Swept-Source Optical Coherence Tomography in the Diagnosis and Monitoring of Optic Nerve Neuropathy in Patients with Wernicke’s Encephalopathy Due to Hyperemesis Gravidarum
Abstract
1. Introduction
2. Materials and Methods
3. Role of Thiamine (Vitamin B1)
4. Diagnosis of WE
- Ocular abnormalities (e.g., ophthalmoplegia, nystagmus, ptosis).
- Dietary deficiency of thiamine.
- Altered mental status (confusion, memory impairment, or coma).
- Cerebellar dysfunction (ataxia, unsteady gait).
5. Treatment and Prognosis of WE
6. Optic Nerve Involvement in WE
7. Optical Coherence Tomography in Diagnosing Optic Neuropathy
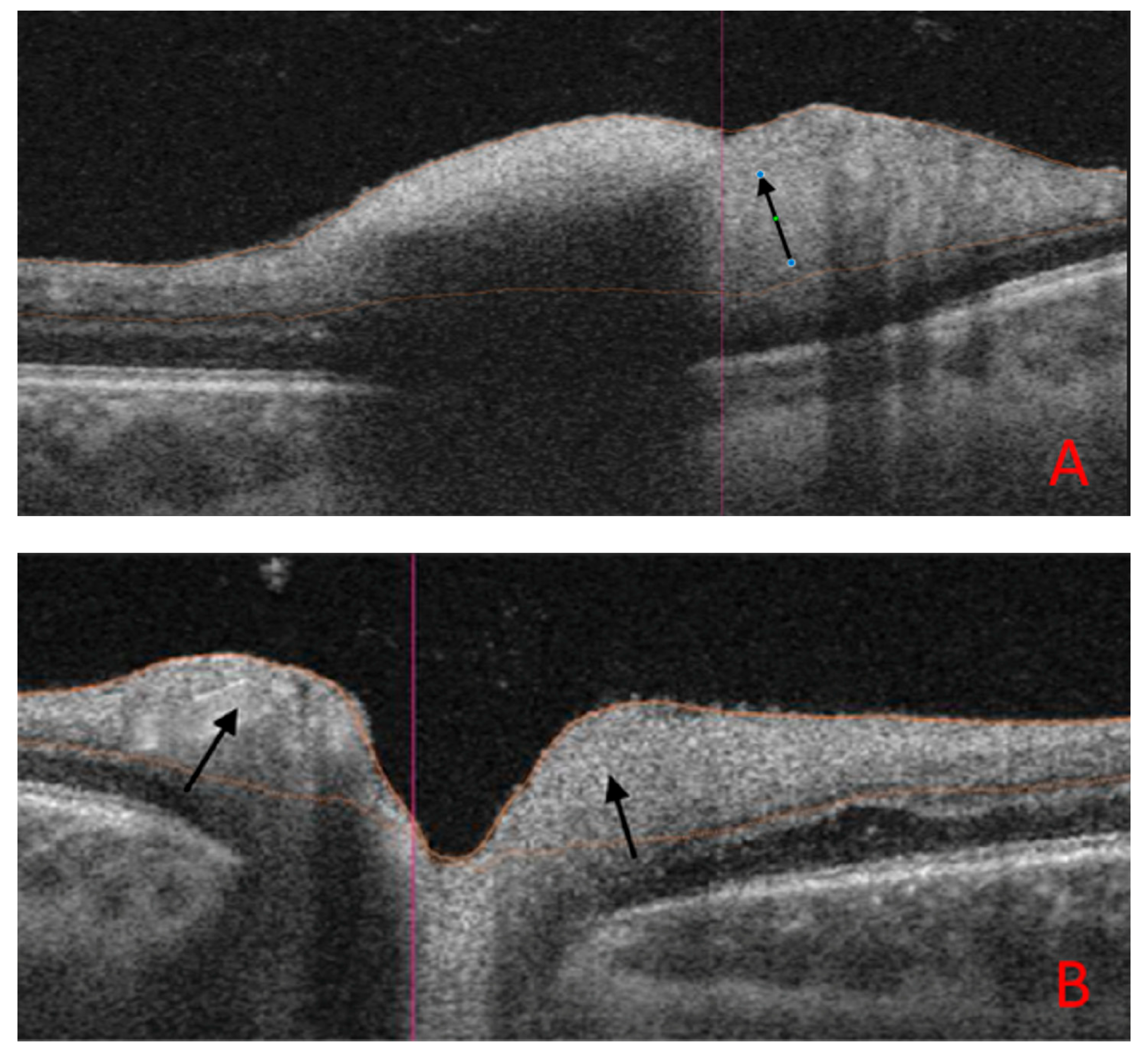
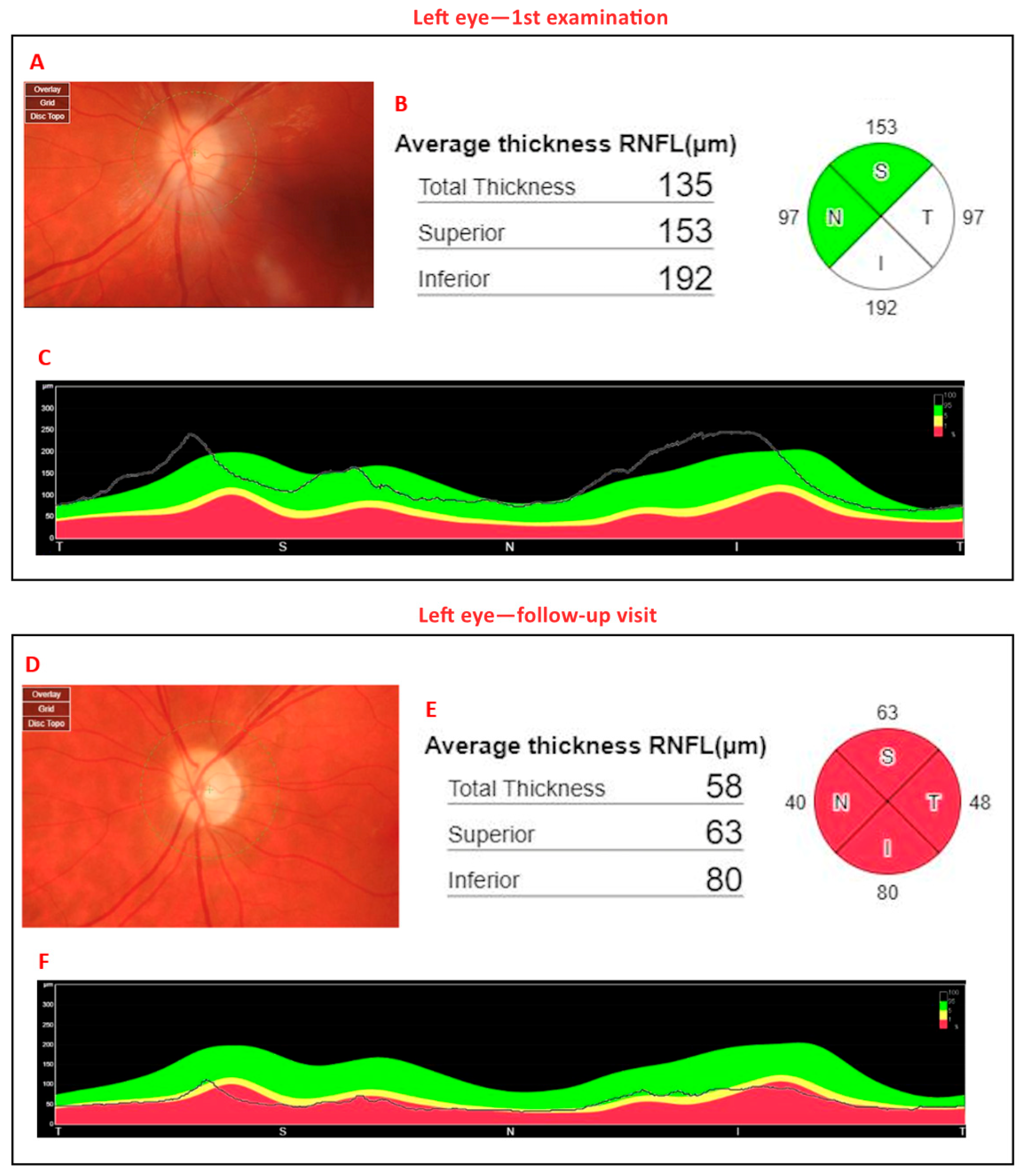
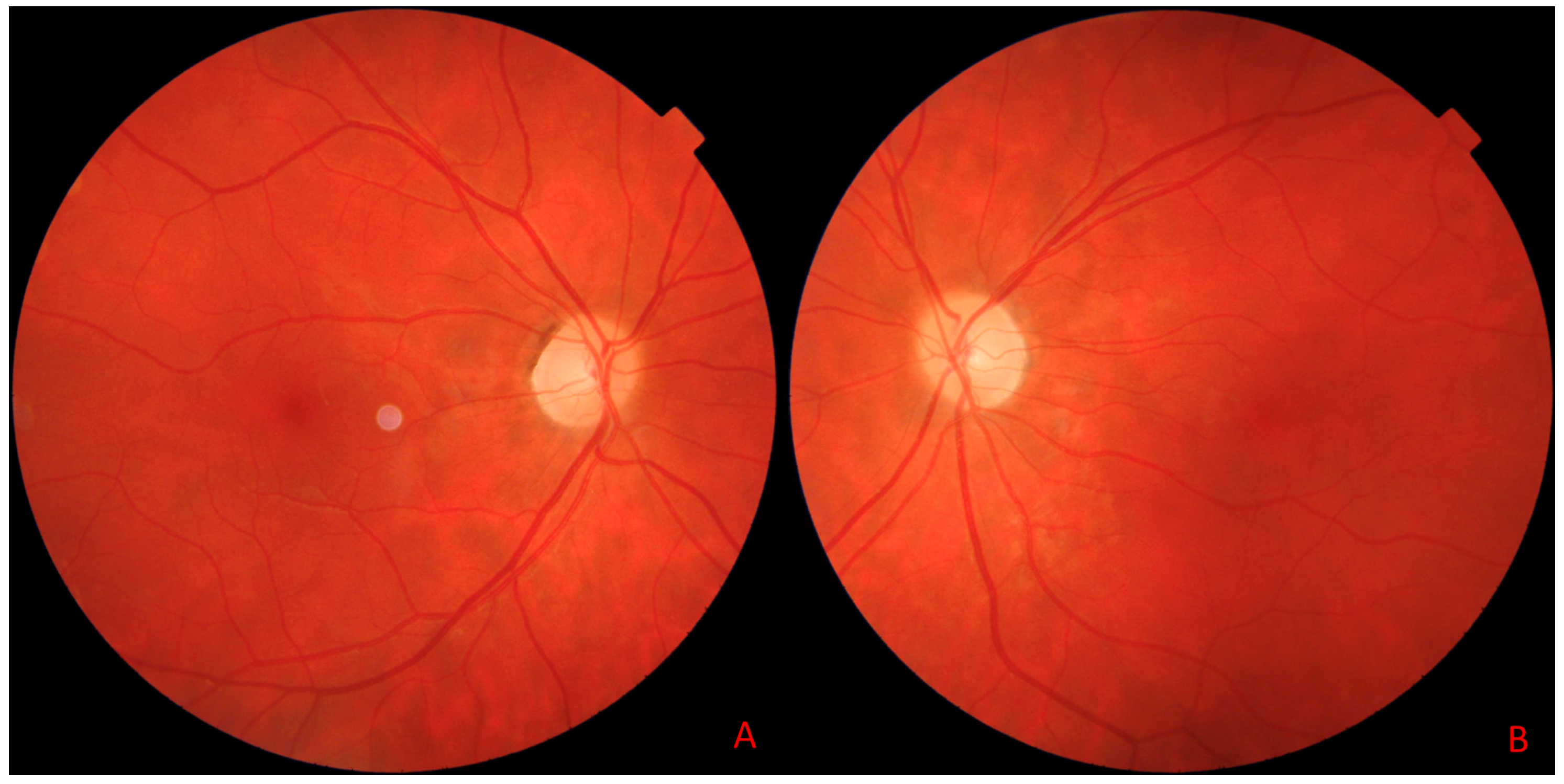
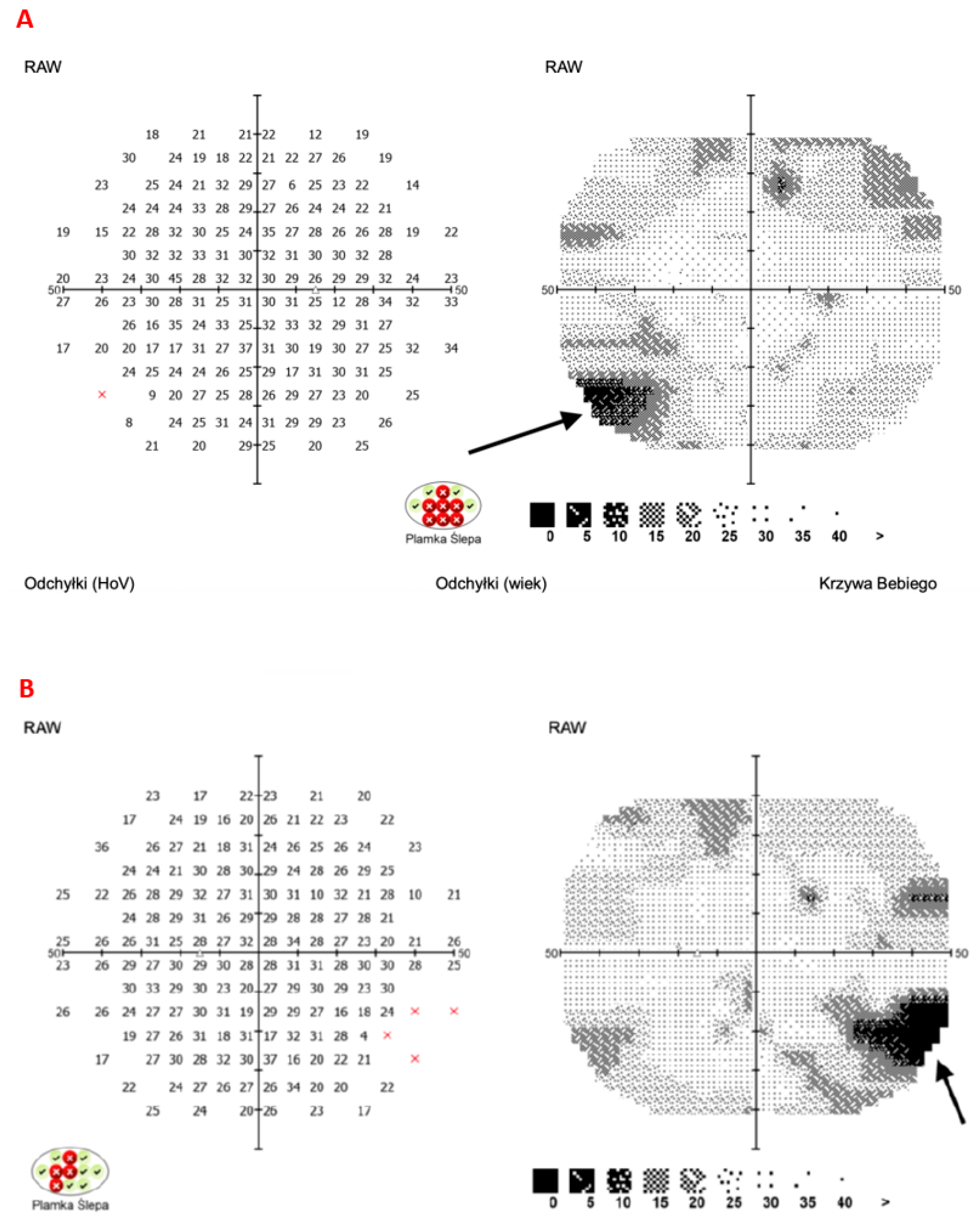
8. Illustrative Case Example
9. Discussion
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALT | Alanine Aminotransferase |
| AST | Aspartate Aminotransferase |
| CMV | Cytomegalovirus |
| EBV | Epstein–Barr Virus |
| FLAIR | Fluid-Attenuated Inversion Recovery (MRI sequence) |
| GGTP | Gamma-Glutamyl Transpeptidase |
| HBV | Hepatitis B Virus |
| HCV | Hepatitis C Virus |
| MRI | Magnetic Resonance Imaging |
| OCT | Optical Coherence Tomography |
| pRNFL | Peripapillary Retinal Nerve Fiber Layer |
| SD-OCT | Spectral-Domain Optical Coherence Tomography |
| SS-OCT | Swept-Source Optical Coherence Tomography |
| WE | Wernicke’s Encephalopathy |
References
- Oudman, E.; Wijnia, J.W.; Oey, M.J.; van Dam, M.J.; Postma, A. Preventing Wernicke’s Encephalopathy in Anorexia Nervosa: A Systematic Review. Psychiatry Clin. Neurosci. 2018, 72, 774–779. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, S.; Tong, O.; Gluck, L.; Robbins, M. Convergence Spasm in Wernicke Encephalopathy. Neurohospitalist 2018, 8, NP1–NP2. [Google Scholar] [CrossRef]
