Semiquantitative Analysis in PET/CT Imaging of Prostate Cancer
Abstract
1. Introduction
2. Materials and Methods
3. Background
3.1. The Use of PET/CT in Prostate Cancer Imaging
3.2. The Role of Different Radiopharmaceuticals in Prostate Cancer PET/CT Imaging
4. Semiquantitative Analysis and PET/CT
Standard Uptake Value (SUV)
5. Clinical Applications
5.1. Primary Diagnosis and Initial Staging of Prostate Cancer
5.2. Biochemical Recurrence and Metastatic Prostate Cancer
6. Limitations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| PET/CT | Positron emission tomography/computed tomography |
| 68Ga-PSMA | 68Gallium-prostate-specific membrane antigen |
| [18F]-FDG | 18F-fluorodeoxyglucose |
| SUV | Standard Uptake Value |
| SUVmax | Standard Uptake Value maximum |
| MTV | Metabolic tumor volume |
| TMTV | Total metabolic tumor volume |
| PSMA | TV PSMA Total Volume |
| PSMA TLU | Psma total lesion uptake |
| TL-PSMA | Total lesion PSMA |
| PSMA-TVp | Prostate PSMA-tumor volume |
| ASP | Tumor asphericity |
| wbPSMA-TV | Whole-body PSMA tumor volume |
| wbTL-PSMA | Whole-body total lesions PSMA uptake |
| OS | Overall Survival |
| SUVmean | Mean standard uptake value |
| SUVpeak | Peak standard uptake value |
| SUL | Lean standard uptake value |
| SUVbsa | Body surface area standard uptake value |
| AUC | Area under the curve |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Affiliations Expand, Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- American Cancer Society. Key Statistics for Prostate Cancer. Available online: https://www.cancer.org/cancer/prostate-cancer/about/key-statistics.html (accessed on 28 February 2025).
- Litwin, M.S.; Tan, H.J. The Diagnosis and Treatment of Prostate Cancer: A Review. JAMA 2017, 317, 2532–2542. [Google Scholar] [CrossRef] [PubMed]
- Ceci, F.; Farulla, L.S.A.; Bonatto, E.; Evangelista, L.; Aliprandi, M.; Cecchi, L.G.; Mattana, F.; Bertocchi, A.; de Vincenzo, F.; Perrino, M.; et al. New target therapies in prostate cancer: From radioligand therapy, to PARP-inhibitors and immunotherapy. Q. J. Nucl. Med. Mol. Imaging 2024, 68, 101–115. [Google Scholar] [CrossRef]
- Li, R.; Ravizzini, G.C.; Gorin, M.A.; Maurer, T.; Eiber, M.; Cooperberg, M.R.; Alemozzaffar, M.; Tollefson, M.K.; Delacroix, S.E.; Chapin, B.F. The use of PET/CT in prostate cancer. Prostate Cancer Prostatic Dis. 2018, 21, 4–21. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.; Singh, H.; Dash, A.K. Clinical Applications of PET and PET-CT. Med. J. Armed Forces India 2009, 65, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Tilki, D.; van den Bergh, R.C.N.; Briers, E.; van den Broeck, T.; Brunckhorst, O.; Darraugh, J.; Eberli, D.; de Meerleer, G.; de Santis, M.; Farolfi, A.; et al. EAU-EANM-ESTRO-ESUR-ISUP-SIOG Guidelines on Prostate Cancer. Part II-2024 Update: Treatment of relapsing and metastatic prostate cancer. Eur. Urol. 2024, 86, 164–182. [Google Scholar] [CrossRef]
