Trends in Depression Among Hospitalized Patients with Type 2 Diabetes in Spain (2017–2023): A Population-Based Analysis with a Focus on Sex Differences and In-Hospital Outcomes
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Data Source
2.2. Study Population
2.3. Study Variables
2.4. Ethical Considerations
2.5. Statistical Analysis
3. Results
4. Discussion
4.1. Time Trends in Prevalence of Depression Among Hospitalized Adults with Type 2 Diabetes Mellitus (T2DM)
4.2. Variables Associated with the Presence of Depression Among Hospitalized Adults with Type 2 Diabetes Mellitus (T2DM)
4.3. Variables Associated with In-Hospital Mortality Among Adults with Type 2 Diabetes Mellitus (T2DM) Hospitalized with Concomitant Depression
4.4. Strengths and Limitations
4.5. Recommendations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| T2DM | type 2 diabetes mellitus |
| RAE-CMBD | Registry of Specialized Care Activity–Minimum Basic Data Set |
| ICD-10 | International Classification of Diseases, Tenth Revision |
| CCI | Charlson Comorbidity Index |
| LOHS | length of hospital stay |
| ICU | intensive care unit |
| IHM | in-hospital mortality |
| APC | annual percent change |
| NHANES | National Health and Nutrition Examination Survey |
References
- World Health Organization. Depressive Disorder (Depression). Available online: https://www.who.int/news-room/fact-sheets/detail/depression (accessed on 17 May 2025).
- Malhi, G.S.; Mann, J.J. Depression. Lancet 2018, 392, 2299–2312. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, S.; Zong, Q.Q.; Zhang, Q.; Ng, C.H.; Ungvari, G.S.; Xiang, Y.T. Prevalence of comorbid major depressive disorder in Type 2 diabetes: A meta-analysis of comparative and epidemiological studies. Diabet. Med. 2019, 36, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Tardif, I.; Guénette, L.; Zongo, A.; Demers, É.; Lunghi, C. Depression and the risk of hospitalization in type 2 diabetes patients: A nested case-control study accounting for non-persistence to antidiabetic treatment. Diabetes Metab. 2022, 48, 101334. [Google Scholar] [CrossRef] [PubMed]
- Simon, G.E.; Katon, W.J.; Lin, E.H.; Ludman, E.; VonKorff, M.; Ciechanowski, P.; Young, B.A. Diabetes complications and depression as predictors of health service costs. Gen. Hosp. Psychiatry 2005, 27, 344–351. [Google Scholar] [CrossRef]
- Nouwen, A.; Adriaanse, M.C.; van Dam, K.; Iversen, M.M.; Viechtbauer, W.; Peyrot, M.; Caramlau, I.; Kokoszka, A.; Kanc, K.; de Groot, M.; et al. European Depression in Diabetes (EDID) Research Consortium. Longitudinal associations between depression and diabetes complications: A systematic review and meta-analysis. Diabet. Med. 2019, 36, 1562–1572. [Google Scholar] [CrossRef]
- Brown, L.C.; Majumdar, S.R.; Newman, S.C.; Johnson, J.A. Type 2 diabetes does not increase risk of depression. CMAJ 2006, 175, 42–46. [Google Scholar] [CrossRef][Green Version]
- Icks, A.; Kruse, J.; Dragano, N.; Broecker-Preuss, M.; Slomiany, U.; Mann, K.; Jöckel, K.H.; Erbel, R.; Giani, G.; Moebus, S. Heinz Nixdorf Recall Study Investigator Group. Are symptoms of depression more common in diabetes? Results from the Heinz Nixdorf Recall study. Diabet. Med. 2008, 25, 1330–1336. [Google Scholar] [CrossRef]
- Tabák, A.G.; Akbaraly, T.N.; Batty, G.D.; Kivimäki, M. Depression and type 2 diabetes: A causal association? Lancet Diabetes Endocrinol. 2014, 2, 236–245. [Google Scholar] [CrossRef]
- Chae, W.R.; Kohring, C.; Rohde, C.; Köhler-Forsberg, O.; Otte, C.; Holstiege, J. Eight-year nationwide study of the bidirectional association between type 2 diabetes and depression in nearly 8 million German outpatients. BMJ Open Diabetes Res. Care 2024, 12, e003903. [Google Scholar] [CrossRef]
- Alzoubi, A.; Abunaser, R.; Khassawneh, A.; Alfaqih, M.; Khasawneh, A.; Abdo, N. The Bidirectional Relationship between Diabetes and Depression: A Literature Review. Korean J. Fam. Med. 2018, 39, 137–146. [Google Scholar] [CrossRef]
- Moulton, C.D.; Pickup, J.C.; Ismail, K. The link between depression and diabetes: The search for shared mechanisms. Lancet Diabetes Endocrinol. 2015, 3, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Naicker, K.; Øverland, S.; Johnson, J.A.; Manuel, D.; Skogen, J.C.; Sivertsen, B.; Colman, I. Symptoms of anxiety and depression in type 2 diabetes: Associations with clinical diabetes measures and self-management outcomes in the Norwegian HUNT study. Psychoneuroendocrinology 2017, 84, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Kuehner, C. Why is depression more common among women than among men? Lancet Psychiatry 2017, 4, 146–158. [Google Scholar] [CrossRef]
- González-Castro, T.B.; Escobar-Chan, Y.M.; Fresan, A.; López-Narváez, M.L.; Tovilla-Zárate, C.A.; Juárez-Rojop, I.E.; Ble-Castillo, J.L.; Genis-Mendoza, A.D.; Arias-Vázquez, P.I. Higher risk of depression in individuals with type 2 diabetes and obesity: Results of a meta-analysis. J. Health Psychol. 2021, 26, 1404–1419. [Google Scholar] [CrossRef]
- Nong, Y.; Wu, G.; Lu, J.; Wei, X.; Yu, D. The mediating role of obesity in the development of depression in individuals with diabetes: A population-based study from NHANES 2005–2014. J. Affect. Disord. 2024, 351, 977–982. [Google Scholar] [CrossRef]
- Shell, A.L.; Crawford, C.A.; Cyders, M.A.; Hirsh, A.T.; Stewart, J.C. Depressive disorder subtypes, depressive symptom clusters, and risk of obesity and diabetes: A systematic review. J. Affect. Disord. 2024, 353, 70–89. [Google Scholar] [CrossRef]
- Iranpour, S.; Sabour, S.; Koohi, F.; Saadati, H.M. The trend and pattern of depression prevalence in the U.S.: Data from National Health and Nutrition Examination Survey (NHANES) 2005 to 2016. J. Affect. Disord. 2022, 298, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.; Osborn, D.; Mathur, R.; Forbes, H.; Parekh, R.; Maini, A.; Neves, A.L.; Gnani, S.; Beaney, T.; Walters, K.; et al. Is alcohol use disorder associated with higher rates of depression and anxiety among people with new onset type 2 diabetes? A cohort study using linked primary care data in England. BMC Prim. Care 2024, 25, 386. [Google Scholar] [CrossRef]
