Translating a Home-Based Breathlessness Service: A Pilot Study of Feasibility, Person-Reported, and Hospital Use Outcomes
Abstract
1. Introduction
- Examine the feasibility and participant experience of the BLIS program;
- Describe pre–post BLIS program changes in patient-reported outcomes of distress due to breathlessness and carer-reported outcomes of burden and wellbeing;
- Describe public hospital-related health care utilization for a COPD-related cause 12 months before and after commencing the BLIS program.
2. Materials and Methods
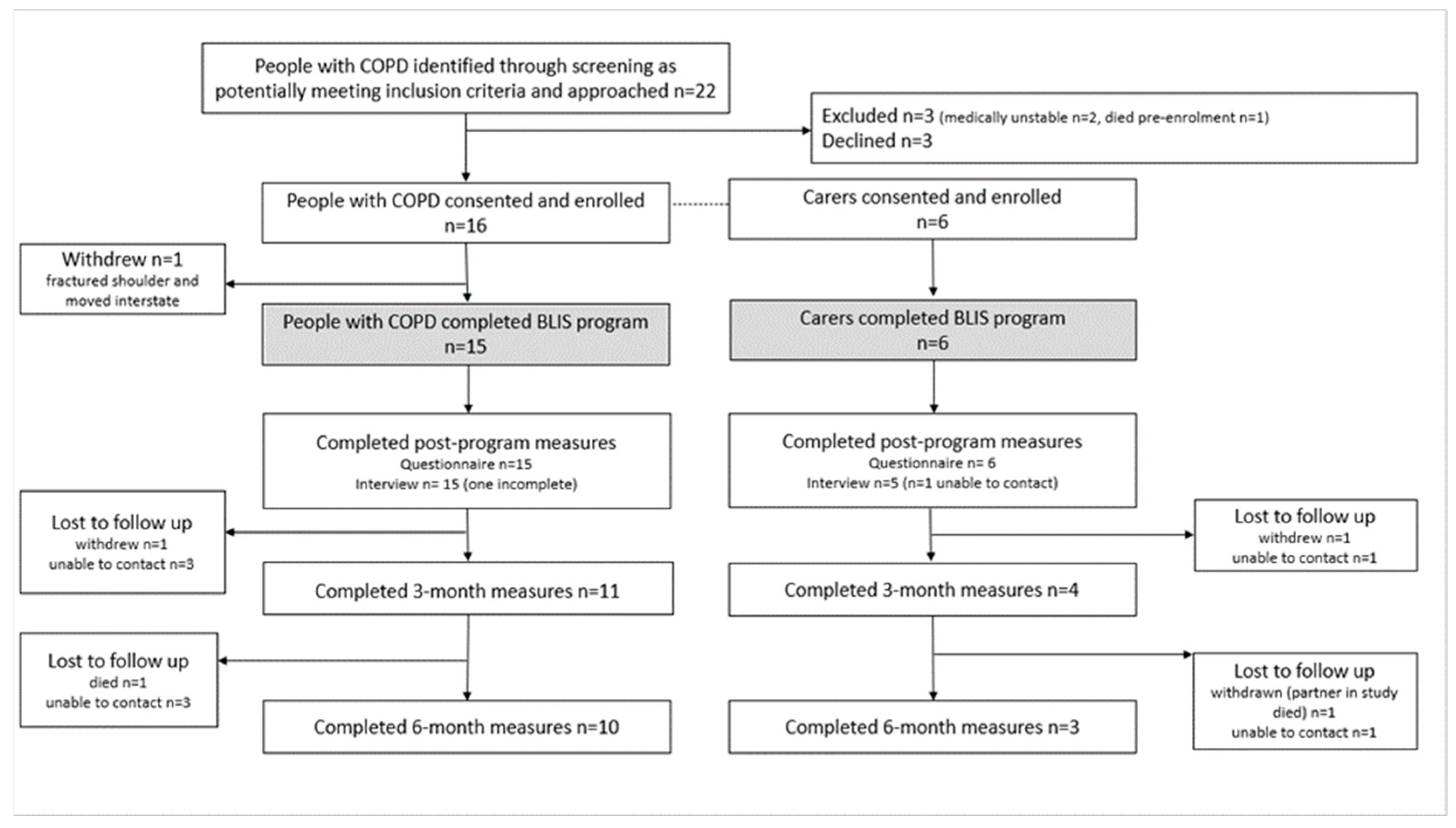
2.1. Study Design and Ethics Approvals
2.2. Consumer and Stakeholder Involvement
2.3. Participant Eligibility Criteria
2.4. Study Recruitment
2.5. Breathlessness Intervention Service (BLIS) Program Intervention
2.6. Program Feasibility and Acceptability
2.7. Person Living with Breathlessness/Carer-Reported Outcomes
2.8. Hospital-Related Health Care Utilization
2.9. Data Management and Synthesis
3. Results
3.1. Participant Baseline Characteristics
3.2. BLIS Program Delivery
3.3. Feasibility and Acceptability Results: Study Objective 1
3.4. Person Living with Breathlessness/Carer-Reported Outcome Results: Study Objective 2
3.5. Hospital-Related Health Care Utilization Results: Study Objective 3
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AQoL8-D | Assessment of Quality of Life |
| B-IPQ | Brief Illness Perception Questionnaire (breathlessness version) |
| BLIS | Breathlessness Intervention Service |
| BTF | Breathing Thinking Functioning |
| CBIS | Cambridge Breathlessness Intervention Service |
| CI | Confidence Interval |
| COPD | Chronic Obstructive Pulmonary Disease |
| CRQ | Chronic Respiratory Questionnaire |
| DASS-21 | Depression, Anxiety, and Stress Scale |
| DRG | Diagnosis-Related Group |
| FEV1%pred | Forced expiratory volume in one second percentage predicted |
| HREC | Human Research Ethics Committee |
| MDP | Multidimensional Dyspnea Profile |
| NHS | National Health Service |
| QALY | Quality-Adjusted Life Years |
| RNP | Respiratory Nurse Practitioner |
| UK | United Kingdom |
| ZBI-12 | Zarit Burden Interview |
References
- van Dijk, M.; Gan, C.T.; Koster, T.D.; Wijkstra, P.J.; Slebos, D.J.; Kerstjens, H.A.M.; van der Vaart, H.; Duiverman, M.L. Treatment of severe stable COPD: The multidimensional approach of treatable traits. ERJ Open Res. 2020, 6, 00322-2019. [Google Scholar] [CrossRef] [PubMed]
