Comorbidity Patterns Among Patients Diagnosed with Sialolithiasis: A Retrospective Analysis
Abstract
1. Introduction
2. Methodology
2.1. Methods
2.2. Study Setting
2.3. Study Period
2.4. Participants
2.5. Data Collection
2.6. Patient Characteristics
2.7. Statistical Analysis
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kao, W.K.; Chole, R.A.; Ogden, M.A. Evidence of a microbial etiology for sialoliths. Laryngoscope 2020, 130, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Kraaij, S.; Brand, H.S.; van der Meij, E.H.; de Visscher, J.G. Biochemical composition of salivary stones in relation to stone- and patient-related factors. Med. Oral Patol. Oral Cir. Bucal 2018, 23, e540–e544. [Google Scholar] [CrossRef] [PubMed]
- Lagha, N.B.; Alantar, A.; Samson, J.; Chapireau, D.; Maman, L. Lithiasis of minor salivary glands: Current data. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2005, 100, 345–348. [Google Scholar] [CrossRef]
- Huang, C.H.; Hwang, M.J.; Lang, M.J.; Chiang, C.P. Minor salivary gland sialolithiasis of the upper lip. J. Dent. Sci. 2022, 17, 1050–1052. [Google Scholar] [CrossRef]
- Pachisia, S.; Mandal, G.; Sahu, S.; Ghosh, S. Submandibular sialolithiasis: A series of three case reports with review of literature. Clin. Pract. 2019, 9, 1119. [Google Scholar] [CrossRef]
- Kraaij, S.; Karagozoglu, K.; Forouzanfar, T.; Veerman, E.; Brand, H. Salivary stones: Symptoms, aetiology, biochemical composition and treatment. Br. Dent. J. 2014, 217, E23. [Google Scholar] [CrossRef]
- Schrøder, S.A.; Homøe, P.; Wagner, N.; Bardow, A. Does saliva composition affect the formation of sialolithiasis? J. Laryngol. Otol. 2017, 131, 162–167. [Google Scholar] [CrossRef]
- Harrison, J.D.; Triantafyllou, A.; Baldwin, D.; Schäfer, H. Histochemical and biochemical determination of calcium in salivary glands with particular reference to chronic submandibular sialadenitis. Virchows Arch. A Pathol. Anat. Histopathol. 1993, 423, 29–32. [Google Scholar] [CrossRef]
- Buhler, A.V.; Huynh, P.; Low, P.; Von, M. Possible Drug-Associated Sialolithiasis From the Bicarbonate Anhydrase Inhibitor Topiramate: A Case Report and Literature Review. J. Oral Maxillofac. Surg. 2016, 74, 2447–2452. [Google Scholar] [CrossRef]
- Hiraide, F.; Nomura, Y. The fine surface structure and composition of salivary calculi. Laryngoscope 1980, 90, 152–158. [Google Scholar] [CrossRef]
- Taher, A.A. The incidence and composition of salivary stones (sialolithiasis) in Iran: Analysis of 95 cases—A short report. Singap. Dent. J. 1989, 14, 33–35. [Google Scholar]
- Sigismund, P.E.; Zenk, J.; Koch, M.; Schapher, M.; Rudes, M.; Iro, H. Nearly 3,000 salivary stones: Some clinical and epidemiologic aspects. Laryngoscope 2015, 125, 1879–1882. [Google Scholar] [CrossRef] [PubMed]
- Schrøder, S.A.; Andersson, M.; Wohlfahrt, J.; Wagner, N.; Bardow, A.; Homøe, P. Incidence of sialolithiasis in Denmark: A nationwide population-based register study. Eur. Arch. Otorhinolaryngol. 2017, 274, 1975–1981. [Google Scholar] [CrossRef]
- Lim, H.-K.; Kim, S.-M.; Kim, M.-J.; Lee, J.-H. Clinical, statistical and chemical study of sialolithiasis. J. Korean Assoc. Oral Maxillofac. Surg. 2012, 38, 44–49. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, H.J.; Lim, H.; Lim, M.S.; Kim, M.; Park, I.S.; Choi, H.G. Association between cholelithiasis and sialolithiasis: Two longitudinal follow-up studies. Medicine 2019, 98, e16153. [Google Scholar] [CrossRef]