- Pan, A.; Wang, Y.; Talaei, M.; Hu, F.B.; Wu, T. Relation of active, passive, and quitting smoking with incident type 2 diabetes: A systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015, 3, 958–967. [Google Scholar] [CrossRef]
- Clyde, M.; Smith, K.J.; Gariépy, G.; Schmitz, N. The association between smoking and depression in a Canadian community-based sample with type 2 diabetes. Can. J. Diabetes 2013, 37, 150–155. [Google Scholar] [CrossRef]
- Wu, L.T.; Ghitza, U.E.; Batch, B.C.; Pencina, M.J.; Rojas, L.F.; Goldstein, B.A.; Schibler, T.; Dunham, A.A.; Rusincovitch, S.; Brady, K.T. Substance use and mental diagnoses among adults with and without type 2 diabetes: Results from electronic health records data. Drug Alcohol Depend. 2015, 156, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Ter Braake, J.G.; Fleetwood, K.J.; Vos, R.C.; Blackbourn, L.; McGurnaghan, S.J.; Wild, S.H.; Jackson, C.A. Scottish Diabetes Research Network Epidemiology Group. Cardiovascular risk management among individuals with type 2 diabetes and severe mental illness: A cohort study. Diabetologia 2024, 67, 1029–1039. [Google Scholar] [CrossRef]
- Wang, Y.; Lopez, J.M.; Bolge, S.C.; Zhu, V.J.; Stang, P.E. Depression among people with type 2 diabetes mellitus, US National Health and Nutrition Examination Survey (NHANES), 2005–2012. BMC Psychiatry 2016, 16, 88. [Google Scholar] [CrossRef]
- Osme, S.F.; Ferreira, L.; Jorge, M.T.; de Souza Andréo, J.; Jorge, M.; de Melo Costa Pinto, R.; Jorge, M.T.; Jorge, P.T. Difference between the prevalence of symptoms of depression and anxiety in non-diabetic smokers and in patients with type 2 diabetes with and without nicotine dependence. Diabetol. Metab. Syndr. 2012, 4, 39. [Google Scholar] [CrossRef]
- Katon, W.J. Epidemiology and treatment of depression in patients with chronic medical illness. Dialogues Clin. Neurosci. 2011, 13, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Bakkedal, C.; Persson, F.; Christensen, M.B.; Kriegbaum, M.; Mohr, G.H.; Andersen, J.S.; Lind, B.S.; Lykkegaard, C.; Siersma, V.; Rozing, M.P. The development of type 2 diabetes management in people with severe mental illness in the Capital Region of Denmark from 2001 to 2015. Acta Psychiatr. Scand. 2024, 149, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Kahl, K.G.; Greggersen, W.; Schweiger, U.; Cordes, J.; Correll, C.U.; Frieling, H.; Balijepalli, C.; Lösch, C.; Moebus, S. Prevalence of the metabolic syndrome in patients with borderline personality disorder: Results from a cross-sectional study. Eur. Arch. Psychiatry Clin. Neurosci. 2013, 263, 205–213. [Google Scholar] [CrossRef]
- Distaso, W.; Malik, M.M.A.H.; Semere, S.; AlHakami, A.; Alexander, E.C.; Hirani, D.; Shah, R.J.; Suba, K.; McKechnie, V.; Nikčević, A.; et al. Diabetes self-management during the COVID-19 pandemic and its associations with COVID-19 anxiety syndrome, depression and health anxiety. Diabet. Med. 2022, 39, e14911. [Google Scholar] [CrossRef]
- Chao, A.M.; Wadden, T.A.; Clark, J.M.; Hayden, K.M.; Howard, M.J.; Johnson, K.C.; Laferrère, B.; McCaffery, J.M.; Wing, R.R.; Yanovski, S.Z.; et al. Changes in the Prevalence of Symptoms of Depression, Loneliness, and Insomnia in U.S. Older Adults with Type 2 Diabetes During the COVID-19 Pandemic: The Look AHEAD Study. Diabetes Care 2022, 45, 74–82. [Google Scholar] [CrossRef]
- Genis-Mendoza, A.D.; González-Castro, T.B.; Tovilla-Vidal, G.; Juárez-Rojop, I.E.; Castillo-Avila, R.G.; López-Narváez, M.L.; Tovilla-Zárate, C.A.; Sánchez-de la Cruz, J.P.; Fresán, A.; Nicolini, H. Increased Levels of HbA1c in Individuals with Type 2 Diabetes and Depression: A Meta-Analysis of 34 Studies with 68,398 Participants. Biomedicines 2022, 10, 1919. [Google Scholar] [CrossRef]
- Farooqi, A.; Khunti, K.; Abner, S.; Gillies, C.; Morriss, R.; Seidu, S. Comorbid depression and risk of cardiac events and cardiac mortality in people with diabetes: A systematic review and meta-analysis. Diabetes Res. Clin. Pract. 2019, 156, 107816. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Stone, M.A.; Peters, J.L.; Davies, M.J.; Khunti, K. The prevalence of co-morbid depression in adults with Type 2 diabetes: A systematic review and meta-analysis. Diabet. Med. 2006, 23, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- Khaledi, M.; Haghighatdoost, F.; Feizi, A.; Aminorroaya, A. The prevalence of comorbid depression in patients with type 2 diabetes: An updated systematic review and meta-analysis on huge number of observational studies. Acta Diabetol. 2019, 56, 631–650. [Google Scholar] [CrossRef]
- Lopez-de-Andres, A.; Jimenez-Garcia, R.; de Miguel-Díez, J.; Hernández-Barrera, V.; Del Barrio, J.L.; Carabantes-Alarcon, D.; Zamorano-Leon, J.J.; Noriega, C. Sex-Related Disparities in the Prevalence of Depression among Patients Hospitalized with Type 2 Diabetes Mellitus in Spain, 2011–2020. J. Clin. Med. 2022, 11, 6260. [Google Scholar] [CrossRef] [PubMed]
- Deischinger, C.; Dervic, E.; Leutner, M.; Kosi-Trebotic, L.; Klimek, P.; Kautzky, A.; Kautzky-Willer, A. Diabetes mellitus is associated with a higher risk for major depressive disorder in women than in men. BMJ Open Diabetes Res. Care 2020, 8, e001430. [Google Scholar] [CrossRef]
- Ministerio de Sanidad. Servicios Sociales e Igualdad Real Decreto 69/2015, de 6 de febrero, por el que se regula el Registro de Actividad de Atención Sanitaria Especializada. Spanish National Hospital Discharge Database. BOE 2015, 35, 10789–10809. Available online: https://www.mscbs.gob.es/estadEstudios/estadisticas/docs/BOE_RD_69_2015_RAE_CMBD.pdf (accessed on 31 January 2025).