- Miravitlles, M.; Ribera, A. Understanding the impact of symptoms on the burden of COPD. Respir. Res. 2017, 18, 67. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.J.; Yorke, J.; Hansen-Flaschen, J.; Lansing, R.; Ekström, M.; Similowski, T.; Currow, D. Towards an expert consensus to delineate a clinical syndrome of chronic breathlessness. Eur. Respir. J. 2017, 49, 602277. [Google Scholar] [CrossRef] [PubMed]
- Disler, R.; Green, A.; Luckett, T.; Newton, P.; Inglis, S.; Currow, D.; Davidson, P. Experience of advanced chronic obstructive pulmonary disease: Metasynthesis of qualitative research. J. Pain Symptom Manag. 2014, 48, 1182–1199. [Google Scholar] [CrossRef]
- Reitzel, T.; Bergmann, A.; Schloesser, K.; Pauli, B.; Eisenmann, Y.; Randerath, W.; Tuchscherer, A.; Frank, K.; Simon, S.T.; Pralong, A. The experience of episodic breathlessness from the perspective of informal caregivers: A qualitative interview study. Ann. Palliat. Med. 2022, 11, 2225–2234. [Google Scholar] [CrossRef]
- Dzingina, M.; Reilly, C.; Bausewein, C.; Jolley, C.; Moxham, J.; McCrone, P.; Higginson, I.; Yi, D. Variations in the cost of formal and informal health care for patients with advanced chronic disease and refractory breathlessness: A cross sectional secondary analysis. Palliat. Med. 2017, 31, 369–377. [Google Scholar] [CrossRef]
- Brighton, L.J.; Miller, S.; Farquhar, M.; Booth, S.; Yi, D.; Gao, W.; Bajwah, S.; Man, W.D.; Higginson, I.J.; Maddocks, M. Holistic services for people with advanced disease and chronic breathlessness: A systematic review and meta-analysis. Thorax 2019, 74, 270–281. [Google Scholar] [CrossRef]
- Spathis, A.; Reilly, C.C.; Bausewein, C.; Reinke, L.F.; Romero, L.; Smallwood, N.E.; Ekström, M.; Holland, A.E. Multicomponent services for symptoms in serious respiratory illness: A systematic review and meta-analysis. Eur. Respir. Rev. 2024, 33, 240054. [Google Scholar] [CrossRef]
- Holland, A.E.; Spathis, A.; Marsaa, K.; Bausewein, C.; Ahmadi, Z.; Burge, A.T.; Pascoe, A.; Gadowski, A.M.; Collis, P.; Jelen, T.; et al. European Respiratory Society Clinical Practice Guideline on symptom management for adults with serious respiratory illness. Eur. Respir. J. 2024, 63, 2400335. [Google Scholar] [CrossRef]
