The Role of Global Physical Capacity Score in Key Parameters of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD)
Abstract
1. Introduction
2. Materials and Methods
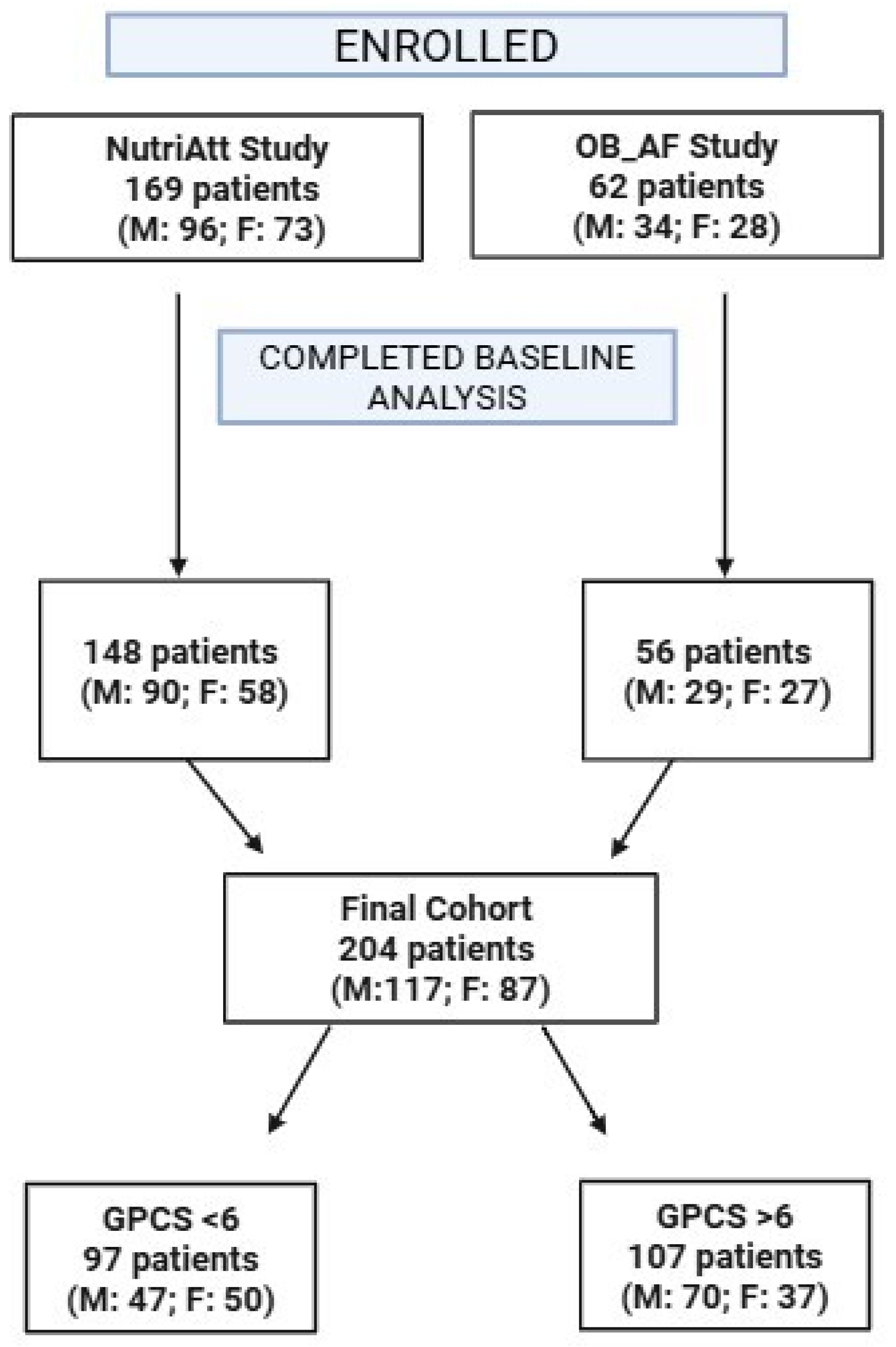
2.1. Participants and Study Design
2.2. Data Collection
2.3. Physical Capacity Evaluation Test
2.4. Definition of MASLD
2.5. Exposure—Global Physical Capacity Score
2.6. Outcome Assessment
2.7. Variables of Exposure and Confounders
2.8. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Regression Analysis
3.3. Logistic Regression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MASLD | Metabolic Dysfunction-Associated Steatotic Liver Disease |
| NAFLD | Non-Alcoholic Fatty Liver Disease |
| MASH | Metabolic Dysfunction-Associated Steatohepatitis |
| PC | Physical Capacity |
| CRF | Cardiorespiratory Fitness |
| HOMA-IR | Homeostasis Model Assessment of Insulin Resistance |
| RCTs | Randomized Clinical Trials |
| BMI | Body Mass Index |
| CAP | Controlled Attenuation Parameter |
| E | Elasticity |
| HbA1c | Glycated Hemoglobin |
| TC | Total Cholesterol |
| HDL | High-Density Lipoprotein |
| LDL | Low-Density Lipoprotein |
| AST | Aspartate Transaminase |
| ALT | Alanine Amino Transferase |
| GGT | Gamma Glutamyl Transferase |
| TG | Triglycerides |
| VIF | Variance Inflation Factor |
| OR | Odds Ratio |
| WHO | World Health Organization |
| CI | Confidence Interval |
References
- Calzadilla-Bertot, L.; Jeffrey, G.P.; Wang, Z.; Huang, Y.; Garas, G.; Wallace, M.; De Boer, B.; George, J.; Eslam, M.; Phu, A.; et al. Predicting Liver-Related Events in NAFLD: A Predictive Model. Hepatology 2023, 78, 1240–1251. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.-A.; Moon, J.H.; Kim, W. Critical Appraisal of Metabolic Dysfunction-Associated Steatotic Liver Disease: Implication of Janus-Faced Modernity. Clin. Mol. Hepatol. 2023, 29, 831–843. [Google Scholar] [CrossRef]
- Kim, D.; Danpanichkul, P.; Wijarnpreecha, K.; Cholankeril, G.; Loomba, R.; Ahmed, A. Current Burden of Steatotic Liver Disease and Fibrosis among Adults in the United States, 2017–2023. Clin. Mol. Hepatol. 2025, 31, 382–393. [Google Scholar] [CrossRef]
- Younossi, Z.M.; AlQahtani, S.A.; Funuyet-Salas, J.; Romero-Gómez, M.; Yilmaz, Y.; Keklikkiran, C.; Alswat, K.; Yu, M.-L.; Liu, C.-J.; Fan, J.-G.; et al. The Impact of Stigma on Quality of Life and Liver Disease Burden among Patients with Nonalcoholic Fatty Liver Disease. JHEP Rep. 2024, 6, 101066. [Google Scholar] [CrossRef]
- Yoon, E.L.; Jun, D.W. Waiting for the Changes after the Adoption of Steatotic Liver Disease. Clin. Mol. Hepatol. 2023, 29, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Powell, E.E.; Wong, V.W.-S.; Rinella, M. Non-Alcoholic Fatty Liver Disease. Lancet 2021, 397, 2212–2224. [Google Scholar] [CrossRef]
- Huttasch, M.; Roden, M.; Kahl, S. Obesity and MASLD: Is Weight Loss the (Only) Key to Treat Metabolic Liver Disease? Metabolism 2024, 157, 155937. [Google Scholar] [CrossRef] [PubMed]