- Tang, L.; Alsulaim, H.A.; Canner, J.K.; Prokopowicz, G.P.; Steele, K.E. Prevalence and Predictors of Postoperative Thiamine Deficiency after Vertical Sleeve Gastrectomy. Surg. Obes. Relat. Dis. 2018, 14, 943–950. [Google Scholar] [CrossRef]
- Galvin, R.; Bråthen, G.; Ivashynka, A.; Hillbom, M.; Tanasescu, R.; Leone, M.A.; EFNS. EFNS Guidelines for Diagnosis, Therapy and Prevention of Wernicke Encephalopathy. Eur. J. Neurol. 2010, 17, 1408–1418. [Google Scholar] [CrossRef] [PubMed]
- Chandrakumar, A.; Bhardwaj, A.; ’t Jong, G.W. Review of Thiamine Deficiency Disorders: Wernicke Encephalopathy and Korsakoff Psychosis. J. Basic Clin. Physiol. Pharmacol. 2018, 30, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Arts, N.; Walvoort, S.; Kessels, R. Korsakoff’s Syndrome: A Critical Review. Neuropsychiatr. Dis. Treat. 2017, 13, 2875–2890. [Google Scholar] [CrossRef]
- Cantu-Weinstein, A.; Branning, R.; Alamir, M.; Weleff, J.; Do, M.; Nero, N.; Anand, A. Diagnosis and Treatment of Wernicke’s Encephalopathy: A Systematic Literature Review. Gen. Hosp. Psychiatry 2024, 87, 48–59. [Google Scholar] [CrossRef]
- Karakonstantis, S.; Galani, D.; Korela, D.; Maragou, S.; Arna, D.; Basta, M. Missing the Early Signs of Thiamine Deficiency. A Case Associated with a Liquid-Only Diet. Nutr. Neurosci. 2020, 23, 384–386. [Google Scholar] [CrossRef]
- Gratton, S.M.; Lam, B.L. Visual Loss and Optic Nerve Head Swelling in Thiamine Deficiency without Prolonged Dietary Deficiency. Clin. Ophthalmol. 2014, 8, 1021–1024. [Google Scholar] [CrossRef]