- Seidl, H.; Schunk, M.; Le, L.; Syunyaeva, Z.; Streitwieser, S.; Berger, U.; Mansmann, U.; Szentes, B.L.; Bausewein, C.; Schwarzkopf, L. Cost-effectiveness of a specialized breathlessness service versus usual care for patients with advanced diseases. Value Health 2023, 26, 81–90. [Google Scholar] [CrossRef]
- Farquhar, M.C.; Prevost, A.T.; McCrone, P.; Brafman-Price, B.; Bentley, A.; Higginson, I.J.; Todd, C.; Booth, S. Is a specialist breathlessness service more effective and cost-effective for patients with advanced cancer and their carers than standard care? Findings of a mixed-method randomised controlled trial. BMC Med. 2014, 12, 194. [Google Scholar] [CrossRef] [PubMed]
- Schunk, M.; Le, L.; Syunyaeva, Z.; Haberland, B.; Tänzler, S.; Mansmann, U.; Schwarzkopf, L.; Seidl, H.; Streitweiser, S.; Hofmann, M.; et al. Effectiveness of a specialised breathlessness service for patients with advanced disease in Germany: A pragmatic fast-track randomised controlled trial (BreathEase). Eur. Respir. J. 2021, 58, 2002139. [Google Scholar] [CrossRef] [PubMed]
- Mooren, K.; Wester, D.; Kerstjens, H.; Bergkamp, E.; Spathis, A.; Engels, Y. Filling the gap: A feasibility study of a COPD-specific breathlessness service. COPD 2022, 19, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Drury, A.; Goss, J.; Afolabi, J.; McHugh, G.; O’Leary, N.; Brady, A. A mixed methods evaluation of a pilot multidisciplinary breathlessness support service. Eval. Rev. 2023, 47, 820–870. [Google Scholar] [CrossRef]
- Qian, M.Y.; Politis, J.; Thompson, M.; Wong, D.; Le, B.; Irving, L.; Smallwood, N. Individualized breathlessness interventions may improve outcomes in patients with advanced COPD. Respirology 2018, 23, 1146–1151. [Google Scholar] [CrossRef]
- Luckett, T.; Roberts, M.M.; Smith, T.; Swami, V.; Cho, J.G.; Wheatley, J.R. Patient perspectives on how to optimise benefits from a breathlessness service for people with COPD. NPJ Prim. Care Respir. Med. 2020, 30, 16. [Google Scholar] [CrossRef]
- Lancaster, G.A.; Thabane, L. Guidelines for reporting non-randomised pilot and feasibility studies. Pilot Feasibility Stud. 2019, 5, 114. [Google Scholar] [CrossRef]
- Yang, I.A.; George, J.; McDonald, C.F.; McDonald, V.; Ordman, R.; Goodwin, A.; Smith, B.; McNamara, R.; Zwar, N.; Dabscheck, E. The COPD-X Plan: Australian and New Zealand Guidelines for the Management of Chronic Obstructive Pulmonary Disease 2024. Version 2.76. Available online: https://copdx.org.au/copd-x-plan/ (accessed on 28 November 2024).