- Targher, G.; Tilg, H.; Byrne, C.D. Non-Alcoholic Fatty Liver Disease: A Multisystem Disease Requiring a Multidisciplinary and Holistic Approach. Lancet Gastroenterol. Hepatol. 2021, 6, 578–588. [Google Scholar] [CrossRef]
- Shen, Y.; Cheng, L.; Xu, M.; Wang, W.; Wan, Z.; Xiong, H.; Guo, W.; Cai, M.; Xu, F. SGLT2 Inhibitor Empagliflozin Downregulates miRNA-34a-5p and Targets GREM2 to Inactivate Hepatic Stellate Cells and Ameliorate Non-Alcoholic Fatty Liver Disease-Associated Fibrosis. Metabolism 2023, 146, 155657. [Google Scholar] [CrossRef]
- Wang, X.-F.; Chen, X.; Tang, Y.; Wu, J.-M.; Qin, D.-L.; Yu, L.; Yu, C.-L.; Zhou, X.-G.; Wu, A.-G. The Therapeutic Potential of Plant Polysaccharides in Metabolic Diseases. Pharmaceuticals 2022, 15, 1329. [Google Scholar] [CrossRef]
- Zhao, F.; Shao, M.; Li, M.; Li, T.; Zheng, Y.; Sun, W.; Ni, C.; Li, L. Sphingolipid Metabolites Involved in the Pathogenesis of Atherosclerosis: Perspectives on Sphingolipids in Atherosclerosis. Cell Mol. Biol. Lett. 2025, 30, 18. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, S.A.; Targher, G. Role of Glucocorticoids in Metabolic Dysfunction-Associated Steatotic Liver Disease. Curr. Obes. Rep. 2024, 13, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Curci, R.; Bonfiglio, C.; Franco, I.; Bagnato, C.B.; Verrelli, N.; Bianco, A. Leisure-Time Physical Activity in Subjects with Metabolic-Dysfunction-Associated Steatotic Liver Disease: An All-Cause Mortality Study. JCM 2024, 13, 3772. [Google Scholar] [CrossRef]
- Horn, P.; Tacke, F. Metabolic Reprogramming in Liver Fibrosis. Cell Metab. 2024, 36, 1439–1455. [Google Scholar] [CrossRef]
- Bagnato, C.B.; Bianco, A.; Bonfiglio, C.; Franco, I.; Verrelli, N.; Carella, N.; Shahini, E.; Zappimbulso, M.; Giannuzzi, V.; Pesole, P.L.; et al. Healthy Lifestyle Changes Improve Cortisol Levels and Liver Steatosis in MASLD Patients: Results from a Randomized Clinical Trial. Nutrients 2024, 16, 4225. [Google Scholar] [CrossRef]
- Piercy, K.L.; Troiano, R.P.; Ballard, R.M.; Carlson, S.A.; Fulton, J.E.; Galuska, D.A.; George, S.M.; Olson, R.D. The Physical Activity Guidelines for Americans. JAMA 2018, 320, 2020. [Google Scholar] [CrossRef]
- Riebe, D.; Franklin, B.A.; Thompson, P.D.; Garber, C.E.; Whitfield, G.P.; Magal, M.; Pescatello, L.S. Updating ACSM’s Recommendations for Exercise Preparticipation Health Screening. Med. Sci. Sports Exerc. 2015, 47, 2473–2479. [Google Scholar] [CrossRef]
- Erikssen, G. Physical Fitness and Changes in Mortality: The Survival of the Fittest. Sports Med. 2001, 31, 571–576. [Google Scholar] [CrossRef]
- Kodama, S. Cardiorespiratory Fitness as a Quantitative Predictor of All-Cause Mortality and Cardiovascular Events in Healthy Men and Women: A Meta-Analysis. JAMA 2009, 301, 2024. [Google Scholar] [CrossRef]
