PSMA PET as a Tool for Active Surveillance of Prostate Cancer—Where Are We at?
Abstract
1. Introduction
2. Materials and Methods
3. Risk Stratification Using PSMA PET
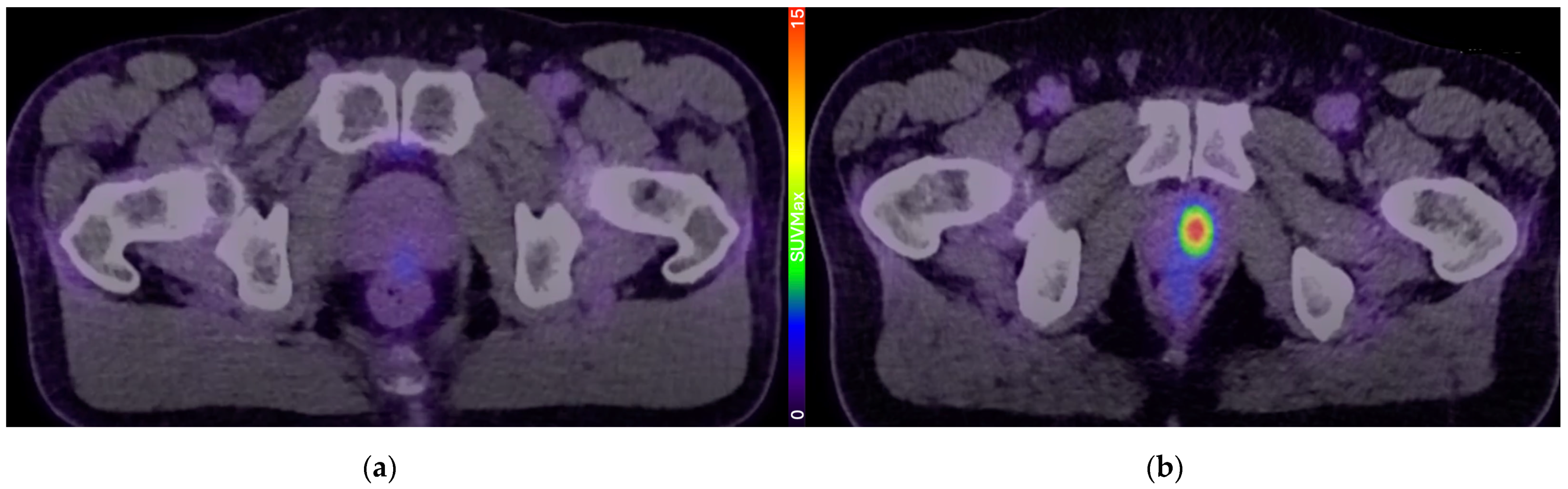
4. Current Studies Examining PSMA PET in Active Surveillance
5. Ongoing Prospective Trials
6. Current Guidelines
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| PSMA | Prostate-Specific Membrane Antigen |
| PET | Positron Emission Tomography |
| mpMRI | Multi-parametric MRI |
| MRI | Magnetic Resonance Imaging |
| ISUP | International Society of Urological Pathology |
| SUVMax | Maximum Standardized Uptake Value |
| AUA | American Urological Association |
| EAU | European Association of Urology |
| NCCN | National Comprehensive Cancer Network |
References
- Bergengren, O.; Pekala, K.R.; Matsoukas, K.; Fainberg, J.; Mungovan, S.F.; Bratt, O.; Bray, F.; Brawley, O.; Luckenbaugh, A.N.; Mucci, L.; et al. 2022 Update on Prostate Cancer Epidemiology and Risk Factors—A Systematic Review. Eur. Urol. 2023, 84, 191–206. [Google Scholar] [CrossRef]
- Ye, Y.; Zheng, Y.; Miao, Q.; Ruan, H.; Zhang, X. Causes of Death Among Prostate Cancer Patients Aged 40 Years and Older in the United States. Front. Oncol. 2022, 12, 914875. [Google Scholar] [CrossRef]
- Jahn, J.L.; Giovannucci, E.L.; Stampfer, M.J. The high prevalence of undiagnosed prostate cancer at autopsy: Implications for epidemiology and treatment of prostate cancer in the Prostate-specific Antigen-era. Int. J. Cancer 2015, 137, 2795–2802. [Google Scholar] [CrossRef]
- Jacklin, C.; Philippou, Y.; Brewster, S.F.; Bryant, R.J. “More men die with prostate cancer than because of it”—An old adage that still holds true in the 21st century. Cancer Treat. Res. Commun. 2021, 26, 100225. [Google Scholar] [CrossRef]
- Klotz, L. Overdiagnosis in urologic cancer: For World Journal of Urology Symposium on active surveillance in prostate and renal cancer. World J. Urol. 2022, 40, 1–8. [Google Scholar] [CrossRef]
- Cornford, D.T.P.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Brunckhorst, O.; Darraugh, J.; Eberli, D.; De Meerleer, G.; De Santis, M.; Farolfi, A.; et al. EAU-EANM-ESTRO-ESUR-ISUP-SIOG Guidelines on Prostate Cancer. Eur. Urol. 2024, 86, 148–163. [Google Scholar] [CrossRef]
- Eastham, J.A.; Auffenberg, G.B.; Barocas, D.A.; Chou, R.; Crispino, T.; Davis, J.W.; Eggener, S.; Horwitz, E.M.; Kane, C.J.; Kirkby, E.; et al. Clinically Localized Prostate Cancer: AUA/ASTRO Guideline, Part II: Principles of Active Surveillance, Principles of Surgery, and Follow-Up. J. Urol. 2022, 208, 19–25. [Google Scholar] [CrossRef]
- Lam, T.B.L.; MacLennan, S.; Willemse, P.-P.M.; Mason, M.D.; Plass, K.; Shepherd, R.; Baanders, R.; Bangma, C.H.; Bjartell, A.; Bossi, A.; et al. EAU-EANM-ESTRO-ESUR-SIOG Prostate Cancer Guideline Panel Consensus Statements for Deferred Treatment with Curative Intent for Localised Prostate Cancer from an International Collaborative Study (DETECTIVE Study). Eur. Urol. 2019, 76, 790–813. [Google Scholar] [CrossRef]