- Booth, S.; Burkin, J.; Moffat, C.; Spathis, A. Managing Breathlessness in Clinical Practice; Springer: London, UK, 2014. [Google Scholar] [CrossRef]
- Spathis, A.; Booth, S.; Moffat, C.; Hurst, R.; Ryan, R.; Chin, C.; Burkin, J. The Breathing, Thinking, Functioning clinical model: A proposal to facilitate evidence-based breathlessness management in chronic respiratory disease. NPJ Prim. Care Respir. Med. 2017, 27, 27. [Google Scholar] [CrossRef]
- Battersby, M.W.; Ask, A.; Reece, M.M.; Markwick, M.J.; Collins, J. A case study using the “Problems and Goals Approach” in a coordinated care trial: SA HealthPlus. Aust. J. Public Health 2001, 7, 45–48. [Google Scholar] [CrossRef]
- Ewing, G.; Grande, G. Development of a Carer Support Needs Assessment Tool (CSNAT) for end-of-life care practice at home: A qualitative study. Palliat. Med. 2013, 27, 244–256. [Google Scholar] [CrossRef]
- Saunderson, E. Family and friends test. Br. J. Gen. Pract. 2014, 64, 615–616. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Banzett, R.B.; O’Donnell, C.R.; Guilfoyle, T.E.; Parshall, M.B.; Schwartzstein, R.M.; Meek, P.M.; Gracely, R.H.; Lansing, R.W. Multidimensional Dyspnea Profile: An instrument for clinical and laboratory research. Eur. Respir. J. 2015, 45, 1681–1691. [Google Scholar] [CrossRef]
- Williams, J.E.; Singh, S.J.; Sewell, L.; Guyatt, G.H.; Morgan, M.D. Development of a self-reported Chronic Respiratory Questionnaire (CRQ-SR). Thorax 2001, 56, 954–959. [Google Scholar] [CrossRef]
- Broadbent, E.; Petrie, K.J.; Main, J.; Weinman, J. The brief illness perception questionnaire. J. Psychosom. Res. 2006, 60, 631–637. [Google Scholar] [CrossRef]
- Henry, J.D.; Crawford, J.R. The short-form version of the Depression Anxiety Stress Scales (DASS-21): Construct validity and normative data in a large non-clinical sample. Br. J. Clin. Psychol. 2005, 44, 227–239. [Google Scholar] [CrossRef]
- Richardson, J.R.; Iezzi, A.; Khan, M.A.; Maxwell, A. Validity and reliability of the Assessment of Quality of Life (AQoL)-8D multi-attribute utility instrument. Patient 2014, 7, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Gratão, A.C.; Brigola, A.G.; Ottaviani, A.C.; Luchesi, B.M.; Souza, É.N.; Rossetti, E.S.; de Oliveira, N.A.; Terassi, M.; Pavarini, S.C. Brief version of Zarit Burden Interview (ZBI) for burden assessment in older caregivers. Dement. Neuropsychol. 2019, 13, 122–129. [Google Scholar] [CrossRef]
- Australian Institute of Health and Welfare [AIHW] 2023. Hospitals Glossary. Available online: https://www.aihw.gov.au/reports-data/myhospitals/content/glossary (accessed on 22 April 2024).
- Independent Health and Aged Care Pricing Authority [IHACPA] (Formerly Independent Hospital Pricing Authority) (2019) Australian Refined Diagnosis Related Groups Version 10.0 Final Report. February 2019. Available online: https://www.ihacpa.gov.au/sites/default/files/2022-08/AR-DRG%20Version%2010.0%20Final%20Report_0.pdf (accessed on 22 April 2024).