- Ross, R.; Blair, S.N.; Arena, R.; Church, T.S.; Després, J.-P.; Franklin, B.A.; Haskell, W.L.; Kaminsky, L.A.; Levine, B.D.; Lavie, C.J.; et al. Importance of Assessing Cardiorespiratory Fitness in Clinical Practice: A Case for Fitness as a Clinical Vital Sign: A Scientific Statement from the American Heart Association. Circulation 2016, 134, e653–e699. [Google Scholar] [CrossRef]
- Katzmarzyk, P.T.; Church, T.S.; Blair, S.N. Cardiorespiratory Fitness Attenuates the Effects of the Metabolic Syndrome on All-Cause and Cardiovascular Disease Mortality in Men. Arch. Intern. Med. 2004, 164, 1092. [Google Scholar] [CrossRef] [PubMed]
- Fraser, B.J.; Huynh, Q.L.; Schmidt, M.D.; Dwyer, T.; Venn, A.J.; Magnussen, C.G. Childhood Muscular Fitness Phenotypes and Adult Metabolic Syndrome. Med. Sci. Sports Exerc. 2016, 48, 1715–1722. [Google Scholar] [CrossRef] [PubMed]
- Silventoinen, K.; Magnusson, P.K.E.; Tynelius, P.; Batty, G.D.; Rasmussen, F. Association of Body Size and Muscle Strength with Incidence of Coronary Heart Disease and Cerebrovascular Diseases: A Population-Based Cohort Study of One Million Swedish Men. Int. J. Epidemiol. 2009, 38, 110–118. [Google Scholar] [CrossRef]
- Kang, S.; Moon, M.K.; Kim, W.; Koo, B.K. Association between Muscle Strength and Advanced Fibrosis in Non-alcoholic Fatty Liver Disease: A Korean Nationwide Survey. J. Cachexia Sarcopenia Muscle 2020, 11, 1232–1241. [Google Scholar] [CrossRef]
- Gan, D.; Wang, L.; Jia, M.; Ru, Y.; Ma, Y.; Zheng, W.; Zhao, X.; Yang, F.; Wang, T.; Mu, Y.; et al. Low Muscle Mass and Low Muscle Strength Associate with Nonalcoholic Fatty Liver Disease. Clin. Nutr. 2020, 39, 1124–1130. [Google Scholar] [CrossRef]
- Hao, X.-Y.; Zhang, K.; Huang, X.-Y.; Yang, F.; Sun, S.-Y. Muscle Strength and Non-Alcoholic Fatty Liver Disease/Metabolic-Associated Fatty Liver Disease. World J. Gastroenterol. 2024, 30, 636–643. [Google Scholar] [CrossRef]
- Mikkelsson, L.O.; Nupponen, H.; Kaprio, J.; Kautiainen, H.; Mikkelsson, M.; Kujala, U.M. Adolescent Flexibility, Endurance Strength, and Physical Activity as Predictors of Adult Tension Neck, Low Back Pain, and Knee Injury: A 25 Year Follow up Study. Br. J. Sports Med. 2006, 40, 107–113. [Google Scholar] [CrossRef]
- Yamamoto, K.; Kawano, H.; Gando, Y.; Iemitsu, M.; Murakami, H.; Sanada, K.; Tanimoto, M.; Ohmori, Y.; Higuchi, M.; Tabata, I.; et al. Poor Trunk Flexibility Is Associated with Arterial Stiffening. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H1314–H1318. [Google Scholar] [CrossRef]
- Bouchard, D.R.; Soucy, L.; Sénéchal, M.; Dionne, I.J.; Brochu, M. Impact of Resistance Training with or without Caloric Restriction on Physical Capacity in Obese Older Women. Menopause 2009, 16, 66–72. [Google Scholar] [CrossRef]
- Sato, T.; Fujihara, K.; Yamada, M.H.; Chou, K.; Yaguchi, Y.; Kitazawa, M.; Ishiguro, H.; Osawa, T.; Yamada, T.; Kodama, S.; et al. Utility of a Physical Fitness Score in Screening for Chronic Diseases. J. Sports Sci. Med. 2023, 22, 98–110. [Google Scholar] [CrossRef]