- Groener, D.; Schneider, S.; Baumgarten, J.; Happel, C.; Klimek, K.; Mader, N.; Ngoc, C.N.; Wichert, J.; Mandel, P.; Tselis, N. Baseline [68Ga]Ga-PSMA-11 PET/CT before [177Lu]Lu-PSMA-617 Radioligand Therapy: Value of PSMA-Uptake Thresholds in Predicting Targetable Lesions. Cancers 2023, 15, 473. [Google Scholar] [CrossRef]
- Subesinghe, M.; Kulkarni, M.; Cook, G.J. The Role of PET-CT Imaging in Prostate Cancer. Semin. Ultrasound CT MRI 2020, 41, 373–391. [Google Scholar] [CrossRef]
- Singh, K.B.; London, K.I.; Wong, V.C.K.; Mansberg, R. Diagnostic accuracy of bone scan at different PSA levels in biochemical recurrence of prostate cancer. J. Med. Imaging Radiat. Sci. 2024, 55, 91–96. [Google Scholar] [CrossRef]
- Huls, S.J.; Burkett, B.; Ehman, E.; Lowe, V.J.; Subramaniam, R.M.; Kendi, A.T. Clinical practice in prostate PET imaging. Ther. Adv. Med. Oncol. 2023, 15, 17588359231213618. [Google Scholar] [CrossRef]
- Sutherland, D.E.K.; Azad, A.A.; Murphy, D.G.; Eapen, R.S.; Kostos, L.; Hofman, M.S. Role of FDG PET/CT in Management of Patients with Prostate Cancer. Semin. Nucl. Med. 2024, 54, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Hennrich, U.; Eder, M. [68Ga]Ga-PSMA-11: The First FDA-Approved 68Ga-Radiopharmaceutical for PET Imaging of Prostate Cancer. Pharmaceuticals 2021, 14, 713. [Google Scholar] [CrossRef] [PubMed]
- Tateishi, U. Prostate-specific membrane antigen (PSMA)-ligand positronemission tomography and radioligand therapy (RLT) of prostate cancer. Jpn. J. Clin. Oncol. 2020, 50, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.C.; Blusztajn, J.K.; Caudill, M.A.; Klatt, K.C.; Zeisel, S.H. Choline: The neurocognitive essential nutrient of interest to obstetricians andgynecologists. J. Diet. Suppl. 2019, 17, 733–752. [Google Scholar] [CrossRef]
- Panagiotidis, E.; Fragkiadaki, V.; Papathanasiou, N.; Kypraios, C.; Liatsikos, E.; Klampatsas, A.; Paschali, A.; Exarhos, D.; Zarokosta, F.; Chatzipavlidou, V.; et al. Comparison of 18F-PSMA-1007 and 18F-Choline PET/CT in prostate cancer patients with biochemical recurrence: A phase 3, prospective, multicenter, randomized study. Nucl. Med. Commun. 2023, 44, 1126–1134. [Google Scholar] [CrossRef]
- Rahbar, K.; Afshar-Oromieh, A.; Seifert, R.; Wagner, S.; Schäfers, M.; Bögemann, M.; Weckesser, M. Diagnostic performance of 18F-PSMA-1007 PET/CT in patients with biochemical recurrent prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 2055–2061. [Google Scholar] [CrossRef]
- Rauscher, I.; Krönke, M.; König, M.; Gafita, A.; Maurer, T.; Horn, T.; Schiller, K.; Weber, W.; Eiber, M. Matched-pair Comparison of 68Ga-PSMA-11 PET/CT and 18F-PSMA-1007 PET/CT: Frequency of pitfalls and detection efficacy in biochemical recurrence after radical prostatectomy. J. Nucl. Med. 2020, 61, 51–57. [Google Scholar] [CrossRef]