- Carrodeguas, L.; Kaidar-Person, O.; Szomstein, S.; Antozzi, P.; Rosenthal, R. Preoperative Thiamine Deficiency in Obese Population Undergoing Laparoscopic Bariatric Surgery. Surg. Obes. Relat. Dis. 2005, 1, 517–522, discussion 522. [Google Scholar] [CrossRef]
- Martin, P.R.; Singleton, C.K.; Hiller-Sturmhöfel, S. The Role of Thiamine Deficiency in Alcoholic Brain Disease. Alcohol Res. Health 2003, 27, 134–142. [Google Scholar] [PubMed]
- Thompson, F.O. Vitamin Profile of 174 Mothers and Newborns at Parturition. Am. J. Clin. Nutr. 1975, 28, 59–65. [Google Scholar]
- Togay-Işikay, C.; Yiğit, A.; Mutluer, N. Wernicke’s Encephalopathy Due to Hyperemesis Gravidarum: An under-Recognised Condition. Aust. N. Z. J. Obstet. Gynaecol. 2001, 41, 453–456. [Google Scholar] [CrossRef]
- Kumar, N. Neurologic Presentations of Nutritional Deficiencies. Neurol. Clin. 2010, 28, 107–170. [Google Scholar] [CrossRef]
- Sinha, S.; Kataria, A.; Kolla, B.P.; Thusius, N.; Loukianova, L. Wernicke Encephalopathy—Clinical Pearls. Mayo Clin. Proc. 2019, 94, 1065–1072. [Google Scholar] [CrossRef]
- Harper, C.G.; Giles, M. Clinical Signs in the Wernmicke-Korsakoff Complex: A Retrospective Analysis of 131 Cases Diagnosed at Necropsy. J. Neurol. Neurosurg. Psychiatry 1986, 49, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Kantor, S.; Prakash, S.; Chandwani, J.; Gokhale, A.; Sarma, K.; Albahrani, M.J. Wernicke’s Encephalopathy Following Hyperemesis Gravidarum. Indian J. Crit. Care Med. 2014, 18, 164–166. [Google Scholar] [CrossRef]
- Kim, W.J.; Kim, M.M. Wernicke’s Encephalopathy Presenting with Bilateral Complete Horizontal and Downward Gaze Palsy in a Malnourished Patient. Korean J. Ophthalmol. 2017, 31, 372. [Google Scholar] [CrossRef]
- Jenkins, P.F. Wernicke Encephalopathy. Am. Orthopt. J. 2015, 65, 104–108. [Google Scholar] [CrossRef]