- Sundararajan, V.; Henderson, T.; Perry, C.; Muggivan, A.; Quan, H.; Ghali, W.A. New ICD-10 version of the Charlson comorbidity index predicted in-hospital mortality. J. Clin. Epidemiol. 2004, 57, 1288–1294. [Google Scholar] [CrossRef]
- Ministerio de Sanidad, Consumo y Bienestar Social Solicitud de Extracción de Datos–Extraction Request (Spanish National Hospital Discharge Database). Available online: https://www.mscbs.gob.es/estadEstudios/estadisticas/estadisticas/estMinisterio/SolicitudCMBDdocs/2018_Formulario_Peticion_Datos_RAE_CMBD.pdf (accessed on 31 January 2025).
- Ley 14/2007, de 3 de julio, de investigación biomédica. Agencia Estatal Boletín Oficial del Estado. 2007. Available online: https://www.boe.es/eli/es/l/2007/07/03/14 (accessed on 31 March 2025).
- Kim, H.J.; Fay, M.P.; Feuer, E.J.; Midthune, D.N. Permutation tests for joinpoint regression with applications to cancer rates. Stat. Med. 2000, 19, 335–351. [Google Scholar] [CrossRef]
- Dibato, J.; Montvida, O.; Ling, J.; Koye, D.; Polonsky, W.H.; Paul, S.K. Temporal trends in the prevalence and incidence of depression and the interplay of comorbidities in patients with young- and usual-onset type 2 diabetes from the USA and the UK. Diabetologia 2022, 65, 2066–2077. [Google Scholar] [CrossRef]
- Lopez-de-Andrés, A.; Jiménez-Trujillo, M.I.; Hernández-Barrera, V.; de Miguel-Yanes, J.M.; Méndez-Bailón, M.; Perez-Farinos, N.; de Burgos Lunar, C.; Cárdenas-Valladolid, J.; Salinero-Fort, M.Á.; Jiménez-García, R.; et al. Trends in the prevalence of depression in hospitalized patients with type 2 diabetes in Spain: Analysis of hospital discharge data from 2001 to 2011. PLoS ONE 2015, 10, e0117346. [Google Scholar] [CrossRef][Green Version]
- Best, J.R.; Gan, D.R.Y.; Wister, A.V.; Cosco, T.D. Age and sex trends in depressive symptoms across middle and older adulthood: Comparison of the Canadian Longitudinal Study on Aging to American and European cohorts. J. Affect. Disord. 2021, 295, 1169–1176. [Google Scholar] [CrossRef] [PubMed]
- Roskoschinski, A.; Liang, W.; Duan, Y.; Al-Salehi, H.; Lippke, S. Loneliness and depression in older adults with multimorbidity: The role of self-efficacy and social support. Front. Psychiatry 2023, 14, 1232067. [Google Scholar] [CrossRef]
- Di Quirico, R. Uncertainty, Anxiety and the Post-Pandemic Economic Environment. Clin. Neuropsychiatry 2023, 20, 227–232. [Google Scholar] [CrossRef]
- Feng, Z.; Tong, W.K.; Zhang, X.; Tang, Z. Prevalence of depression and association with all-cause and cardiovascular mortality among individuals with type 2 diabetes: A cohort study based on NHANES 2005–2018 data. BMC Psychiatry 2023, 23, 490. [Google Scholar] [CrossRef] [PubMed]
- Zghebi, S.S.; Steinke, D.T.; Rutter, M.K.; Ashcroft, D.M. Eleven year multimorbidity burden among 637,255 people with and without type 2 diabetes: A population-based study using primary care and linked hospitalisation data. BMJ Open 2020, 10, e033866. [Google Scholar] [CrossRef]
- Bojanić, I.; Sund, E.R.; Sletvold, H.; Bjerkeset, O. Prevalence trends of depression and anxiety symptoms in adults with cardiovascular diseases and diabetes 1995–2019: The HUNT studies, Norway. BMC Psychol. 2021, 9, 130. [Google Scholar] [CrossRef]
- Alvarez-Cisneros, T.; Roa-Rojas, P.; Garcia-Peña, C. Longitudinal relationship of diabetes and depressive symptoms in older adults from Mexico: A secondary data analysis. BMJ Open Diabetes Res. Care 2020, 8, e001789. [Google Scholar] [CrossRef]
- Fu, X.; Wang, Y.; Zhao, F.; Cui, R.; Xie, W.; Liu, Q.; Yang, W. Shared biological mechanisms of depression and obesity: Focus on adipokines and lipokines. Aging 2023, 15, 5917–5950. [Google Scholar] [CrossRef] [PubMed]