- Maisto, C.; Aurilio, M.; Morisco, A.; de Marino, R.; Recchimuzzo, M.J.B.; Carideo, L.; d’Ambrosio, L.; di Gennaro, F.; Esposito, A.; Gaballo, P.; et al. Analysis of pros and cons in using [68Ga]Ga-PSMA-11 and [18F]PSMA-1007: Production, costs, and PET/CT applications in patients with prostate cancer. Molecules 2022, 27, 3862. [Google Scholar] [CrossRef]
- Jussing, E.; Milton, S.; Samén, E.; Moein, M.M.; Bylund, L.; Axelsson, R.; Siikanen, J.; Tran, T.A. Clinically applicable cyclotron-produced gallium-68 gives high-yield radiolabeling of DOTA-based tracers. Biomolecules 2021, 11, 1118. [Google Scholar] [CrossRef]
- Pattison, D.A.; Debowski, M.; Gulhane, B.; Arnfield, E.G.; Pelecanos, A.M.; Garcia, P.L.; Latter, M.J.; Lin, C.Y.; Roberts, M.J.; Ramsay, S.C.; et al. Prospective intra-individual blinded comparison of [18F]PSMA-1007 and [68 Ga]Ga-PSMA-11 PET/CT imaging in patients with confirmed prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 763–776. [Google Scholar] [CrossRef]
- Kuten, J.; Fahoum, I.; Savin, Z.; Shamni, O.; Gitstein, G.; Hershkovitz, D.; Mabjeesh, N.J.; Yossepowitch, O.; Mishani, E.; Even-Sapir, E. Head-to-head comparison of 68Ga-PSMA-11 with 18F-PSMA-1007 PET/CT in staging prostate cancer using histopathology and immunohistochemical analysis as a reference standard. J. Nucl. Med. 2020, 61, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.A.; von Eyben, F.E.; Fischer, N.; Rosar, F.; Muller-Hubenthal, J.; Buchholz, H.G.; Wieler, H.J.; Schreckenberger, M. Comparison of [18F]PSMA-1007 with [68Ga]Ga-PSMA-11 PET/CT in restaging of prostate cancer patients with PSA relapse. Cancers 2022, 14, 1479. [Google Scholar] [CrossRef] [PubMed]
- Hoberuck, S.; Lock, S.; Borkowetz, A.; Sommer, U.; Winzer, R.; Zophel, K.; Fedders, D.; Michler, E.; Kotzerke, J.; Kopket, K.; et al. Intraindividual comparison of [68 Ga]-Ga-PSMA-11 and [18F]-F-PSMA-1007 in prostate cancer patients: A retrospective single-center analysis. EJNMMI Res. 2021, 11, 109. [Google Scholar] [CrossRef]
- Dietlein, F.; Kobe, C.; Neubauer, S.; Schmidt, M.; Stockter, S.; Fischer, T.; Schomäcker, K.; Heidenreich, A.; Zlatopolskiy, B.D.; Neumaier, B.; et al. PSA-stratified performance of 18F- and 68Ga-PSMA PET in patients with biochemical recurrence of prostate cancer. J. Nucl. Med. 2017, 58, 947–952. [Google Scholar] [CrossRef]
- Ferreira, G.; Iravani, A.; Hofman, M.S.; Hicks, R.J. Intra-individual comparison of 68Ga-PSMA-11 and 18F-DCFPyL normal-organ biodistribution. Cancer Imaging 2019, 19, 23. [Google Scholar] [CrossRef]