- Manzo, G.; De Gennaro, A.; Cozzolino, A.; Serino, A.; Fenza, G.; Manto, A. MR Imaging Findings in Alcoholic and Nonalcoholic Acute Wernicke’s Encephalopathy: A Review. BioMed Res. Int. 2014, 2014, 503596. [Google Scholar] [CrossRef]
- Habas, E.; Farfar, K.; Errayes, N.; Rayani, A.; Elzouki, A.-N. Wernicke Encephalopathy: An Updated Narrative Review. Saudi J. Med. Med. Sci. 2023, 11, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Hope, L.C.; Cook, C.C.; Thomson, A.D. A Survey of the Current Clinical Practice of Psychiatrists and Accident and Emergency Specialists in the United Kingdom Concerning Vitamin Supplementation for Chronic Alcohol Misusers. Alcohol Alcohol. 1999, 34, 862–867. [Google Scholar] [CrossRef] [PubMed]
- Schabelman, E.; Kuo, D. Glucose before Thiamine for Wernicke Encephalopathy: A Literature Review. J. Emerg. Med. 2012, 42, 488–494. [Google Scholar] [CrossRef]
- Cogan, D.G.; Victor, M. Ocular Signs of Wernicke’s Disease. AMA Arch. Ophthalmol. 1954, 51, 204–211. [Google Scholar] [CrossRef]
- Kattah, J.C. The Spectrum of Vestibular and Ocular Motor Abnormalities in Thiamine Deficiency. Curr. Neurol. Neurosci. Rep. 2017, 17, 40. [Google Scholar] [CrossRef]
- Li, J.-M.; Rucker, J.C. Irreversible Optic Neuropathy in Wernicke Encephalopathy and Leber Hereditary Optic Neuropathy. J. Neuroophthalmol. 2010, 30, 49–53. [Google Scholar] [CrossRef]
- Nakamura, Z.M.; Tatreau, J.R.; Rosenstein, D.L.; Park, E.M. Clinical Characteristics and Outcomes Associated with High-Dose Intravenous Thiamine Administration in Patients with Encephalopathy. Psychosomatics 2018, 59, 379–387. [Google Scholar] [CrossRef]
- Wernicke, C. Lehrbuch der Gehirnkrankheiten fur Aertze und Studirende; Outlook Verlag: Frankfurt, Germany, 1881; Volume 2, pp. 229–242. [Google Scholar]