- Bianco, A.; Russo, F.; Franco, I.; Riezzo, G.; Donghia, R.; Curci, R.; Bonfiglio, C.; Prospero, L.; D’Attoma, B.; Ignazzi, A.; et al. Enhanced Physical Capacity and Gastrointestinal Symptom Improvement in Southern Italian IBS Patients Following Three Months of Moderate Aerobic Exercise. JCM 2023, 12, 6786. [Google Scholar] [CrossRef] [PubMed]
- Franco, I.; Bianco, A.; Mirizzi, A.; Campanella, A.; Bonfiglio, C.; Sorino, P.; Notarnicola, M.; Tutino, V.; Cozzolongo, R.; Giannuzzi, V.; et al. Physical Activity and Low Glycemic Index Mediterranean Diet: Main and Modification Effects on NAFLD Score. Results from a Randomized Clinical Trial. Nutrients 2020, 13, 66. [Google Scholar] [CrossRef] [PubMed]
- Laukkanen, R.; Oja, P.; Pasanen, M.; Vuori, I. Validity of a Two Kilometre Walking Test for Estimating Maximal Aerobic Power in Overweight Adults. Int. J. Obes. Relat. Metab. Disord. 1992, 16, 263–268. [Google Scholar] [PubMed]
- Roberts, H.C.; Denison, H.J.; Martin, H.J.; Patel, H.P.; Syddall, H.; Cooper, C.; Sayer, A.A. A Review of the Measurement of Grip Strength in Clinical and Epidemiological Studies: Towards a Standardised Approach. Age Ageing 2011, 40, 423–429. [Google Scholar] [CrossRef]
- Lemmink, K.A.P.M.; Kemper, H.C.G.; Greef, M.H.G.; Rispens, P.; Stevens, M. The Validity of the Sit-and-Reach Test and the Modified Sit-and-Reach Test in Middle-Aged to Older Men and Women. Res. Q. Exerc. Sport. 2003, 74, 331–336. [Google Scholar] [CrossRef]
- Stenholm, S.; Mehta, N.K.; Elo, I.T.; Heliövaara, M.; Koskinen, S.; Aromaa, A. Obesity and muscle strength as long-term determinants of all-cause mortality—a 33-year follow-up of the Mini-Finland Health Examination Survey. Int. J. Obes. 2014, 38, 1126–1132. [Google Scholar] [CrossRef]
- Jackson, A.W.; Morrow, J.R., Jr.; Bril, P.A.; Kohl, H.W., III; Gordon, N.F.; Blair, S.N. Relations of sit-up and sit-and-reach tests to low back pain in adults. J. Orthop. Sports Phys. Ther. 1998, 27, 22–26. [Google Scholar] [CrossRef]
- Mayorga-Vega, D.; Merino-Marban, R.; Viciana, J. Criterion-Related Validity of Sit-and-Reach Tests for Estimating Hamstring and Lumbar Extensibility: A Meta-Analysis. J. Sports Sci. Med. 2014, 13, 1–14. [Google Scholar]
- Rance, M.; Boussuge, P.Y.; Lazaar, N.; Bedu, M.; Van Praagh, E.; Dabonneville, M.; Duché, P. Validity of a V.O2 max prediction equation of the 2-km walk test in female seniors. Int. J. Sports Med. 2005, 26, 453–456. [Google Scholar] [CrossRef]