- Li, E.V.; Schaeffer, E.M.; Kumar, S.K.S.R.; Zhou, R.; Yang, X.J.; Mana-Ay, M.; Vescovo, M.; Ho, A.; Keeter, M.K.; Carr, J.; et al. Utility of 18F-DCFPyL PET for local staging for high or very high risk prostate cancer for patients undergoing radical prostatectomy. Eur. J. Nucl. Med. Mol. Imaging 2025. ahead of printing. [Google Scholar] [CrossRef] [PubMed]
- Dietlein, F.; Hohberg, M.; Kobe, C.; Zlatopolskiy, B.D.; Krapf, P.; Endepols, H.; Täger, P.; Hammes, J.; Heidenreich, A.; Neumaier, B.; et al. An 18F-labeled PSMA ligand for PET/CT of prostate cancer: First-in-humans observational study and clinical experience with 18F-JK-PSMA-7 during the first year of application. J. Nucl. Med. 2020, 61, 202–209. [Google Scholar] [CrossRef]
- Kroenke, M.; Mirzoyan, L.; Horn, T.; Peeken, J.C.; Wurzer, A.; Wester, H.J.; Makowski, M.; a Weber, W.; Eiber, M.; Rauscher, I. Matched-pair comparison of 68Ga-PSMA-11 and 18F-rhPSMA-7 PET/CT in patients with primary and biochemical recurrence of prostate cancer: Frequency of non-tumor-related uptake and tumor positivity. J. Nucl. Med. 2021, 62, 1082–1088. [Google Scholar] [CrossRef]
- Huang, S.; Ong, S.; McKenzie, D.; Mirabelli, A.; Chen, D.C.; Chengodu, T.; Murphy, D.G.; Hofman, M.S.; Lawrentschuk, N.; Perera, M. Comparison of 18F-based PSMA radiotracers with [68Ga]Ga-PSMA-11 in PET/CT imaging of prostate cancer-a systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2024, 27, 654–664. [Google Scholar] [CrossRef]
- Basu, S.; Zaidi, H.; Houseni, M.; Bural, G.; Udupa, J.; Acton, P.; a Torigian, D.; Alavi, A. Novel quantitative techniques for assessing regional and global function and structure based on modern imaging modalities: Implications for normal variation, aging and diseased states. Semin. Nucl. Med. 2007, 37, 223–239. [Google Scholar] [CrossRef]
- Ziai, P.; Hayeri, M.R.; Salei, A.; Salavati, A.; Houshmand, S.; Alavi, A.; Teytelboym, O.M. Role of Optimal Quantification of FDG PET Imaging in the Clinical Practice of Radiology. Radiographics 2016, 36, 481–496. [Google Scholar] [CrossRef] [PubMed]
- Fragkiadaki, V.; Panagiotidis, E.; Vlontzou, E.; Kalathas, T.; Paschali, A.; Kypraios, C.; Chatzipavlidou, V.; Datseris, I. Correlation of PSA blood levels with standard uptake value maximum (SUVmax) and total metabolic tumor volume (TMTV) in 18F-PSMA-1007 and 18F-choline PET/CT in patients with biochemically recurrent prostate cancer. Nucl. Med. Commun. 2024, 45, 924–930. [Google Scholar] [CrossRef] [PubMed]
- Im, H.J.; Bradshaw, T.; Solaiyappan, M.; Cho, S.Y. Current Methods to Define Metabolic Tumor Volume in Positron Emission Tomography: Which One is Better? Nucl. Med. Mol. Imaging. 2017, 52, 5–15. [Google Scholar] [CrossRef]
- Dong, S.; Li, Y.; Chen, J.; Li, Y.; Yang, P.; Li, J. 18F-PSMA-1007 PET/CT-derived semi-quantitative parameters for risk stratification of newly diagnosed prostate cancer. Front. Oncol. 2022, 12, 1025930. [Google Scholar] [CrossRef] [PubMed]