- De Wardener, H.E.; Lennox, B. Cerebral Beriberi: Review of 52 Cases in a Singapore Prisoner-of-War Camp. Lancet 1947, 1, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Dinkin, M. Trans-Synaptic Retrograde Degeneration in the Human Visual System: Slow, Silent, and Real. Curr. Neurol. Neurosci. Rep. 2017, 17, 16. [Google Scholar] [CrossRef]
- Mun-Wei, L.; Gayathri, G.; Kwang Hwee, G.; Ruban, K.; Suresh Kumar, V.; Shatriah, I. Optic Discs Swelling Procrastinates Wernicke’s Encephalopathy Associated with Hyperemesis Gravidarum: A Case Report and Review of Literature. Cureus 2018, 10, e2793. [Google Scholar] [CrossRef]
- Isen, D.R.; Kline, L.B. Neuro-Ophthalmic Manifestations of Wernicke Encephalopathy. Eye Brain 2020, 12, 49–60. [Google Scholar] [CrossRef]
- Victor, M.; Adams, R.D.; Collins, G.H. The Wernicke-Korsakoff Syndrome: A Clinical and Pathological Study of 245 Patients, 82 with Post-Mortem Examinations. Contemp. Neurol. Ser. 1971, 7, 1–206. [Google Scholar]
- Bvega, M.W.; Miller, J.; Wlsh, J. Myths and Misconception of Wernicke’s Encephalopathy: What Every Emergency Physician Should Know. Ann. Emerg. Med. 2007, 50, 715–721. [Google Scholar]
- Kattah, J.C. Early Signs of Thiamine Deficiency: A Case Report. Ann. Intern. Med. 2020, 173, 72–73. [Google Scholar] [CrossRef]
- Reuler, J.B.; Girard, D.E.; Cooney, T.G. Current Concepts. Wernicke’s Encephalopathy. N. Engl. J. Med. 1985, 312, 1035–1039. [Google Scholar] [CrossRef]
- Deramo, V.A.; Jayamanne, D.G.; Auerbach, D.B.; Danesh-Meyer, H. Acute Bilateral Ophthalmoplegia in a Young Woman. Surv. Ophthalmol. 2000, 44, 513–517. [Google Scholar] [CrossRef]