- Willemse, P.M.; Davis, N.F.; Grivas, N.; Zattoni, F.; Lardas, M.; Briers, E.; Cumberbatch, M.G.; De Santis, M.; Dell’Oglio, P.; Donaldson, J.F.; et al. Systematic Review of Active Surveillance for Clinically Localised Prostate Cancer to Develop Recommendations Regarding Inclusion of Intermediate-risk Disease, Biopsy Characteristics at Inclusion and Monitoring, and Surveillance Repeat Biopsy Strategy. Eur. Urol. 2022, 81, 337–346. [Google Scholar] [CrossRef]
- Porten, S.P.; Whitson, J.M.; Cowan, J.E.; Cooperberg, M.R.; Shinohara, K.; Perez, N.; Greene, K.L.; Meng, M.V.; Carroll, P.R. Changes in prostate cancer grade on serial biopsy in men undergoing active surveillance. J. Clin. Oncol. 2011, 29, 2795–2800. [Google Scholar] [CrossRef]
- King, A.C.; Livermore, A.; Laurila, T.A.J.; Huang, W.; Jarrard, D.F. Impact of immediate TRUS rebiopsy in a patient cohort considering active surveillance for favorable risk prostate cancer. Urol. Oncol. 2013, 31, 739–743. [Google Scholar] [CrossRef] [PubMed]
- de Rooij, M.; Hamoen, E.H.; Witjes, J.A.; Barentsz, J.O.; Rovers, M.M. Accuracy of Magnetic Resonance Imaging for Local Staging of Prostate Cancer: A Diagnostic Meta-analysis. Eur. Urol. 2016, 70, 233–245. [Google Scholar] [CrossRef]
- Schoots, I.G.; Roobol, M.J.; Nieboer, D.; Bangma, C.H.; Steyerberg, E.W.; Hunink, M.G.M. Magnetic Resonance Imaging–targeted Biopsy May Enhance the Diagnostic Accuracy of Significant Prostate Cancer Detection Compared to Standard Transrectal Ultrasound-guided Biopsy: A Systematic Review and Meta-analysis. Eur. Urol. 2015, 68, 438–450. [Google Scholar] [CrossRef]
- Drost, F.H.; Osses, D.F.; Nieboer, D.; Steyerberg, E.W.; Bangma, C.H.; Roobol, M.J.; Schoots, I.G. Prostate MRI, with or without MRI-targeted biopsy, and systematic biopsy for detecting prostate cancer. Cochrane Database Syst. Rev. 2019, 4, Cd012663. [Google Scholar] [CrossRef]
- Epstein, J.I.; Egevad, L.; Amin, M.B.; Delahunt, B.; Srigley, J.R.; Humphrey, P.A. The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma: Definition of Grading Patterns and Proposal for a New Grading System. Am. J. Surg. Pathol. 2016, 40, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Mellinger, G.T.; Gleason, D.; Bailar, J., 3rd. The histology and prognosis of prostatic cancer. J. Urol. 1967, 97, 331–337. [Google Scholar] [CrossRef]
- Wright, G.L., Jr.; Haley, C.; Beckett, M.L.; Schellhammer, P.F. Expression of prostate-specific membrane antigen in normal, benign, and malignant prostate tissues. Urol. Oncol. 1995, 1, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Hofman, M.S.; Lawrentschuk, N.; Francis, R.J.; Tang, C.; Vela, I.; Thomas, P.; Rutherford, N.; Martin, J.M.; Frydenberg, M.; Shakher, R.; et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): A prospective, randomised, multicentre study. Lancet 2020, 395, 1208–1216. [Google Scholar] [CrossRef]