- Nefs, G.; Pouwer, F.; Denollet, J.; Pop, V. The course of depressive symptoms in primary care patients with type 2 diabetes: Results from the Diabetes, Depression, Type D Personality Zuidoost-Brabant (DiaDDZoB) Study. Diabetologia 2012, 55, 608–616. [Google Scholar] [CrossRef]
- Webb, R.T.; Kontopantelis, E.; Doran, T.; Qin, P.; Creed, F.; Kapur, N. Suicide risk in primary care patients with major physical diseases: A case-control study. Arch. Gen. Psychiatry 2012, 69, 256–264. [Google Scholar] [CrossRef]
- Paudel, S.; Khanal, S.P.; Gautam, S.; Chalise, A.; Koirala, T.N.; Marahatta, S.B. Anxiety and depression among people with type 2 diabetes visiting diabetes clinics of Pokhara Metropolitan, Nepal: A cross-sectional study. BMJ Open 2023, 13, e064490. [Google Scholar] [CrossRef] [PubMed]
- Steenblock, C.; Schwarz, P.E.H.; Perakakis, N.; Brajshori, N.; Beqiri, P.; Bornstein, S.R. The interface of COVID-19, diabetes, and depression. Discov. Ment. Health 2022, 2, 5. [Google Scholar] [CrossRef]
- Forde, R.; Arente, L.; Ausili, D.; De Backer, K.; Due-Christensen, M.; Epps, A.; Fitzpatrick, A.; Grixti, M.; Groen, S.; Halkoaho, A.; et al. FEND COVID-19 consortium. The impact of the COVID-19 pandemic on people with diabetes and diabetes services: A pan-European survey of diabetes specialist nurses undertaken by the Foundation of European Nurses in Diabetes survey consortium. Diabet. Med. 2021, 38, e14498. [Google Scholar] [CrossRef]
- Khunti, K.; Aroda, V.R.; Aschner, P.; Chan, J.C.N.; Del Prato, S.; Hambling, C.E.; Harris, S.; Lamptey, R.; McKee, M.; Tandon, N.; et al. The impact of the COVID-19 pandemic on diabetes services: Planning for a global recovery. Lancet Diabetes Endocrinol. 2022, 10, 890–900. [Google Scholar] [CrossRef] [PubMed]
- Czeisler, M.É.; Barrett, C.E.; Siegel, K.R.; Weaver, M.D.; Czeisler, C.A.; Rajaratnam, S.M.W.; Howard, M.E.; Bullard, K.M. Health Care Access and Use Among Adults with Diabetes During the COVID-19 Pandemic—United States, February–March 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1597–1602. [Google Scholar] [CrossRef] [PubMed]
- García-Lara, R.A.; Suleiman-Martos, N.; Membrive-Jiménez, M.J.; García-Morales, V.; Quesada-Caballero, M.; Guisado-Requena, I.M.; Gómez-Urquiza, J.L. Prevalence of Depression and Related Factors among Patients with Chronic Disease during the COVID-19 Pandemic: A Systematic Review and Meta-Analysis. Diagnostics 2022, 12, 3094. [Google Scholar] [CrossRef]
- Wu, C.S.; Hsu, L.Y.; Wang, S.H. Association of depression and diabetes complications and mortality: A population-based cohort study. Epidemiol. Psychiatr. Sci. 2020, 29, e96. [Google Scholar] [CrossRef]
- Naskar, S.; Victor, R.; Nath, K. Depression in diabetes mellitus-A comprehensive systematic review of literature from an Indian perspective. Asian J. Psychiatr. 2017, 27, 85–100. [Google Scholar] [CrossRef]
- Engelmann, J.; Manuwald, U.; Rubach, C.; Kugler, J.; Birkenfeld, A.L.; Hanefeld, M.; Rothe, U. Determinants of mortality in patients with type 2 diabetes: A review. Rev. Endocr. Metab. Disord. 2016, 17, 129–137. [Google Scholar] [CrossRef]
- Huang, C.J.; Hsieh, H.M.; Chiu, H.C.; Wang, P.W.; Lee, M.H.; Li, C.Y.; Lin, C.H. Impact of Anxiety Disorders on Mortality for Persons With Diabetes: A National Population-Based Cohort Study. Psychosomatics 2017, 58, 266–273. [Google Scholar] [CrossRef]
- Wu, C.; Li, Y.; Li, N.; Chan, K.K.; Piao, C. Body Mass Index and Risk of All-Cause and Cardiovascular Disease Mortality in Patients with Type 2 Diabetes Mellitus. Endocrinology 2025, 166, bqaf040. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.; Kim, H.J.; Park, S.; Park, Y.G.; Cho, K.H. Body Mass Index-Related Mortality in Patients with Type 2 Diabetes and Heterogeneity in Obesity Paradox Studies: A Dose-Response Meta-Analysis. PLoS ONE 2017, 12, e0168247. [Google Scholar] [CrossRef]