- Ronk, F.R.; Korman, J.R.; Hooke, G.R.; Page, A.C. Assessing clinical significance of treatment outcomes using the DASS-21. Psychol. Assess. 2013, 25, 1103–1110. [Google Scholar] [CrossRef] [PubMed]
- Booth, S.; Farquhar, M.; Gysels, M.; Bausewein, C.; Higginson, I.J. The impact of a breathlessness intervention service (BIS) on the lives of patients with intractable dyspnea: A qualitative phase 1 study. Palliat. Support Care 2006, 4, 287–293. [Google Scholar] [CrossRef]
- Hill, K.; Hug, S.; Smith, A.; O’Sullivan, P. The role of illness perceptions in dyspnoea-related fear in chronic obstructive pulmonary disease. J. Clin. Med. 2024, 13, 200. [Google Scholar] [CrossRef]
- Williams, M.T.; Lewthwaite, H.; Paquet, C.; Cafarella, P.; Frith, P. Pulmonary rehabilitation with and without a cognitive behavioral intervention for breathlessness in people living with chronic obstructive pulmonary disease: Randomized controlled trial. J. Clin. Med. 2023, 12, 7286. [Google Scholar] [CrossRef] [PubMed]
- Higginson, I.J.; Gao, W.; Jackson, D.; Murray, J.; Harding, R. Short-form Zarit Caregiver Burden Interviews were valid in advanced conditions. J. Clin. Epidemiol. 2010, 63, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Micklewright, K.; Farquhar, M. Face and content validity of the Carer Support Needs Assessment Tool (CSNAT), and feasibility of the CSNAT intervention, for carers of patients with chronic obstructive pulmonary disease. Chronic Illn. 2022, 18, 532–548. [Google Scholar] [CrossRef] [PubMed]
- Ekström, M.; Bornefalk, H.; Sköld, C.M.; Janson, C.; Blomberg, A.; Sandberg, J.; Bornefalk-Hermansson, A.; Currow, D.; Johnson, M.; Sundh, J. Minimal clinically important differences for Dyspnea-12 and MDP scores are similar at 2 weeks and 6 months: Follow-up of a longitudinal clinical study. Eur. Respir. J. 2021, 57, 2002823. [Google Scholar] [CrossRef]
- Alqahtani, J.S.; Oyelade, T.; Aldhahir, A.M.; Mendes, R.G.; Alghamdi, S.M.; Miravitlles, M.; Mandal, S.; Hurst, J. Reduction in hospitalised COPD exacerbations during COVID-19: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0255659. [Google Scholar] [CrossRef]
- So, J.Y.; O’Hara, N.N.; Kenaa, B.; Williams, J.G.; deBorja, C.L.; Slejko, J.F.; Zafari, Z.; Sokolow, M.; Zimand, P.; Deming, M.; et al. Population decline in COPD admissions during the COVID-19 pandemic associated with lower burden of community respiratory viral infections. Am. J. Med. 2021, 134, 1252–1259. [Google Scholar] [CrossRef]
| Objective 1: Feasibility and Acceptability | Week 0 | BLIS | Week 9 | 3 mths | 6 mths | |
|---|---|---|---|---|---|---|
| Program uptake | Number consented as a % of those eligible and approached [75% uptake] a | X | ||||
| Program retention | Number completing post-program assessment as a % of those who commenced [80% retention] a | X | ||||
| Measure completion | Number of completed questionnaires (pre- and post-program) as a % of questionnaires [75% completed] a | X | ||||
| Program fidelity | Videoed assessment/intervention versus CBIS approach [19] [75% congruent] a | X | ||||
| Program acceptability | NHS Family and Friends Test [23] [80% positive experience] a; semi-structured telephone interview | X | ||||
| Objective 2: Person living with breathlessness/carer reported outcomes | ||||||
| Domain | Instrument | |||||
| Breathlessness discomfort | Multidimensional Dyspnoea Profile (A1 scale) [24] | X | X | X | X | |
| Perceived control of breathlessness | CRQ Self-Administered (mastery subscale) [25] | X | X | X | X | |