- Eurofit for adults: Assessment of Health-Related Fitness; Oja, Pekka.; Tuxworth, Bill.; Council of Europe. Committee for the Development of Sport; UKK Institute for Health Promotion Research; Council of Europe, Committee for the Development of Sport; UKK Institute for Health Promotion Research; Croton-on-Hudson, N.Y.: Manhattan Pub. Co. distributor; 1995 Library Catalog; MMS ID 9911531813406676. Available online: https://catalog.nlm.nih.gov/discovery/fulldisplay?docid=alma9911531813406676&context=L&vid=01NLM_INST:01NLM_INST&lang=en&search_scope=MyInstitution&adaptor=Local%20Search%20Engine&tab=LibraryCatalog&query=lds56,contains,Physical%20Fitness%20--%20physiology,AND&mode=advanced&offset=40 (accessed on 13 April 2025).
- Liao, C.-D.; Tsauo, J.-Y.; Huang, S.-W.; Ku, J.-W.; Hsiao, D.-J.; Liou, T.-H. Effects of Elastic Band Exercise on Lean Mass and Physical Capacity in Older Women with Sarcopenic Obesity: A Randomized Controlled Trial. Sci. Rep. 2018, 8, 2317. [Google Scholar] [CrossRef]
- Belsley, D.A.; Kuh, E.; Welsch, R.E. Regression Diagnostics: Identifying Influential Data and Sources of Collinearity, 1st ed.; Wiley Series in Probability and Statistics; Wiley: Hoboken, NJ, USA, 1980; ISBN 978-0-471-05856-4. [Google Scholar]
- Hastie, T.; Tibshirani, R.; Wainwright, M. Statistical Learning with Sparsity: The Lasso and Generalizations; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2015; 6000 Broken Sound Parkway NW, Suite 300. [Google Scholar]
- Schneider, A.; Hommel, G.; Blettner, M. Linear Regression Analysis. Dtsch. Ärzteblatt Int. 2010, 107, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Simon, S.D. Understanding the Odds Ratio and the Relative Risk. J. Androl. 2001, 22, 533–536. [Google Scholar] [CrossRef]
- Soares-Miranda, L.; Siscovick, D.S.; Psaty, B.M.; Longstreth, W.T.; Mozaffarian, D. Physical Activity and Risk of Coronary Heart Disease and Stroke in Older Adults: The Cardiovascular Health Study. Circulation 2016, 133, 147–155. [Google Scholar] [CrossRef]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.-P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 Guidelines on Physical Activity and Sedentary Behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef]
- Bowden Davies, K.A.; Sprung, V.S.; Norman, J.A.; Thompson, A.; Mitchell, K.L.; Harrold, J.A.; Finlayson, G.; Gibbons, C.; Wilding, J.P.H.; Kemp, G.J.; et al. Physical Activity and Sedentary Time: Association with Metabolic Health and Liver Fat. Med. Sci. Sports Exerc. 2019, 51, 1169–1177. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Saltin, B. Exercise as Medicine—Evidence for Prescribing Exercise as Therapy in 26 Different Chronic Diseases. Scand. Med. Sci. Sports 2015, 25, 1–72. [Google Scholar] [CrossRef]
- Park, S.W.; Goodpaster, B.H.; Strotmeyer, E.S.; De Rekeneire, N.; Harris, T.B.; Schwartz, A.V.; Tylavsky, F.A.; Newman, A.B. Decreased Muscle Strength and Quality in Older Adults with Type 2 Diabetes. Diabetes 2006, 55, 1813–1818. [Google Scholar] [CrossRef]
- Konyn, P.; Ahmed, A.; Kim, D. Causes and Risk Profiles of Mortality among Individuals with Nonalcoholic Fatty Liver Disease. Clin. Mol. Hepatol. 2023, 29, S43–S57. [Google Scholar] [CrossRef]