- Atkins, J.L.; Masoli, J.A.H.; Delgado, J.; Pilling, L.C.; Kuo, C.L.; Kuchel, G.A.; Melzer, D. Preexisting Comorbidities Predicting COVID-19 and Mortality in the UK Biobank Community Cohort. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 2224–2230. [Google Scholar] [CrossRef]
- Lee, K.; Hwang, J. Impact of underlying diseases and complications on COVID-19 mortality in South Korea: Analysis of national health insurance service data. Arch. Public Health 2025, 83, 20. [Google Scholar] [CrossRef] [PubMed]
- Clouston, S.A.P.; Luft, B.J.; Sun, E. Clinical risk factors for mortality in an analysis of 1375 patients admitted for COVID treatment. Sci. Rep. 2021, 11, 23414. [Google Scholar] [CrossRef]
- Fiest, K.M.; Jette, N.; Quan, H.; St Germaine-Smith, C.; Metcalfe, A.; Patten, S.B.; Beck, C.A. Systematic review and assessment of validated case definitions for depression in administrative data. BMC Psychiatry 2014, 14, 289. [Google Scholar] [CrossRef]
- Pena-Gralle, A.P.B.; Talbot, D.; Trudel, X.; Aubé, K.; Lesage, A.; Lauzier, S.; Milot, A.; Brisson, C. Validation of case definitions of depression derived from administrative data against the CIDI-SF as reference standard: Results from the PROspective Québec (PROQ) study. BMC Psychiatry 2021, 21, 491. [Google Scholar] [CrossRef] [PubMed]
- Doktorchik, C.; Patten, S.; Eastwood, C.; Peng, M.; Chen, G.; Beck, C.A.; Jetté, N.; Williamson, T.; Quan, H. Validation of a case definition for depression in administrative data against primary chart data as a reference standard. BMC Psychiatry 2019, 19, 9. [Google Scholar] [CrossRef]
- Menéndez Torre, E.L.; Ares Blanco, J.; Conde Barreiro, S.; Rojo Martínez, G.; Delgado Alvarez, E.; en representación del Grupo de Epidemiología de la Sociedad Española de Diabetes (SED). Prevalence of diabetes mellitus in Spain in 2016 according to the Primary Care Clinical Database (BDCAP). Endocrinol. Diabetes Nutr. (Engl. Ed.) 2021, 68, 109–115. [Google Scholar] [CrossRef]
- Lipscombe, L.L.; Hwee, J.; Webster, L.; Shah, B.R.; Booth, G.L.; Tu, K. Identifying diabetes cases from administrative data: A population-based validation study. BMC Health Serv. Res. 2018, 18, 316. [Google Scholar] [CrossRef]
- Lascar, N.; Brown, J.; Pattison, H.; Barnett, A.H.; Bailey, C.J.; Bellary, S. Type 2 diabetes in adolescents and young adults. Lancet Diabetes Endocrinol. 2018, 6, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Maimaitituerxun, R.; Chen, W.; Xiang, J.; Kaminga, A.C.; Wu, X.Y.; Chen, L.; Yang, J.; Liu, A.; Dai, W. Prevalence of comorbid depression and associated factors among hospitalized patients with type 2 diabetes mellitus in Hunan, China. BMC Psychiatry 2023, 23, 158. [Google Scholar] [CrossRef] [PubMed]
- Joseph, L.M.; Berry, D.C.; Jessup, A.; Davison, J.; Schneider, B.J.; Twersky, J.I. Implementing and feasibility testing depression screening using the electronic medical record for patients with type 2 diabetes admitted to the hospital. Nurs. Open 2018, 6, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Messina, R.; Iommi, M.; Rucci, P.; Reno, C.; Fantini, M.P.; Lunghi, C.; Altini, M.; Bravi, F.; Rosa, S.; Nicolucci, A.; et al. Is it time to consider depression as a major complication of type 2 diabetes? Evidence from a large population-based cohort study. Acta Diabetol. 2022, 59, 95–104. [Google Scholar] [CrossRef]