- Winters, J.; Niespodzany, E.; Kini, T.A.; Othman, B.A.; Lee, A.G. Rapid Same-Day Resolution of Internuclear Ophthalmoplegia in Wernicke Encephalopathy Following Parental High Dose Thiamine. Can. J. Ophthalmol. 2020, 55, e69–e70. [Google Scholar] [CrossRef]
- De La Paz, M.A.; Chung, S.M.; McCrary, J.A., 3rd. Bilateral Internuclear Ophthalmoplegia in a Patient with Wernicke’s Encephalopathy. J. Clin. Neuroophthalmol. 1992, 12, 116–120. [Google Scholar]
- Lee, S.-H.; Kim, S.-H.; Kim, J.-M.; Tarnutzer, A.A. Vestibular Dysfunction in Wernicke’s Encephalopathy: Predominant Impairment of the Horizontal Semicircular Canals. Front. Neurol. 2018, 9, 141. [Google Scholar] [CrossRef]
- Kulkarni, S.; Lee, A.G.; Holstein, S.A.; Warner, J.E.A. You Are What You Eat. Surv. Ophthalmol. 2005, 50, 389–393. [Google Scholar] [CrossRef]
- Sia, P.I.; Sia, D.I.T.; Crompton, J.L.; Casson, R.J. Nerve Fiber Layer Infarcts in Thiamine Deficiency. J. Neuroophthalmol. 2015, 35, 274–276. [Google Scholar] [CrossRef]
- Thompson, R.A.; Lynde, R.H. Convergence Spasm Associated with Wernicke’s Encephalopathy. Neurology 1969, 19, 711–712. [Google Scholar] [CrossRef]
- Costello, F.; Chen, J.J. The Role of Optical Coherence Tomography in the Diagnosis of Afferent Visual Pathway Problems: A Neuroophthalmic Perspective. Handb. Clin. Neurol. 2021, 178, 97–113. [Google Scholar] [CrossRef]
- Costello, F.; Hamann, S. Publisher Correction to: Advantages and Pitfalls of the Use of Optical Coherence Tomography for Papilledema. Curr. Neurol. Neurosci. Rep. 2024, 24, 65. [Google Scholar] [CrossRef]
- Nakayama, L.F.; Zago Ribeiro, L.; de Oliveira, J.A.E.; de Matos, J.C.R.G.; Mitchell, W.G.; Malerbi, F.K.; Celi, L.A.; Regatieri, C.V.S. Fairness and Generalizability of OCT Normative Databases: A Comparative Analysis. Int. J. Retina Vitreous 2023, 9, 48. [Google Scholar] [CrossRef]