| Manage/live with breathlessness | Visual Analog Scale (0 = not able to manage at all, 10 = able to manage extremely well) | X | X | X | X | |
| Breathlessness threat | Brief Illness Perception Questionnaire (breathlessness version) [26] | X | X | X | X | |
| Depression, anxiety, and stress | Depression, Anxiety, and Stress Scale (DASS-21) [27] | X | X | X | X | |
| Quality of Life | Assessment of Quality of Life (AQoL-8D) [28] | X | X | X | X | |
| Carer burden | Zarit Burden Interview (ZBI-12) [29] | X | X | X | X | |
| Managing/living with their relative’s breathlessness | Visual Analog Scale (0 = not able to manage at all, 10 = able to manage extremely well) | X | X | X | X | |
| Depression, anxiety, and stress | Depression, Anxiety, and Stress Scale (DASS-21) [27] | X | X | X | X | |
| Objective 3: Hospital-related health care utilization | ||||||
| Health care use | 12 months before and after the program Number of hospital admissions and length of stay b Number of emergency department presentations b Cost estimates based on the diagnosis-related group b | ← | → | |||
| Frequency (%) or Mean (SD) | |
|---|---|
| Demographic features | |
| Gender, male/female | 7 (47)/8 (53) |
| Age, years | 73 (9) |
| Living arrangements | |
| Alone | 7 (47%) |
| With spouse | 6 (40%) |
| With other family/friend | 2 (13%) |
| Housing arrangements | |
| Lived in their own home | 9 (60%) |
| Rented accommodation | 6 (40%) |
| Primary respiratory diagnosis | |
| COPD | 10 (67%) |
| Asthma/COPD overlap | 5 (33%) |
| Lung function test results, post-bronchodilator (n = 14) | |
| FEV1% predicted | 42 (15) |
| FVC% predicted | 64 (25) |
| FEV1/FVC% | 44 (9) |
| Prescribed long-term oxygen therapy | |
| Yes | 10 (67) |
| No | 5 (33) |
| Modified Medical Research Council Dyspnea Scale (n = 14) | |
| 0: “I only get breathless with strenuous exercise” | 0 |
| 1: “I get short of breath when hurrying on the level or walking up a slight hill” | 0 |
| 2: “I walk slower than people my own age on the level because of my breathlessness or have to stop for breath when walking at my own pace on the level” | 5 (33) |
| 3: “I stop for breath after walking about 100 yards or after a few minutes on the level” | 10 (67) |
| 4: “I am too breathless to leave the house” | 0 |
| Comorbidities | |
| Number of comorbid conditions per participant | 9 (3) |
| Mental health comorbidities | |
| Depression | 7 (47) |
| Anxiety | 4 (27) |
| Number of current medications at study commencement | 9(4) |
| Polypharmacy (5 or more medications) | 14 (93) |
| Hospital encounters in the previous 12 months | median (IQR) |
| COPD-related emergency department presentations | 0 (0, 1) |
| COPD-related admissions | 3 (2.5, 3) |
| COPD-related total length of stay (days) | 15 (10.5, 31.5) |
| Mean (SD)/n (%) | ||
|---|---|---|
| BLIS program visits | Number of therapist home visits per participant | 6.6 (1.5) |
| Duration of therapist home visit (minutes) | 63 (8) | |
| Total therapist contacts per participant | 7.6 (2.1) | |
| Program duration (weeks) | 6.7 (2.2) | |
| Program-related non-clinical time a by respiratory NP, hours per participant | 5.2(1.7) | |
| Types of contacts provided in the BLIS program, % of the total of contacts n = 109 | ||
| Respiratory NP individual home visit | 67 (61%) | |
| Physiotherapist individual home visit | 19 (17%) | |
| Joint respiratory NP/physiotherapist home visit | 8 (7%) | |