- Xue, A.L.; Kalapara, A.A.; Ballok, Z.E.; Levy, S.M.; Sivaratnam, D.; Ryan, A.; Ramdave, S.; O’Sullivan, R.; Moon, D.; Grummet, J.P.; et al. (68)Ga-Prostate-Specific Membrane Antigen Positron Emission Tomography Maximum Standardized Uptake Value as a Predictor of Gleason Pattern 4 and Pathological Upgrading in Intermediate-Risk Prostate Cancer. J. Urol. 2022, 207, 341–349. [Google Scholar] [CrossRef]
- Raveenthiran, S.; Yaxley, W.J.; Franklin, T.; Coughlin, G.; Roberts, M.; Gianduzzo, T.; Kua, B.; Samaratunga, H.; Delahunt, B.; Egevad, L.; et al. Findings in 1,123 Men with Preoperative (68)Ga-Prostate-Specific Membrane Antigen Positron Emission Tomography/Computerized Tomography and Multiparametric Magnetic Resonance Imaging Compared to Totally Embedded Radical Prostatectomy Histopathology: Implications for the Diagnosis and Management of Prostate Cancer. J. Urol. 2022, 207, 573–580. [Google Scholar] [CrossRef]
- Emmett, L.; Buteau, J.; Papa, N.; Moon, D.; Thompson, J.; Roberts, M.J.; Rasiah, K.; Pattison, D.A.; Yaxley, J.; Thomas, P.; et al. The Additive Diagnostic Value of Prostate-specific Membrane Antigen Positron Emission Tomography Computed Tomography to Multiparametric Magnetic Resonance Imaging Triage in the Diagnosis of Prostate Cancer (PRIMARY): A Prospective Multicentre Study. Eur. Urol. 2021, 80, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Emmett, L.; Papa, N.; Buteau, J.; Ho, B.; Liu, V.; Roberts, M.; Thompson, J.; Moon, D.; Sheehan-Dare, G.; Alghazo, O.; et al. The PRIMARY Score: Using Intraprostatic (68)Ga-PSMA PET/CT Patterns to Optimize Prostate Cancer Diagnosis. J. Nucl. Med. 2022, 63, 1644–1650. [Google Scholar] [CrossRef]
- Stabile, A.; Pellegrino, A.; Mazzone, E.; Cannoletta, D.; de Angelis, M.; Barletta, F.; Scuderi, S.; Cucchiara, V.; Gandaglia, G.; Raggi, D.; et al. Can Negative Prostate-specific Membrane Antigen Positron Emission Tomography/Computed Tomography Avoid the Need for Pelvic Lymph Node Dissection in Newly Diagnosed Prostate Cancer Patients? A Systematic Review and Meta-analysis with Backup Histology as Reference Standard. Eur. Urol. Oncol. 2022, 5, 1–17. [Google Scholar] [CrossRef]
- Mazzone, E.; Cannoletta, D.; Quarta, L.; Chen, D.C.; Thomson, A.; Barletta, F.; Stabile, A.; Moon, D.; Eapen, R.; Lawrentschuk, N.; et al. A Comprehensive Systematic Review and Meta-analysis of the Role of Prostate-specific Membrane Antigen Positron Emission Tomography for Prostate Cancer Diagnosis and Primary Staging before Definitive Treatment. Eur. Urol. 2025, in press. [CrossRef]