- Lindekilde, N.; Scheuer, S.H.; Diaz, L.J.; Rubin, K.H.; Plana-Ripoll, O.; Henriksen, J.E.; Lasgaard, M.; Andersen, G.S.; Pouwer, F. Risk of Developing Type 2 Diabetes in Individuals With a Psychiatric Disorder: A Nationwide Register-Based Cohort Study. Diabetes Care 2022, 45, 724–733. [Google Scholar] [CrossRef] [PubMed]

| 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | 2023 | Time Trend Analysis p | |
|---|---|---|---|---|---|---|---|---|
| T2DM, n | 620,395 | 650,396 | 663,794 | 611,899 | 653,517 | 688,486 | 709,181 | |
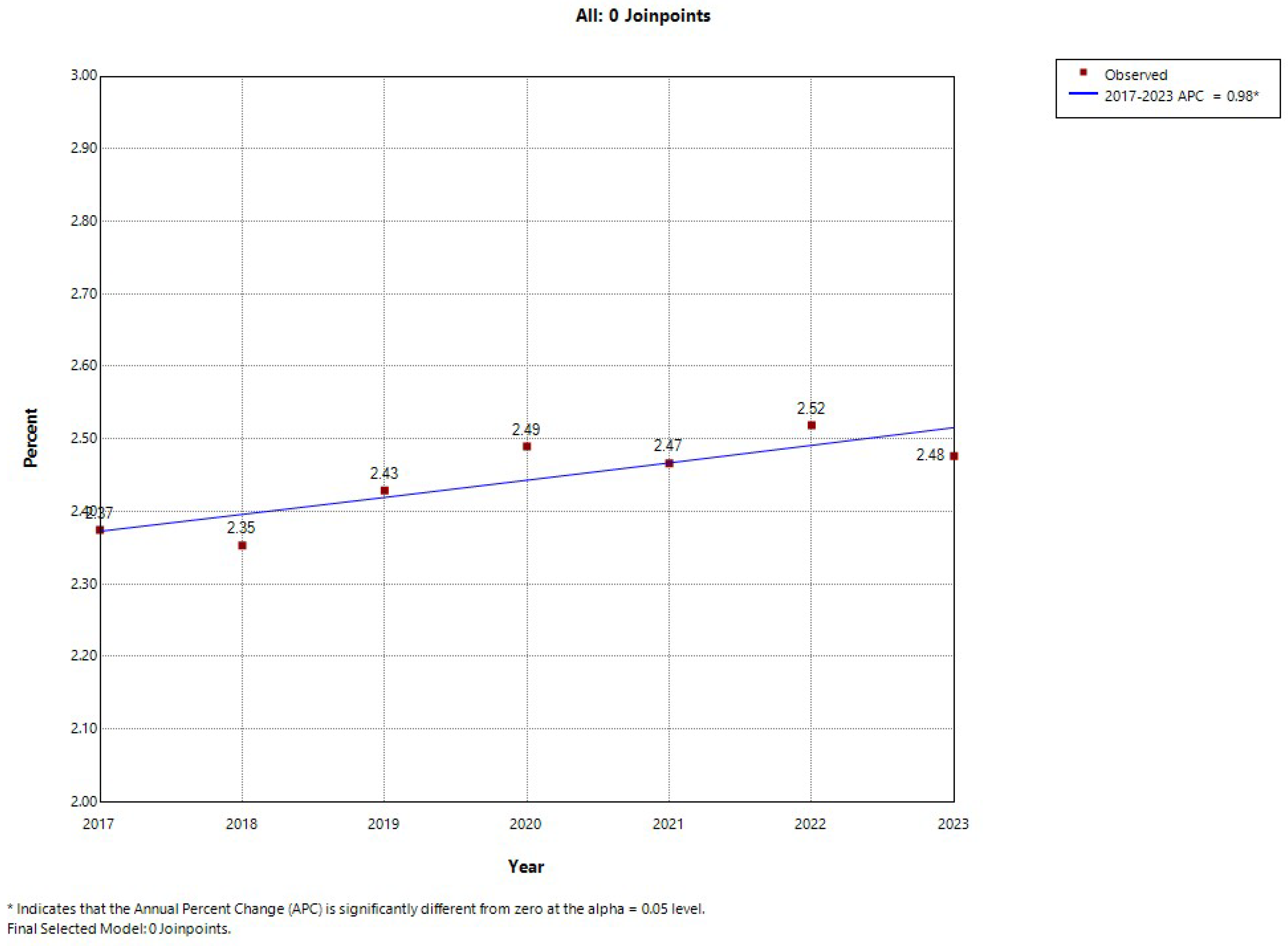
| Prevalence of depression, n (%) | 26,889 (4.33) | 27,446 (4.22) | 29,136 (4.39) | 27,091 (4.43) | 28,928 (4.43) | 30,932 (4.49) | 31,672 (4.47) | <0.001 |
| Men, n (%) | 8460 (31.46) | 8888 (32.38) | 9388 (32.22) | 8963 (33.08) | 9481 (32.77) | 10,175 (32.89) | 10,382 (32.78) | <0.001 |
| Women, n (%) | 18,429 (68.54) | 18,558 (67.62) | 19,748 (67.78) | 18,128 (66.92) | 19,447 (67.23) | 20,757 (67.11) | 21,290 (67.22) | |
| Age, mean (SD) | 74.77 (11.07) | 74.99 (11.11) | 75.19 (11.1) | 75.48 (11.14) | 75.48 (11.17) | 76.05 (11.14) | 76.02 (11.26) | <0.001 |
| CCI, mean (SD) | 0.95 (0.91) | 0.95 (0.91) | 0.97 (0.92) | 0.97 (0.92) | 0.98 (0.93) | 0.99 (0.93) | 1 (0.92) | <0.001 |
| Admission to ICU, n (%) | 1289 (4.79) | 1368 (4.98) | 1481 (5.08) | 1405 (5.19) | 1449 (5.01) | 1621 (5.24) | 1773 (5.6) | <0.001 |
| LOHS, median (IQR) | 6 (8) | 6 (7) | 6 (7) | 6 (8) | 6 (8) | 6 (7) | 6 (7) | 0.089 |
| IHM, n (%) | 1717 (6.39) | 1776 (6.47) | 1933 (6.63) | 2389 (8.82) | 2251 (7.78) | 2328 (7.53) | 2173 (6.86) | <0.001 |
| 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | 2023 | Time Trend Analysis p | |
|---|---|---|---|---|---|---|---|---|
| Age, mean (SD) | 76.04 (10.75) | 76.25 (10.75) | 76.38 (10.84) | 76.81 (10.8) | 76.9 (10.87) | 77.47 (10.82) | 77.43 (10.98) | <0.001 |
| CCI, mean (SD) | 0.89 (0.88) | 0.88 (0.88) | 0.91 (0.89) | 0.91 (0.89) | 0.93 (0.89) | 0.94 (0.9) | 0.96 (0.9) | <0.001 |
| Hypoglycemia, n (%) | 171 (0.93) | 253 (1.36) | 249 (1.26) | 263 (1.45) | 267 (1.37) | 289 (1.39) | 273 (1.28) | <0.001 |
| Obesity, n (%) | 4176 (22.66) | 4035 (21.74) | 4617 (23.38) | 4236 (23.37) | 4771 (24.53) | 4829 (23.26) | 4787 (22.48) | <0.001 |
| Anxiety, n (%) | 240 (1.3) | 255 (1.37) | 319 (1.62) | 320 (1.77) | 395 (2.03) | 472 (2.27) | 453 (2.13) | <0.001 |
| Specific personality disorders, n (%) | 187 (1.01) | 185 (1) | 225 (1.14) | 190 (1.05) | 231 (1.19) | 249 (1.2) | 297 (1.4) | 0.002 |
| Intentionally self-inflicted injuries, n (%) | 84 (0.46) | 167 (0.9) | 163 (0.83) | 149 (0.82) | 140 (0.72) | 131 (0.63) | 159 (0.75) | <0.001 |
| Suicide attempts, n (%) | 15 (0.08) | 11 (0.06) | 9 (0.05) | 9 (0.05) | 7 (0.04) | 14 (0.07) | 17 (0.08) | 0.439 |
| Alcohol, n (%) | 273 (1.48) | 281 (1.51) | 372 (1.88) | 347 (1.91) | 398 (2.05) | 417 (2.01) | 402 (1.89) | <0.001 |
| Tobacco use, n (%) | 1773 (9.62) | 1884 (10.15) | 2211 (11.2) | 2003 (11.05) | 2278 (11.71) | 2662 (12.82) | 2975 (13.97) | <0.001 |
| All-cause dementia, n (%) | 2091 (11.35) | 2139 (11.53) | 2346 (11.88) | 2289 (12.63) | 2394 (12.31) | 2678 (12.9) | 2661 (12.5) | <0.001 |
| Alzheimer’s dementia, n (%) | 771 (4.18) | 826 (4.45) | 914 (4.63) | 886 (4.89) | 871 (4.48) | 1002 (4.83) | 1019 (4.79) | 0.012 |
| Vascular dementia, n (%) | 412 (2.24) | 365 (1.97) | 445 (2.25) | 407 (2.25) | 448 (2.3) | 484 (2.33) | 490 (2.3) | 0.247 |
| COVID-19 infection, n (%) | 0 (0) | 0 (0) | 0 (0) | 929 (5.12) | 1525 (7.84) | 2030 (9.78) | 881 (4.14) | <0.001 |
| Admission to ICU, n (%) | 784 (4.25) | 828 (4.46) | 880 (4.46) | 828 (4.57) | 834 (4.29) | 924 (4.45) | 1046 (4.91) | 0.035 |
| LOHS, median (IQR) | 6 (7) | 6 (7) | 6 (7) | 6 (7) | 6 (7) | 6 (7) | 6 (7) | 0.102 |