- Yi, N.; Wang, Y.; Zang, S.; Yang, L.; Liu, H.; Sun, H.; Wang, L.; Wang, F. Ability of 68 Ga-PSMA PET/CT SUVmax to differentiate ISUP GG2 from GG3 in intermediate-risk prostate cancer: A single-center retrospective study of 147 patients. Cancer Med. 2023, 12, 7140–7148. [Google Scholar] [CrossRef]
- Heetman, J.G.; Pereira, L.J.P.; Kelder, J.C.; Soeterik, T.F.W.; Wever, L.; Lavalaye, J.; van der Hoeven, E.J.R.J.; Lam, M.G.E.H.; van Melick, H.H.E.; van den Bergh, R.C.N. The additional value of 68Ga-PSMA PET/CT SUVmax in predicting ISUP GG ≥ 2 and ISUP GG ≥ 3 prostate cancer in biopsy. Prostate 2024, 84, 1025–1032. [Google Scholar] [CrossRef]
- Rogic, I.; Golubic, A.T.; Zuvic, M.; Smitran, T.; Jukic, N.; Gamulin, M.; Kastelan, Z.; Huic, D. Clinical utility of [68Ga]Ga-PSMA-11 PET/CT in initial staging of patients with prostate cancer and importance of intraprostatic SUVmax values. Nucl. Med. Rev. Cent. East. Eur. 2024, 27, 6–12. [Google Scholar] [CrossRef]
- Ali, H.; Rashid-Ul-Amin, S.; Hai, A. Standardised Uptake Value in Organ Confined Prostate Cancer in 68-Ga- Prostate-Specific Membrane Antigen Positron Emission Tomography-Computed Tomography Scan and its Correlation with Prostate Specific Antigen Level and Gleason Score. J. Cancer Allied Spec. 2023, 9, 529. [Google Scholar] [CrossRef]
- Andela, S.B.; Amthauer, H.; Furth, C.; Rogasch, J.M.; Beck, M.; Mehrhof, F.; Ghadjar, P.; van den Hoff, J.; Klatte, T.; Tahbaz, R. Quantitative PSMA-PET parameters in localized prostate cancer: Prognostic and potential predictive value. Radiat. Oncol. 2024, 19, 97. [Google Scholar] [CrossRef]
- Bodar, Y.J.L.; Veerman, H.; Meijer, D.; de Bie, K.; van Leeuwen, P.J.; Donswijk, M.L.; van Moorselaar, R.J.A.; Hendrikse, N.H.; Boellaard, R.; Oprea-Lager, D.E.; et al. Standardised uptake values as determined on prostate-specific membrane antigen positron emission tomography/computed tomography is associated with oncological outcomes in patients with prostate cancer. BJU Int. 2022, 129, 768–776. [Google Scholar] [CrossRef]
- Cardoza-Ochoa, D.R.; Cristancho-Rojas, C.; Pérez, D.J.; Moreno-Izaguirre, P.; Guzman, M.; Gutiérrez-Rivera, M.C.; Gaxiola-Mascareño, A.P.; Avila-Rodríguez, M.A.; Rivera-Bravo, B. Semiautomatic assessment of whole-body tumor burden with 18F-PSMA-1007 in biochemical recurrent prostate cancer. Nucl. Med. Commun. 2022, 43, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, J.; Wang, X.; Yang, P.; Yang, J.; Zhao, Q.; Li, J. Predictive value of volumetric parameters based on 18F-PSMA-1007 PET/CT for prostate cancer metastasis. Front. Oncol. 2024, 14, 1335205. [Google Scholar] [CrossRef]