- Eliakim, R.; Abulafia, O.; Sherer, D.M. Hyperemesis Gravidarum: A Current Review. Am. J. Perinatol. 2000, 17, 207–218. [Google Scholar] [CrossRef]
- Ferdinands, M.D.; Seneviratne, J.; White, O. Visual Deterioration in Hyperemesis Gravidarum. Med. J. Aust. 2005, 182, 585–586. [Google Scholar] [CrossRef]
- Serlin, T.; Moisseiev, E. Fundus Findings in Wernicke Encephalopathy. Case Rep. Ophthalmol. 2017, 8, 406–409. [Google Scholar] [CrossRef]
- Bohnsack, B.L.; Patel, S.S. Peripapillary Nerve Fiber Layer Thickening, Telangiectasia, and Retinal Hemorrhages in Wernicke Encephalopathy. J. Neuroophthalmol. 2010, 30, 54–58. [Google Scholar] [CrossRef]
- Lawton, A.W.; Frisard, N.E. Visual Loss, Retinal Hemorrhages, and Optic Disc Edema Resulting from Thiamine Deficiency Following Bariatric Surgery Complicated by Prolonged Vomiting. Ochsner J. 2017, 17, 112–114. [Google Scholar]
- Wang, M.Y.; Sadun, A.A. Drug-Related Mitochondrial Optic Neuropathies. J. Neuroophthalmol. 2013, 33, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Guler, A.; Alpaydin, S.; Sirin, H.; Calli, C.; Celebisoy, N. A Non-Alcoholic Wernicke’s Encephalopathy Case with Atypical MRI Findings: Clinic versus Radiology. Neuroradiol. J. 2015, 28, 474–477. [Google Scholar] [CrossRef]
- Ahuja, S.; Kumar, P.S.; Kumar, V.P.; Kattimani, S.; Akkilagunta, S. Effect of Chronic Alcohol and Tobacco Use on Retinal Nerve Fibre Layer Thickness: A Case–Control Study. BMJ Open Ophthalmol. 2016, 1, e000003. [Google Scholar] [CrossRef]
- Vieira, L.M.C.; Silva, N.F.A.; Dias dos Santos, A.M.; dos Anjos, R.S.; Pinto, L.A.P.A.; Vicente, A.R.; Borges, B.I.C.C.J.; Ferreira, J.P.T.; Amado, D.M.; da Cunha, J.P.P.B. Retinal Ganglion Cell Layer Analysis by Optical Coherence Tomography in Toxic and Nutritional Optic Neuropathy. J. Neuroophthalmol. 2015, 35, 242–245. [Google Scholar] [CrossRef]