- Vilar-Gomez, E.; Martinez-Perez, Y.; Calzadilla-Bertot, L.; Torres-Gonzalez, A.; Gra-Oramas, B.; Gonzalez-Fabian, L.; Friedman, S.L.; Diago, M.; Romero-Gomez, M. Weight Loss Through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterology 2015, 149, 367–378.e5. [Google Scholar] [CrossRef]
- Li, M. Association of Physical Activity with MAFLD/MASLD and LF among Adults in NHANES, 2017–2020. Wien. Klin. Wochenschr. 2024, 136, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.-K.; Chuah, K.-H.; Rajaram, R.B.; Lim, L.-L.; Ratnasingam, J.; Vethakkan, S.R. Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): A State-of-the-Art Review. J. Obes. Metab. Syndr. 2023, 32, 197–213. [Google Scholar] [CrossRef] [PubMed]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wai-Sun Wong, V.; Dufour, J.-F.; Schattenberg, J.M.; et al. A New Definition for Metabolic Dysfunction-Associated Fatty Liver Disease: An International Expert Consensus Statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef]
- Richter, E.A.; Hargreaves, M. Exercise, GLUT4, and Skeletal Muscle Glucose Uptake. Physiol. Rev. 2013, 93, 993–1017. [Google Scholar] [CrossRef]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-Dependent Myokine That Drives Brown-Fat-like Development of White Fat and Thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef]
- Takeda, R.; Nonaka, Y.; Kakinoki, K.; Miura, S.; Kano, Y.; Hoshino, D. Effect of Endurance Training and PGC-1α Overexpression on Calculated Lactate Production Volume during Exercise Based on Blood Lactate Concentration. Sci. Rep. 2022, 12, 1635. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Vachliotis, I.D.; Mantzoros, C.S. Sarcopenia, Sarcopenic Obesity and Nonalcoholic Fatty Liver Disease. Metabolism 2023, 147, 155676. [Google Scholar] [CrossRef]
- Alabdul Razzak, I.; Fares, A.; Stine, J.G.; Trivedi, H.D. The Role of Exercise in Steatotic Liver Diseases: An Updated Perspective. Liver Int. 2025, 45, e16220. [Google Scholar] [CrossRef]

| All Sample | GPCS Median | |||
|---|---|---|---|---|
| <6 | ≥6 | p-Value ¥ | ||
| N | 204 | 97 (47.6%) | 107 (52.4%) | |
| Gender | ||||
| Female | 87 (42.4%) | 50 (57.5%) | 37 (42.5%) | 0.014 |
| Male | 117 (57.6%) | 47 (40.2%) | 70 (59.8%) | |
| Age * | 50.04 (42.61–58.20) | 48.20 (40.05–56.73) | 50.45 (43.95–59.52) | 0.12 |
| Outcome Variables: | ||||
| CAP (dB/m) ** | 324.5 (289.5–364.0) | 337 (293–364) | 313 (282–353) | 0.006 |
| CAP median categories | ||||
| <324 dB/m | 100 (49.0%) | 39 (39.0%) | 61 (61.0%) | 0.017 |
| ≥324 dB/m | 104 (51.0%) | 58 (55.8%) | 46 (44.2%) | |
| BMI (kg/m2) * | 33.87 (4.92) | 35.74 (5.37) | 32.18 (3.77) | <0.001 |
| BMI median categories | ||||
| <33 | 102 (50.0%) | 35 (34.3%) | 67 (65.7%) | <0.001 |
| ≥33 | 102 (50.0%) | 62 (60.8%) | 40 (39.2%) | |
| HOMA-IR * | 3.41 (2.03) | 4.02 (2.29) | 2.86 (1.57) | <0.001 |
| HOMA-IR categories | ||||
| <2.5 | 75 (36.8%) | 24 (32.0%) | 51 (68.0%) | <0.001 |