| Telephone call | 10 (9%) | |
| Outpatient attendance | 5 (5%) | |
| Intervention Strategy | Mean (SD)/n (%) | |
|---|---|---|
| Breathing/Thinking/Functioning Intervention strategies | ||
| Breathing strategies | Hand-held fan | 15 (100%) |
| Fan/forward lean or flop/focus on the breath out | 15 (100%) | |
| Body positioning | 13 (87%) | |
| Breathing exercises | 13 (87%) | |
| Airway clearance | 5 (33%) | |
| Thinking strategies | Challenging unhelpful thoughts | 9 (60%) |
| Managing expectations | 5 (33%) | |
| Managing anxiety | 4 (27%) | |
| Self-talk/mantra for recovery | 4 (27%) | |
| Mindfulness/relaxation script | 2 (13%) | |
| Functioning strategies | Home exercise program | 12 (80%) |
| Energy conservation | 9 (60%) | |
| Pedometer program/use | 8 (53%) | |
| Priority diary | 1 (7%) | |
| BLIS program resources use | ||
| Individualized breathlessness action plan | 15 (100%) | |
| BLIS program toolkit booklet | 13 (87%) | |
| Videos (St Christophers’ a/Cambridge BIS b) | 13 (87%) | |
| Disease management strategies | ||
| Disease education | 12 (80%) | |
| Disease-printed resources | 10 (67%) | |
| Inhaler device assessment | 9 (60%) | |
| Coordinate health and social care services | 4 (27%) | |
| Advance care planning discussion/printed resource | 4 (27%) | |
| Symptom diary | 1 (7%) | |
| Nutrition education | 1 (7%) | |
| COPD exacerbation management strategy | ||
| Exacerbation management education | 10 (67%) | |
| Home exacerbation management | 7 (47%) | |
| Facilitate urgent GP/respiratory physician review | 7 (47%) | |
| Common Themes from Interview Responses | Number of Responses |
|---|---|
| Impacts of the program | |
| Changed the way I cope with breathlessness; I’m more in control | 11 |
| More confident and not as frightened, worried, or panicked | 11 |
| Able to do more of what matters to me | 6 |
| Made a positive difference in relationships between person and carer | 4 |
| How BLIS made that impact/most helpful aspects of the BLIS program | |
| Using the fan | 13 |
| Changes to breathing and posture | 13 |
| Changed the way I think | 10 |
| Now I know something can be done; what to do to cope | 3 |
| Walking/exercising more | 13 |
| Using the BLIS toolkit and resources | 6 |
| Using oxygen and medicines more effectively now | 5 |
| Helpful aspects of the way the BLIS program was delivered | |
| Program being at home a | 13 |
| Positive qualities of the care provided b | 9 |
| The way things were explained c | 6 |
| Length of time and frequency of visits | 6 |
| Working with both the person and the carer | 3 |
| Links to and provision of other services | 1 |
| Doing questionnaires over the phone d | 1 |
| Unhelpful aspects of the BLIS program | |
| Nothing | 16 |
| Doing questionnaires over the phone e | 1 |
| Might be helpful for someone newly diagnosed | 1 |
| Areas for improvement | |
| Nothing | 17 |
| Think about getting people together f | 1 |
| Pre-Program n = 15 | Post-Program n = 15 | Difference Post–Pre 95% CI n = 15 | 3 mth Post-Program n = 11 | Difference 3 mth–Pre 95% CI n = 11 | 6 mths Post-Program n = 10 | Difference 6 mth–Pre 95% CI n = 10 | |
|---|---|---|---|---|---|---|---|
| MDP A1 scale a | 4.9 (2.3) | 3.9 (1.8) | −1.0 (1.8) −2.0, −0.1 | 4.7 (2.4) | −1.2 (1.9) (−2.5, 0.1) | 5.0 (2.1) | −1.1 (1.4) −2.1, −0.1 |
| CRQ mastery b | 5.2 (1.6) | 5.4 (1.2) | 0.3 (1.4) −0.5, 1.1 | 5.2 (1.2) | −0.1 (0.9) −0.7, 0.5 | 5.2 (1.2) | 0.3 (1.7) −1.0, 1.4 |