| IHM, n (%) | 1152 (6.25) | 1174 (6.33) | 1280 (6.48) | 1584 (8.74) | 1527 (7.85) | 1602 (7.72) | 1516 (7.12) | <0.001 |
| 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | 2023 | Time Trend Analysis p | |
|---|---|---|---|---|---|---|---|---|
| Age, mean (SD) | 71.99 (11.26) | 72.36 (11.4) | 72.7 (11.25) | 72.79 (11.33) | 72.55 (11.21) | 73.15 (11.22) | 73.13 (11.26) | <0.001 |
| CCI, mean (SD) | 1.07 (0.95) | 1.09 (0.96) | 1.1 (0.98) | 1.09 (0.97) | 1.08 (0.99) | 1.09 (0.97) | 1.08 (0.96) | 0.494 |
| Hypoglycemia, n (%) | 96 (1.13) | 108 (1.22) | 112 (1.19) | 118 (1.32) | 112 (1.18) | 157 (1.54) | 115 (1.11) | 0.097 |
| Obesity, n (%) | 1143 (13.51) | 1249 (14.05) | 1336 (14.23) | 1330 (14.84) | 1578 (16.64) | 1664 (16.35) | 1649 (15.88) | <0.001 |
| Anxiety, n (%) | 81 (0.96) | 107 (1.2) | 120 (1.28) | 132 (1.47) | 156 (1.65) | 167 (1.64) | 216 (2.08) | <0.001 |
| Specific personality disorders, n (%) | 137 (1.62) | 125 (1.41) | 149 (1.59) | 171 (1.91) | 178 (1.88) | 180 (1.77) | 192 (1.85) | 0.086 |
| Intentionally self-inflicted injuries, n (%) | 33 (0.39) | 84 (0.95) | 57 (0.61) | 57 (0.64) | 76 (0.8) | 66 (0.65) | 71 (0.68) | <0.001 |
| Suicide attempts, n (%) | 13 (0.15) | 20 (0.23) | 25 (0.27) | 14 (0.16) | 14 (0.15) | 16 (0.16) | 18 (0.17) | 0.395 |
| Alcohol, n (%) | 1144 (13.52) | 1200 (13.5) | 1264 (13.46) | 1214 (13.54) | 1427 (15.05) | 1543 (15.16) | 1632 (15.72) | <0.001 |
| Tobacco use, n (%) | 3319 (39.23) | 3445 (38.76) | 3864 (41.16) | 3509 (39.15) | 3717 (39.2) | 4054 (39.84) | 4230 (40.74) | 0.004 |
| All-cause dementia, n (%) | 793 (9.37) | 905 (10.18) | 875 (9.32) | 893 (9.96) | 980 (10.34) | 1092 (10.73) | 1067 (10.28) | 0.01 |
| Alzheimer’s dementia, n (%) | 229 (2.71) | 262 (2.95) | 251 (2.67) | 191 (2.13) | 263 (2.77) | 279 (2.74) | 294 (2.83) | 0.028 |
| Vascular dementia, n (%) | 170 (2.01) | 198 (2.23) | 217 (2.31) | 211 (2.35) | 226 (2.38) | 249 (2.45) | 237 (2.28) | 0.57 |
| COVID-19 infection, n (%) | 0 (0) | 0 (0) | 0 (0) | 472 (5.27) | 703 (7.41) | 1054 (10.36) | 448 (4.32) | <0.001 |
| Admission to ICU, n (%) | 505 (5.97) | 540 (6.08) | 601 (6.4) | 577 (6.44) | 615 (6.49) | 697 (6.85) | 727 (7) | 0.041 |
| LOHS, median (IQR) | 6 (8) | 6 (8) | 6 (8) | 6 (8) | 6 (8) | 6 (7) | 6 (7) | 0.091 |
| IHM, n (%) | 565 (6.68) | 602 (6.77) | 653 (6.96) | 805 (8.98) | 724 (7.64) | 726 (7.14) | 657 (6.33) | <0.001 |
| Presence of Depression | IHM in Patients with T2DM and Depression | ||||||
|---|---|---|---|---|---|---|---|
| Men | Women | Both Sexes | Men | Women | Both Sexes | ||
| OR (95%CI) | OR (95%CI) | OR (95%CI) | OR (95%CI) | OR (95%CI) | OR (95%CI) | ||
| Year of admission | 2017 | 1 | 1 | 1 | 1 | 1 | 1 |
| 2018 | 0.99 (0.96–1.02) | 0.98 (0.96–1) | 0.99 (0.97–1) | 0.99 (0.88–1.12) | 1 (0.92–1.09) | 0.99 (0.93–1.07) | |
| 2019 | 1.02 (0.99–1.05) | 1.04 (1.01–1.06) | 1.03 (1.01–1.05) | 1.01 (0.9–1.14) | 1 (0.92–1.09) | 1 (0.94–1.07) | |
| 2020 | 1.04 (1.01–1.07) | 1.05 (1.03–1.07) | 1.05 (1.03–1.07) | 1.28 (1.14–1.44) | 1.32 (1.22–1.43) | 1.31 (1.22–1.4) | |
| 2021 | 1.03 (1–1.06) | 1.06 (1.04–1.08) | 1.05 (1.03–1.07) | 1.06 (0.94–1.19) | 1.14 (1.05–1.24) | 1.11 (1.04–1.19) | |
| 2022 | 1.05 (1.02–1.08) | 1.07 (1.05–1.1) | 1.07 (1.05–1.09) | 0.92 (0.82–1.04) | 1.05 (0.97–1.14) | 1.01 (0.94–1.08) | |
| 2023 | 1.03 (1–1.06) | 1.08 (1.06–1.1) | 1.07 (1.05–1.08) | 0.87 (0.77–0.98) | 1 (0.92–1.08) | 0.96 (0.9–1.02) | |
| Age group | 40–64 years | 1 | 1 | 1 | 1 | 1 | 1 |
| 65–74 years | 0.91 (0.9–0.93) | 1.16 (1.14–1.19) | 1.04 (1.02–1.05) | 1.47 (1.32–1.64) | 1.53 (1.37–1.7) | 1.49 (1.38–1.6) | |
| 75–84 years | 0.91 (0.89–0.93) | 1.15 (1.13–1.17) | 1.03 (1.02–1.05) | 2.12 (1.91–2.35) | 2.41 (2.18–2.67) | 2.25 (2.09–2.42) | |