- Lowrance, W.T.; Breau, R.H.; Chou, R.; Chapin, B.F.; Crispino, T.; Dreicer, R.; Jarrard, D.F.; Kibel, A.S.; Morgan, T.M.; Morgans, A.K.; et al. Advanced Prostate Cancer: AUA/ASTRO/SUO Guideline PART I. J. Urol. 2021, 205, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, M.; Lutz, M.; Hoeh, B.; Koll, F.; Garcia, C.C.; Siech, C.; Steuber, T.; Graefen, M.; Tilki, D.; Kluth, L.A.; et al. Influence of Tumor Characteristics and Time to Metastatic Disease on Oncological Outcomes in Metachronous Metastatic Prostate Cancer Patients. Clin. Genitourin. Cancer 2024, 22, 102158. [Google Scholar] [CrossRef]
- National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) Program. Cancer Stat Facts Prostate Cancer. Available online: https://seer.cancer.gov/statfacts/html/prost.html (accessed on 28 February 2025).
- Weiner, A.B.; Matulewicz, R.S.; Eggener, S.E.; Schaeffer, E.M. Increasing incidence of metastatic prostate cancer in the United States (2004–2013). Prostate Cancer Prostatic Dis. 2016, 19, 395–397. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.; Smith, S.; Shamash, J. Metastatic Hormone-Sensitive Prostate Cancer (mHSPC): Advances and Treatment Strategies in the First-Line Setting. Oncol. Ther. 2020, 8, 209–230. [Google Scholar] [CrossRef]
- Raychaudhuri, R.; Lin, D.W.; Montgomery, R.B. Prostate Cancer: A Review. JAMA 2025, 333, 1433. [Google Scholar] [CrossRef]
- Jadvar, H.; Calais, J.; Fanti, S.; Feng, F.; Greene, K.L.; Gulley, J.L.; Hofman, M.; Koontz, B.F.; Lin, D.W.; Morris, M.J.; et al. Appropriate Use Criteria for Prostate-Specific Membrane Antigen PET Imaging. J. Nucl. Med. 2022, 63, 59–68. [Google Scholar] [CrossRef]
- Seifert, R.; Emmett, L.; Rowe, S.P.; Herrmann, K.; Hadaschik, B.; Calais, J.; Giesel, F.L.; Reiter, R.; Maurer, T.; Heck, M.; et al. Second Version of the Prostate Cancer Molecular Imaging Standardized Evaluation Framework Including Response Evaluation for Clinical Trials (PROMISE V2). Eur. Urol. 2023, 83, 405–412. [Google Scholar] [CrossRef]
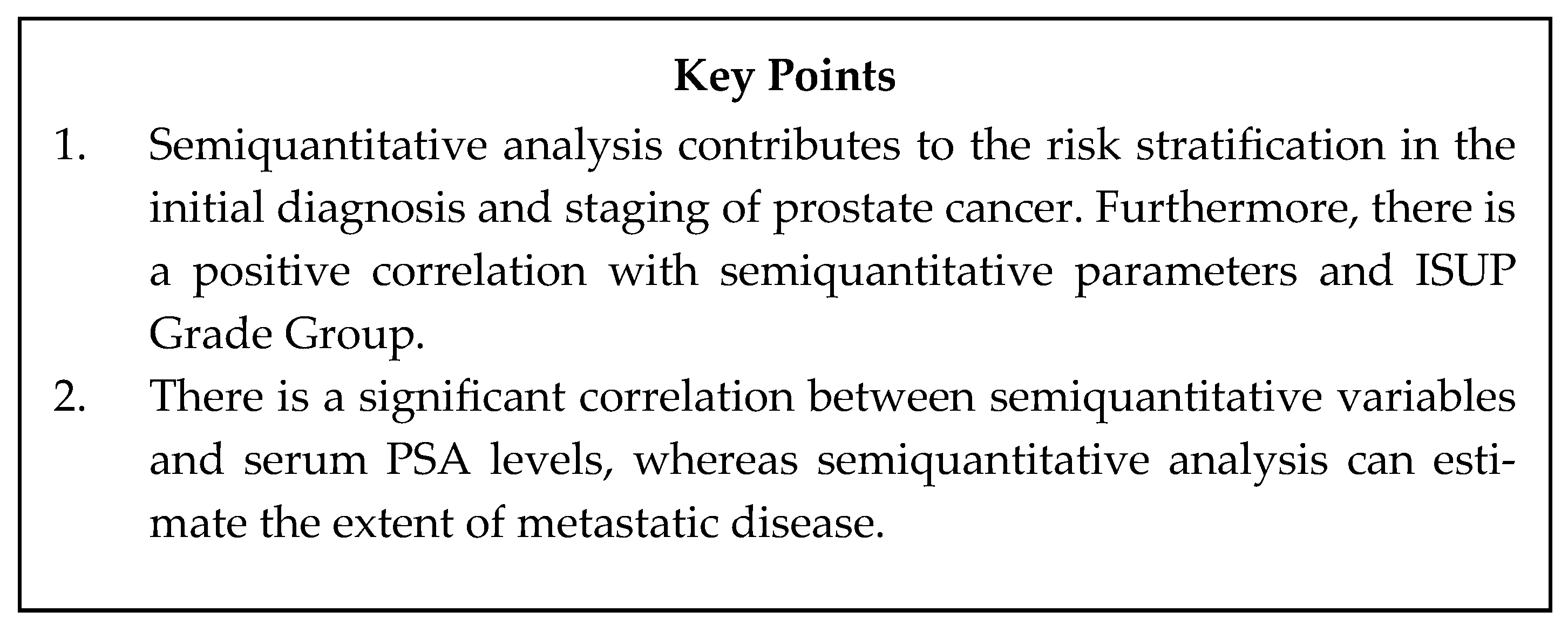
| Basic Characteristics | ||
|---|---|---|
| PSMA based radiotracers | [68Ga]Ga-PSMA-11 [18F]DCFPyL, [18F]PSMA-1007, [18F]JK-PSMA-7, [18F]rhPSMA-7, [18F]AlF-PSMA-11 | 68Ga based radiotracers: on-site generator short half-life lower radiation exposure 18F-based radiotracers: higher image resolution, lower end-point positron energy, longer half-life, cheaper production, more false positives findings, greater locoregional lesion detection rate, accuracy in the delineation of the local lesion, greater lesion SUV uptake |
| FDG based radiotracers | 18F-fluorodeoxyglucose | Prognostic biomarker in advanced prostate cancer use in PSMA-negative disease and in theragnostics approaches |
| Authors (Year) | Number of Patients Included | Radiotracers | Semiquantitative PET/CT Parameters | Main Findings |
|---|---|---|---|---|
