Replacement: Hemodynamics, Reverse Remodeling, and Long-Term Outcomes. Journal of Clinical Medicine, 14(11), 3899. https://doi.org/10.3390/jcm14113899