| ≥85 years | 0.99 (0.96–1.02) | 0.98 (0.96–1) | 0.93 (0.91–0.94) | 3.3 (2.96–3.68) | 4.11 (3.71–4.56) | 3.75 (3.49–4.03) | |
| CCI | 0.97 (0.96–0.98) | 0.94 (0.93–0.95) | 0.95 (0.95–0.95) | 1.41 (1.37–1.45) | 1.46 (1.43–1.49) | 1.44 (1.42–1.47) | |
| Hypoglycemia | 1.23 (1.14–1.32) | 0.99 (0.95–1.04) | 1.06 (1.02–1.11) | 1.45 (1.16–1.81) | 1.17 (0.99–1.38) | 1.26 (1.1–1.44) | |
| Obesity | 1.13 (1.1–1.15) | 1.25 (1.24–1.27) | 1.22 (1.21–1.23) | 0.67 (0.61–0.74) | 0.84 (0.79–0.88) | 0.79 (0.76–0.83) | |
| Anxiety | 0.79 (0.74–0.84) | 0.29 (0.28–0.31) | 0.36 (0.35–0.37) | 0.6 (0.42–0.85) | 0.82 (0.68–0.98) | 0.76 (0.64–0.89) | |
| Specific personality disorders | 5.73 (5.37–6.11) | 2.85 (2.7–3.02) | 3.77 (3.61–3.94) | 0.8 (0.59–1.09) | 0.69 (0.51–0.92) | 0.74 (0.59–0.91) | |
| Intentionally self-inflicted injuries | 1.41 (1.28–1.55) | 1.04 (0.98–1.12) | 1.14 (1.08–1.21) | 1.8 (1.3–2.48) | 0.62 (0.45–0.86) | 0.94 (0.75–1.18) | |
| Suicide attempts | 8.52 (6.84–10.62) | 4 (3.06–5.22) | 6.47 (5.44–7.69) | 0.62 (0.23–1.7) | 1.11 (0.35–3.55) | 0.82 (0.38–1.76) | |
| Alcohol | 1.29 (1.26–1.32) | 1.24 (1.19–1.29) | 1.26 (1.23–1.28) | 1.13 (1.02–1.24) | 1.11 (0.91–1.35) | 1.14 (1.04–1.24) | |
| Tobacco use | 0.94 (0.93–0.96) | 1.19 (1.17–1.21) | 1.03 (1.02–1.04) | 0.84 (0.79–0.9) | 0.9 (0.82–0.98) | 0.86 (0.82–0.91) | |
| Alzheimer’s dementia | 1.44 (1.37–1.51) | 1.1 (1.07–1.13) | 1.16 (1.13–1.18) | 1.46 (1.26–1.69) | 1.63 (1.51–1.76) | 1.59 (1.48–1.7) | |
| Vascular dementia | 1.86 (1.77–1.96) | 1.41 (1.36–1.47) | 1.54 (1.5–1.59) | 1.23 (1.05–1.45) | 1.06 (0.94–1.19) | 1.11 (1.01–1.22) | |
| COVID-19 infection | 1 (0.96–1.04) | 0.97 (0.95–1) | 0.98 (0.96–1.01) | 2.11 (1.86–2.38) | 1.97 (1.81–2.15) | 2.01 (1.87–2.16) | |
| Sex | Men | NA | NA | 1 | NA | NA | 1 |
| Women | NA | NA | 3.21 (3.18–3.24) | NA | NA | 1.11 (1.07–1.16) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez-Sierra, L.; Cuadrado-Corrales, N.; Hernández-Barrera, V.; Jiménez-Garcia, R.; López-de-Andres, A.; de Miguel-Diez, J.; Bodas-Pinedo, A.; Zamorano-León, J.J. Trends in Depression Among Hospitalized Patients with Type 2 Diabetes in Spain (2017–2023): A Population-Based Analysis with a Focus on Sex Differences and In-Hospital Outcomes. J. Clin. Med. 2025, 14, 3895. https://doi.org/10.3390/jcm14113895
Jiménez-Sierra L, Cuadrado-Corrales N, Hernández-Barrera V, Jiménez-Garcia R, López-de-Andres A, de Miguel-Diez J, Bodas-Pinedo A, Zamorano-León JJ. Trends in Depression Among Hospitalized Patients with Type 2 Diabetes in Spain (2017–2023): A Population-Based Analysis with a Focus on Sex Differences and In-Hospital Outcomes. Journal of Clinical Medicine. 2025; 14(11):3895. https://doi.org/10.3390/jcm14113895
Chicago/Turabian StyleJiménez-Sierra, Lucia, Natividad Cuadrado-Corrales, Valentín Hernández-Barrera, Rodrigo Jiménez-Garcia, Ana López-de-Andres, Javier de Miguel-Diez, Andrés Bodas-Pinedo, and José J. Zamorano-León. 2025. "Trends in Depression Among Hospitalized Patients with Type 2 Diabetes in Spain (2017–2023): A Population-Based Analysis with a Focus on Sex Differences and In-Hospital Outcomes" Journal of Clinical Medicine 14, no. 11: 3895. https://doi.org/10.3390/jcm14113895
APA StyleJiménez-Sierra, L., Cuadrado-Corrales, N., Hernández-Barrera, V., Jiménez-Garcia, R., López-de-Andres, A., de Miguel-Diez, J., Bodas-Pinedo, A., & Zamorano-León, J. J. (2025). Trends in Depression Among Hospitalized Patients with Type 2 Diabetes in Spain (2017–2023): A Population-Based Analysis with a Focus on Sex Differences and In-Hospital Outcomes. Journal of Clinical Medicine, 14(11), 3895. https://doi.org/10.3390/jcm14113895