| Dong et al. (2022) [35] | 60 | 18F-PSMA | SUVmax TL-PSMAp, PSMA-TVp | Semiquantitative analysis of the primary tumor on 18F-PSMA-1007 PET/CT imaging contributes to the risk stratification of prostate cancer |
| Yi et al. (2023) [36] | 147 | 68Ga-PSMA | SUVmax | SUVmax is a predicting factor of intermediate and high-risk prostate cancer |
| Heetman et al. (2024) [37] | 386 | 68Ga-PSMA | SUVmax | SUVmax ameliorates diagnostic accuracy in predicting the likelihood of clinically significant prostate cancer in biopsy material |
| Rogic et al. (2024) [38] | 34 | 68Ga-PSMA | SUVmax | A positive correlation was found between intraprostatic SUVmax and ISUP group |
| Ali et al. (2023) [39] | 154 | 68Ga-PSMA | SUVmax | SUVmax was both correlated with Gleason score and prostate-specific antigen (PSA) in organ-confined prostate cancer The median SUVmax and PSA directly related to Gleason score |
| Bela Andela et al. (2024) [40] | 86 | 68Ga-PSMA | SUVmax ASP PSMA-TV PSMA-TLU | Significant association of PSMA-TV PSMA-TLU and ASP with overall survival (OS) |
| Bodar et al. (2022) [41] | 318 | 68Ga-PSMA | SUVmax | SUVmax is correlated with with pISUP score and pathological tumor stage |
| Fragkiadaki et al. (2024) [33] | 104 | 18F-PSMA | SUVmax TMTV | There is a positive correlation between PSA levels with the semiquantitative parameters SUVmax and TMTV of the metastatic foci |
| Cardoza-Ochoa et al. (2022) [42] | 110 | 18F-PSMA | wbTl-PSMA wbPSMA-TV | Statistically significant correlation of between wbTL-PSMA and wbPSMA-TV with serum PSA. |
| Li, Y. et al. (2024) [43] | 110 | 18F-PSMA | TL-PSMAp PSMA-TVp SUVmax | TL-PSMAp and PSMA-TVp can distinguish between oligometastatic and extensive metastatic disease and can predict oligometastatic disease |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fragkiadaki, V.; Ntanasis-Stathopoulos, I.; Liontos, M.; Zagouri, F.; Dimopoulos, M.-A.; Gavriatopoulou, M. Semiquantitative Analysis in PET/CT Imaging of Prostate Cancer. J. Clin. Med. 2025, 14, 3828. https://doi.org/10.3390/jcm14113828
Fragkiadaki V, Ntanasis-Stathopoulos I, Liontos M, Zagouri F, Dimopoulos M-A, Gavriatopoulou M. Semiquantitative Analysis in PET/CT Imaging of Prostate Cancer. Journal of Clinical Medicine. 2025; 14(11):3828. https://doi.org/10.3390/jcm14113828
Chicago/Turabian StyleFragkiadaki, Vasiliki, Ioannis Ntanasis-Stathopoulos, Michalis Liontos, Flora Zagouri, Meletios-Athanasios Dimopoulos, and Maria Gavriatopoulou. 2025. "Semiquantitative Analysis in PET/CT Imaging of Prostate Cancer" Journal of Clinical Medicine 14, no. 11: 3828. https://doi.org/10.3390/jcm14113828
APA StyleFragkiadaki, V., Ntanasis-Stathopoulos, I., Liontos, M., Zagouri, F., Dimopoulos, M.-A., & Gavriatopoulou, M. (2025). Semiquantitative Analysis in PET/CT Imaging of Prostate Cancer. Journal of Clinical Medicine, 14(11), 3828. https://doi.org/10.3390/jcm14113828