| ≥2.5 | 129 (63.2%) | 73 (56.6%) | 56 (43.4%) | |
| Anthropometric parameters: | ||||
| Weight (kg) * | 94.59 (16.09) | 98.32 (18.03) | 91.21 (13.30) | 0.001 |
| Waist (cm) * | 100.52 (19.31) | 103.11 (20.19) | 98.17 (18.25) | 0.067 |
| Hips (cm) * | 111.91 (10.35) | 115.54 (10.66) | 108.62 (8.91) | <0.001 |
| Fat Mass (Kg) * | 53.93 (14.70) | 51.42 (14.17) | 56.09 (14.88) | 0.032 |
| Fat-Free Mass (kg) * | 28.41 (9.95) | 29.96 (11.81) | 27.09 (7.85) | 0.051 |
| E (Kpa) ** | 5.70 (4.40–7.00) | 6.00 (4.40–7.70) | 5.50 (4.40–6.70) | 0.26 |
| Blood Tests: | ||||
| Glucose (mg/dL) * | 99.37 (16.84) | 98.37 (13.63) | 100.27 (19.32) | 0.42 |
| Insulin (µU/mL) * | 13.72 (7.15) | 16.26 (7.94) | 11.40 (5.41) | <0.001 |
| HbA1 (%) * | 5.74 (0.57) | 5.71 (0.50) | 5.76 (0.64) | 0.50 |
| TC (mg/dL) * | 199.42 (39.27) | 195.04 (39.03) | 203.39 (39.24) | 0.13 |
| HDL (mg/dL) * | 46.14 (11.87) | 45.79 (12.21) | 46.46 (11.60) | 0.69 |
| LDL (mg/dL) * | 119.83 (35.11) | 115.62 (35.60) | 123.65 (34.38) | 0.10 |
| AST (U/L) * | 24.55 (8.43) | 24.62 (10.06) | 24.49 (6.67) | 0.91 |
| ALT (U/L) * | 31.82 (16.82) | 31.87 (18.66) | 31.79 (15.05) | 0.97 |
| GGT (U/L)* | 27.73 (18.97) | 27.34 (15.57) | 28.07 (21.66) | 0.78 |
| TG (mg/dL) * | 133.78 (78.83) | 134.52 (74.76) | 133.12 (82.70) | 0.90 |
| Cortisol (µg/dL) * | 12.47 (6.63) | 13.05 (7.18) | 11.92 (5.93) | 0.22 |
| Ferritin (ng/mL) * | 148.59 (161.06) | 140.01 (144.02) | 156.36 (175.38) | 0.47 |
| WBC (103/μL) * | 6.48 (1.61) | 6.60 (1.50) | 6.37 (1.70) | 0.32 |
| Haemoglobin (g/L) * | 14.73 (2.55) | 14.85 (3.47) | 14.62 (1.26) | 0.53 |
| RBC (106/μL) * | 5.02 (0.47) | 5.04 (0.48) | 5.01 (0.47) | 0.61 |
| Haematocrit (%) | 43.25 (3.18) | 43.39 (3.46) | 43.13 (2.91) | 0.57 |
| Demographic and lifestyle characteristics: | ||||
| Smoker | ||||
| Never | 134 (65.7%) | 69 (71.1%) | 65 (60.7%) | 0.12 |
| Current | 70 (34.3%) | 28 (28.9%) | 42 (39.3%) | |
| Marital Status | ||||
| Single | 24 (11.9%) | 16 (16.8%) | 8 (7.5%) | 0.18 |
| Married or Cohabiting | 163 (81.1%) | 74 (77.9%) | 89 (84.0%) | |
| Separated or Divorced | 10 (5.0%) | 4 (4.2%) | 6 (5.7%) | |
| Widower | 4 (2.0%) | 1 (1.1%) | 3 (2.8%) | |
| Education | ||||
| Primary School | 6 (2.9%) | 2 (2.1%) | 4 (3.7%) | 0.91 |
| Secondary School | 46 (22.5%) | 22 (22.7%) | 24 (22.4%) | |
| High School | 107 (52.5%) | 52 (53.6%) | 55 (51.4%) | |
| Graduation | 45 (22.1%) | 21 (21.6%) | 24 (22.4%) | |
| CAP | BMI | HOMA-IR-IR | ||||
|---|---|---|---|---|---|---|
| Model a | β | 95%CI | β | 95%CI | β | 95%CI |
| GPCS | −5.55 * | −9.04; −2.05 | −0.98 ** | −1.37; −0.60 | −0.28 ** | −0.44; −0.14 |
| Model b | β | 95%CI | β | 95%CI | β | 95%CI |
| GPCS | −5.30 * | −8.72; −1.89 | −0.96 ** | −1.35; −0.57 | −0.28 ** | −0.42; −0.13 |
| GPCS Score | CAP Mean Predicted | Std. Error | 95% CI |
|---|---|---|---|
| 3 | 339.17 | 5.79 | 327.75, 350.59 |
| 4 | 333.86 | 4.36 | 325.25, 342.47 |