| Perceived ability c to manage/live with breathlessness in the last week | 8 (4) n = 14, med (IQR) | 8 (3) med (IQR) | 0.1 (2.1) −1.1, 1.2 n = 14 | 8 (2) med (IQR) | 0.7 (1.7) −0.4, 1.8 | 8 (3) med (IQR) | 0.9 (1.9) −0.6, 2.4 n = 9 |
| Pre-Program n = 6 | Post-Program n = 6 | Difference Post–Pre 95% CI n = 6 | 3 mths Post-Program n = 4 | Difference 3 mth–Pre 95% CI n = 4 | 6 mths Post-Program n = 3 | Difference 6 mth–Pre 95% CI n = 3 | |
|---|---|---|---|---|---|---|---|
| Carer burden Zarit Burden Interview | 17.6 (7.4) | 13.7 (10.9) | −4.0 (4.4) −8.6, 0.6 | 17.5 (9.0) | 0 (2.7) −4.3, 4.3 | 8.6 (8.0) | −7 (2.6) −13.6, −0.4 |
| Perceptions of managing breathlessness | |||||||
| Rate the ability of the person you are caring for to manage/live with their breathlessness a | 6.7 (1.5) | 6.0 (2.5) | −0.7 (3.0) −3.8, 2.5 | 5.0 (2.3) | −1.5 (3.1) −6.4, 3.4 | 6.7 (2.9) | 0.3 (1.5) −3.5, 4.1 |
| Rate your own ability to manage/live with the person you are caring for with breathlessness a | 7.5 (0.5) | 7.0 (3.2) | −0.5 (3.0) −3.7, 2.7 | 6.3 (2.9) | −1.0 (2.4) −4.9, 2.9 | 8.3 (1.5) | 0.7 (1.5) −3.1, 4.5 |
| DASS21 | Pre-Program med (IQR) n = 6 | Post-Program med (IQR) n = 6 | Difference Post–Pre 95% CI n = 6 | 3 mth Post-Program med (IQR) n = 4 | Difference 3 mth–Pre 95% CI n = 4 | 6 mths Post-Program med (Range) n = 3 | Difference 6 mth–Pre 95% CI n = 3 |
| Depression subscale | 2.0 (11.0) | 2.0 (13.0) | 1.0 (2.8) −1.9, 3.4 | 5.5 (13.0) | 2.3 (2.1) −1.0, 5.5 | 0 (6) | −2.0 (2.0) −7.0, 3.0 |
| Anxiety subscale | 1 (7) | 3 (9) | 0.6 (5.8) −5.4, 6.7 | 5.0 (9.0) | 3.5 (4.1) −3.1, 10.1 | 0 (10) | −2.7 (3.1) −10.3, 4.9 |
| Stress subscale | 9 (11) | 3 (21) | 0.7 (8.4) −8.1, 9.4 | 10.0 (11.0) | 4.0 (1.6) 1.4, 6.6 | 8 (12) | 2.0 (5.3) −11.1, 15.1 |
| Pre-BLIS Program (12 Months) | Post-BLIS Program (12 Months) | |
|---|---|---|
| Hospital admissions for COPD | ||
| Number of participants who were admitted (n, %) | 14/14 (100%) | 8/14 (57%) |
| Total number of admissions a | 47 | 18 |
| Total length of stay (days) | 270 | 89 |
| Average length of stay per admission (days) | 5.7 | 5.6 |
| Total hospitalization cost (AUD) | AUD 500,676 | AUD 163,756 |
| Emergency department presentations for COPD | ||
| Number of participants who presented (n, %) | 2/14 (14%) | 3/14 (21%) |
| Total number of presentations | 2 | 5 |
| Total presentation costs (AUD) | AUD 1524 | AUD 6100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnston, K.N.; Young, M.; Kay, D.; Williams, M.T. Translating a Home-Based Breathlessness Service: A Pilot Study of Feasibility, Person-Reported, and Hospital Use Outcomes. J. Clin. Med. 2025, 14, 3894. https://doi.org/10.3390/jcm14113894
Johnston KN, Young M, Kay D, Williams MT. Translating a Home-Based Breathlessness Service: A Pilot Study of Feasibility, Person-Reported, and Hospital Use Outcomes. Journal of Clinical Medicine. 2025; 14(11):3894. https://doi.org/10.3390/jcm14113894
Chicago/Turabian StyleJohnston, Kylie N., Mary Young, Debra Kay, and Marie T. Williams. 2025. "Translating a Home-Based Breathlessness Service: A Pilot Study of Feasibility, Person-Reported, and Hospital Use Outcomes" Journal of Clinical Medicine 14, no. 11: 3894. https://doi.org/10.3390/jcm14113894
APA StyleJohnston, K. N., Young, M., Kay, D., & Williams, M. T. (2025). Translating a Home-Based Breathlessness Service: A Pilot Study of Feasibility, Person-Reported, and Hospital Use Outcomes. Journal of Clinical Medicine, 14(11), 3894. https://doi.org/10.3390/jcm14113894