- Yaxley, J.W.; Raveenthiran, S.; Nouhaud, F.X.; Samartunga, H.; Yaxley, A.J.; Coughlin, G.; Delahunt, B.; Egevad, L.; McEwan, L.; Wong, D. Outcomes of Primary Lymph Node Staging of Intermediate and High Risk Prostate Cancer with (68)Ga-PSMA Positron Emission Tomography/Computerized Tomography Compared to Histological Correlation of Pelvic Lymph Node Pathology. J. Urol. 2019, 201, 815–820. [Google Scholar] [CrossRef]
- Cacciamani, G.E.; Maas, M.; Nassiri, N.; Ortega, D.; Gill, K.; Dell’Oglio, P.; Thalmann, G.N.; Heidenreich, A.; Eastham, J.A.; Evans, C.P.; et al. Impact of Pelvic Lymph Node Dissection and Its Extent on Perioperative Morbidity in Patients Undergoing Radical Prostatectomy for Prostate Cancer: A Comprehensive Systematic Review and Meta-analysis. Eur. Urol. Oncol. 2021, 4, 134–149. [Google Scholar] [CrossRef]
- Fossati, N.; Willemse, P.M.; Van den Broeck, T.; van den Bergh, R.C.N.; Yuan, C.Y.; Briers, E.; Bellmunt, J.; Bolla, M.; Cornford, P.; De Santis, M.; et al. The Benefits and Harms of Different Extents of Lymph Node Dissection During Radical Prostatectomy for Prostate Cancer: A Systematic Review. Eur. Urol. 2017, 72, 84–109. [Google Scholar] [CrossRef] [PubMed]
- Perera, M.; Papa, N.; Christidis, D.; Wetherell, D.; Hofman, M.S.; Murphy, D.G.; Bolton, D.; Lawrentschuk, N. Sensitivity, Specificity, and Predictors of Positive 68Ga–Prostate-specific Membrane Antigen Positron Emission Tomography in Advanced Prostate Cancer: A Systematic Review and Meta-analysis. Eur. Urol. 2016, 70, 926–937. [Google Scholar] [CrossRef] [PubMed]
- Pozdnyakov, A.; Kulanthaivelu, R.; Bauman, G.; Ortega, C.; Veit-Haibach, P.; Metser, U. The impact of PSMA PET on the treatment and outcomes of men with biochemical recurrence of prostate cancer: A systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2023, 26, 240–248. [Google Scholar] [CrossRef]
- Banerjee, S.; Pillai, M.R.A.; Knapp, F.F. Lutetium-177 Therapeutic Radiopharmaceuticals: Linking Chemistry, Radiochemistry, and Practical Applications. Chem. Rev. 2015, 115, 2934–2974. [Google Scholar] [CrossRef]
- Sartor, O.; Bono, J.d.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Haddad, G.; et al. Lutetium-177–PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2021, 385, 1091–1103. [Google Scholar] [CrossRef]
- Azad, A.A.; Bressel, M.; Tan, H.; Voskoboynik, M.; Suder, A.; Weickhardt, A.J.; Guminski, A.; Francis, R.J.; Saghebi, J.; Dhiantravan, N.; et al. Sequential [177 Lu]Lu-PSMA-617 and docetaxel versus docetaxel in patients with metastatic hormone-sensitive prostate cancer (UpFrontPSMA): A multicentre, open-label, randomised, phase 2 study. Lancet Oncol. 2024, 25, 1267–1276. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.J.; Castellano, D.; Herrmann, K.; de Bono, J.S.; Shore, N.D.; Chi, K.N.; Crosby, M.; Piulats, J.M.; Fléchon, A.; Wei, X.X.; et al. 177Lu-PSMA-617 versus a change of androgen receptor pathway inhibitor therapy for taxane-naive patients with progressive metastatic castration-resistant prostate cancer (PSMAfore): A phase 3, randomised, controlled trial. Lancet 2024, 404, 1227–1239. [Google Scholar] [CrossRef]