| 5 | 328.56 | 3.25 | 322.14, 334.97 |
| 6 | 323.25 | 2.85 | 317.63, 328.88 |
| 7 | 317.95 | 3.42 | 311.21, 324.69 |
| 8 | 312.65 | 4.61 | 303.56, 321.73 |
| 9 | 307.34 | 6.06 | 295.38, 319.30 |
| 10 | 302.04 | 7.64 | 286.97, 317.10 |
| 11 | 296.73 | 9.27 | 278.45, 315.01 |
| 12 | 291.43 | 10.93 | 269.87, 312.99 |
| GPCS Score | BMI Predicted Mean | Std. Error | 95% CI |
|---|---|---|---|
| 3 | 36.61 | 0.66 | 35.32, 37.91 |
| 4 | 35.65 | 0.49 | 34.68, 36.63 |
| 5 | 34.69 | 0.37 | 33.97, 35.42 |
| 6 | 33.73 | 0.32 | 33.10, 34.37 |
| 7 | 32.77 | 0.39 | 32.01, 33.54 |
| 8 | 31.81 | 0.52 | 30.79, 32.84 |
| 9 | 30.85 | 0.69 | 29.50, 32.21 |
| 10 | 29.89 | 0.86 | 28.19, 31.60 |
| 11 | 28.93 | 1.05 | 26.87, 31.00 |
| 12 | 27.97 | 1.24 | 25.53, 30.41 |
| GPCS Score | HOMA-IR Predicted Mean | Std. Error | 95% CI |
|---|---|---|---|
| 3 | 4.20 | 0.24 | 3.71, 4.68 |
| 4 | 3.92 | 0.18 | 3.55, 4.28 |
| 5 | 3.64 | 0.14 | 3.37, 3.91 |
| 6 | 3.35 | 0.12 | 3.12, 3.59 |
| 7 | 3.07 | 0.14 | 2.79, 3.36 |
| 8 | 2.79 | 0.20 | 2.41, 3.18 |
| 9 | 2.51 | 0.26 | 2.00, 3.02 |
| 10 | 2.23 | 0.32 | 1.59, 2.87 |
| 11 | 1.95 | 0.39 | 1.17, 2.73 |
| 12 | 1.67 | 0.46 | 0.75, 2.59 |
| CAP Median ≥324 vs. <324 | BMI Median ≥33 vs. <33 | HOMA-IR ≥2.5 vs. <2.5 | ||||
|---|---|---|---|---|---|---|
| Model a | OR | 95%CI | OR | 95%CI | OR | 95%CI |
| GPCS | ||||||
| <6 | 1.00 | 1.00 | 1.00 | |||
| ≥6 | 0.44 * | 0.24; 0.78 | 0.36 ** | 0.20; 0.64 | 0.32 ** | 0.17; 0.60 |
| Model b | OR | 95%CI | OR | 95%CI | OR | 95%CI |
| GPCS | ||||||
| <6 | 1.00 | 1.00 | 1.00 | |||
| ≥6 | 0.44 * | 0.24; 0.81 | 0.39 * | 0.21; 0.70 | 0.32 ** | 0.16; 0.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verrelli, N.; Bonfiglio, C.; Franco, I.; Bagnato, C.B.; Stabile, D.; Shahini, E.; Bianco, A. The Role of Global Physical Capacity Score in Key Parameters of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). J. Clin. Med. 2025, 14, 3821. https://doi.org/10.3390/jcm14113821
Verrelli N, Bonfiglio C, Franco I, Bagnato CB, Stabile D, Shahini E, Bianco A. The Role of Global Physical Capacity Score in Key Parameters of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). Journal of Clinical Medicine. 2025; 14(11):3821. https://doi.org/10.3390/jcm14113821
Chicago/Turabian StyleVerrelli, Nicola, Caterina Bonfiglio, Isabella Franco, Claudia Beatrice Bagnato, Dolores Stabile, Endrit Shahini, and Antonella Bianco. 2025. "The Role of Global Physical Capacity Score in Key Parameters of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD)" Journal of Clinical Medicine 14, no. 11: 3821. https://doi.org/10.3390/jcm14113821
APA StyleVerrelli, N., Bonfiglio, C., Franco, I., Bagnato, C. B., Stabile, D., Shahini, E., & Bianco, A. (2025). The Role of Global Physical Capacity Score in Key Parameters of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). Journal of Clinical Medicine, 14(11), 3821. https://doi.org/10.3390/jcm14113821