- Grzybowski, A.; Obuchowska, I.; Arndt, C. OCT in Toxic and Nutritional Optic Neuropathies. In OCT and Imaging in Central Nervous System Diseases; Springer International Publishing: Cham, Switzerland, 2020; pp. 375–400. ISBN 9783030262686. [Google Scholar]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kal, M.; Brzdęk, M.; Tracz, J.; Szadkowski, P.; Zarębska-Michaluk, D. Swept-Source Optical Coherence Tomography in the Diagnosis and Monitoring of Optic Nerve Neuropathy in Patients with Wernicke’s Encephalopathy Due to Hyperemesis Gravidarum. J. Clin. Med. 2025, 14, 3849. https://doi.org/10.3390/jcm14113849
Kal M, Brzdęk M, Tracz J, Szadkowski P, Zarębska-Michaluk D. Swept-Source Optical Coherence Tomography in the Diagnosis and Monitoring of Optic Nerve Neuropathy in Patients with Wernicke’s Encephalopathy Due to Hyperemesis Gravidarum. Journal of Clinical Medicine. 2025; 14(11):3849. https://doi.org/10.3390/jcm14113849
Chicago/Turabian StyleKal, Magdalena, Michał Brzdęk, Justyna Tracz, Paweł Szadkowski, and Dorota Zarębska-Michaluk. 2025. "Swept-Source Optical Coherence Tomography in the Diagnosis and Monitoring of Optic Nerve Neuropathy in Patients with Wernicke’s Encephalopathy Due to Hyperemesis Gravidarum" Journal of Clinical Medicine 14, no. 11: 3849. https://doi.org/10.3390/jcm14113849
APA StyleKal, M., Brzdęk, M., Tracz, J., Szadkowski, P., & Zarębska-Michaluk, D. (2025). Swept-Source Optical Coherence Tomography in the Diagnosis and Monitoring of Optic Nerve Neuropathy in Patients with Wernicke’s Encephalopathy Due to Hyperemesis Gravidarum. Journal of Clinical Medicine, 14(11), 3849. https://doi.org/10.3390/jcm14113849