- Groener, D.; Schneider, S.; Baumgarten, J.; Happel, C.; Klimek, K.; Mader, N.; Nguyen Ngoc, C.; Wichert, J.; Mandel, P.; Tselis, N.; et al. Baseline [(68)Ga]Ga-PSMA-11 PET/CT before [(177)Lu]Lu-PSMA-617 Radioligand Therapy: Value of PSMA-Uptake Thresholds in Predicting Targetable Lesions. Cancers 2023, 15, 473. [Google Scholar] [CrossRef]
- Dondi, F.; Antonelli, A.; Suardi, N.; Treglia, G.; Bertagna, F. The Role of PSMA PET Imaging in the Classification of the Risk of Prostate Cancer Patients: A Systematic Review on the Insights to Guide an Active Surveillance Approach. Cancers 2024, 16, 1122. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Zhang, M.; Huang, H.; Zhang, C.; Ruan, X.; Lin, W.; Li, B.; Chen, L.; Xu, D. Clinical Utility of (18)F-PSMA-1007 Positron Emission Tomography/Magnetic Resonance Imaging in Prostate Cancer: A Single-Center Experience. Front. Oncol. 2020, 10, 612701. [Google Scholar] [CrossRef]
- Grubmüller, B.; Baltzer, P.; Hartenbach, S.; D’Andrea, D.; Helbich, T.H.; Haug, A.R.; Goldner, G.M.; Wadsak, W.; Pfaff, S.; Mitterhauser, M.; et al. PSMA Ligand PET/MRI for Primary Prostate Cancer: Staging Performance and Clinical Impact. Clin. Cancer Res. 2018, 24, 6300–6307. [Google Scholar] [CrossRef]
- Pepe, P.; Pepe, L.; Tamburo, M.; Marletta, G.; Savoca, F.; Pennisi, M.; Fraggetta, F. 68Ga-PSMA PET/CT evaluation in men enrolled in prostate cancer Active Surveillance. Arch. Ital. Urol. Androl. 2023, 95, 11322. [Google Scholar] [CrossRef]
- Heetman, J.G.; Lavalaye, J.; Polm, P.D.; Soeterik, T.F.W.; Wever, L.; Paulino Pereira, L.J.; van der Hoeven, E.; van Melick, H.H.E.; van den Bergh, R.C.N. Gallium-68 Prostate-specific Membrane Antigen Positron Emission Tomography/Computed Tomography in Active Surveillance for Prostate Cancer Trial (PASPoRT). Eur. Urol. Oncol. 2024, 7, 204–210. [Google Scholar] [CrossRef]
- Jain, A.; Nassour, A.J.; Dean, T.; Patterson, I.; Tarlinton, L.; Kim, L.; Woo, H. Expanding the role of PSMA PET in active surveillance. BMC Urol. 2023, 23, 77. [Google Scholar] [CrossRef]
- Gondoputro, W.; Doan, P.; Katelaris, A.; Scheltema, M.J.; Geboers, B.; Agrawal, S.; Liu, Z.; Yaxley, J.; Savdie, R.; Rasiah, K.; et al. (68)Ga-PSMA-PET/CT in addition to mpMRI in men undergoing biopsy during active surveillance for low- to intermediate-risk prostate cancer: Study protocol for a prospective cross-sectional study. Transl. Androl. Urol. 2023, 12, 1598–1606. [Google Scholar] [CrossRef] [PubMed]
- Bagguley, D.; Harewood, L.; McKenzie, D.; Ptasznik, G.; Ong, S.; Chengodu, T.; Woon, D.; Sim, K.; Sheldon, J.; Lawrentschuk, N. The CONFIRM trial protocol: The utility of prostate-specific membrane antigen positron emission tomography/computed tomography in active surveillance for prostate cancer. BJU Int. 2024, 133 (Suppl. S4), 27–36. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Harewood, L.; Bagguley, D.; Dundee, P.; Mirmilstein, G.; Murphy, D.G.; Chan, Y.; Moon, D.; Kearns, P.; Satasivam, P.; et al. Early Results from the CONFIRM Trial: Utility of Prostate-specific Membrane Antigen Positron Emission Tomography/Computed Tomography in Active Surveillance for Prostate Cancer. Eur. Urol. Onc. 2025, in press. [CrossRef] [PubMed]
- Spratt, D.E.; Srinivas, S.; Schaeffer, E.M.; Adra, N.; Ahmed, B.; An, Y.; Bitting, R.; Chapin, B.; Cheng, H.; Cho, S.Y.; et al. NCCN Clinical Practice Guidelines in Oncology for Prostate Cancer Version 1.2025. 2025. Available online: https://www.nccn.org/guidelines/guidelines-detail?id=1459 (accessed on 20 April 2025).
| Paper | Study Design | Patients (N) | Inclusion Criteria | PET Timing | Key Findings |
|---|---|---|---|---|---|
| Pepe et al. [38] | Prospective | 40 | Very-low-risk prostate cancer patients on active surveillance | 48–60 months after initial diagnosis | PSMA PET is superior to MRI at detecting patients that can safely be excluded from a repeat biopsy. |
| Heetman et al. [39] | Prospective | 141 | Newly diagnosed low-risk or favourable intermediate-risk disease suitable for active surveillance | 6 months after initial diagnosis | Up to 9% of patients had pathological upgrading detected on PSMA PET-targeted biopsy. |
| Jain et al. [40] | Retrospective | 30 | Newly diagnosed low-risk or favourable intermediate-risk disease suitable for active surveillance | 50% of men suitable for active surveillance had concerning findings on PSMA PET. PSMA can help inform management of men considering active surveillance. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carll, J.; Bonaddio, J.; Lawrentschuk, N. PSMA PET as a Tool for Active Surveillance of Prostate Cancer—Where Are We at? J. Clin. Med. 2025, 14, 3580. https://doi.org/10.3390/jcm14103580
Carll J, Bonaddio J, Lawrentschuk N. PSMA PET as a Tool for Active Surveillance of Prostate Cancer—Where Are We at? Journal of Clinical Medicine. 2025; 14(10):3580. https://doi.org/10.3390/jcm14103580
Chicago/Turabian StyleCarll, Jonathon, Jacinta Bonaddio, and Nathan Lawrentschuk. 2025. "PSMA PET as a Tool for Active Surveillance of Prostate Cancer—Where Are We at?" Journal of Clinical Medicine 14, no. 10: 3580. https://doi.org/10.3390/jcm14103580
APA StyleCarll, J., Bonaddio, J., & Lawrentschuk, N. (2025). PSMA PET as a Tool for Active Surveillance of Prostate Cancer—Where Are We at? Journal of Clinical Medicine, 14(10), 3580. https://doi.org/10.3390/jcm14103580