- Jonas, E.; Gillman, L.; Masri, D.; Rosenfeld, E.; Chaushu, G.; Avishai, G. Systemic risk factors contributing to sialolithiasis: A big-data retrospective analysis. Clin. Oral Investig. 2025, 29, 119. [Google Scholar] [CrossRef]
- Schapher, M.; Koch, M.; Weidner, D.; Scholz, M.; Wirtz, S.; Mahajan, A.; Herrmann, I.; Singh, J.; Knopf, J.; Leppkes, M. Neutrophil extracellular traps promote the development and growth of human salivary stones. Cells 2020, 9, 2139. [Google Scholar] [CrossRef]
- Marchal, F.; Kurt, A.M.; Dulguerov, P.; Lehmann, W. Retrograde theory in sialolithiasis formation. Arch. Otolaryngol. Head Neck Surg. 2001, 127, 66–68. [Google Scholar] [CrossRef]
- Takeshita, H.; Ishihara, A.; Yamashita, T.; Itoh, A.; Yoshida, K.; Fukaya, M. A case of a salivary calculus containing a limb of a shrimp—The structural analysis. Aichi Gakuin Dent. Sci. 1990, 3, 49–58. [Google Scholar]
- Harrison, J.D. Causes, natural history, and incidence of salivary stones and obstructions. Otolaryngol. Clin. N. Am. 2009, 42, 927–947. [Google Scholar] [CrossRef]
- Huoh, K.C.; Eisele, D.W. Etiologic factors in sialolithiasis. Otolaryngol. Head Neck Surg. 2011, 145, 935–939. [Google Scholar] [CrossRef] [PubMed]
- Kraaij, S.; Karagozoglu, K.H.; Kenter, Y.A.; Pijpe, J.; Gilijamse, M.; Brand, H.S. Systemic diseases and the risk of developing salivary stones: A case control study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2015, 119, 539–543. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yiu, A.J.; Kalejaiye, A.; Amdur, R.L.; Todd Hesham, H.N.; Bandyopadhyay, B.C. Association of serum electrolytes and smoking with salivary gland stone formation. Int. J. Oral Maxillofac. Surg. 2016, 45, 764–768. [Google Scholar] [CrossRef]
- Morita, I.; Okamoto, Y.; Yoshii, S.; Nakagaki, H.; Mizuno, K.; Sheiham, A.; Sabbah, W. Five-Year Incidence of Periodontal Disease Is Related to Body Mass Index. J. Dent. Res. 2011, 90, 199–202. [Google Scholar] [CrossRef]
- Wellen, K.E.; Hotamisligil, G.S. Inflammation, stress, and diabetes. J. Clin. Investig. 2005, 115, 1111–1119. [Google Scholar] [CrossRef]
- Jonas, E.; Muchnik, D.; Rabinovich, I.; Masri, D.; Chaushu, G.; Avishai, G. Characteristics of sialolithiasis in Israel, a big-data retrospective study of 5100 cases. Oral Dis. 2025, 31, 503–509. [Google Scholar] [CrossRef]
- Laforgia, P.D.; Favia, G.F.; Chiaravalle, N.; Lacaita, M.G.; Laforgia, A. Clinico-statistical, morphologic and microstructural analysis of 400 cases of sialolithiasis. Minerva Stomatol. 1989, 38, 1329–1336. [Google Scholar]
- Zenk, J.; Constantinidis, J.; Kydles, S.; Hornung, J.; Iro, H. Clinical and diagnostic findings of sialolithiasis. HNO 1999, 47, 963–969. [Google Scholar] [CrossRef]
- Wahabi, H.; Fayed, A.A.; Shata, Z.; Esmaeil, S.; Alzeidan, R.; Saeed, E.; Amer, Y.; Titi, M.; Bahkali, K.; Hneiny, L. The Impact of Age, Gender, Temporality, and Geographical Region on the Prevalence of Obesity and Overweight in Saudi Arabia: Scope of Evidence. Healthcare 2023, 11, 1143. [Google Scholar] [CrossRef]
- Alenazi, A.M.; Alqahtani, B.A. National and regional prevalence rates of hypertension in Saudi Arabia: A descriptive analysis using the national survey data. Front. Public Health 2023, 11, 1092905. [Google Scholar] [CrossRef]
- Alqurashi, K.A.; Aljabri, K.S.; Bokhari, S.A. Prevalence of diabetes mellitus in a Saudi community. Ann. Saudi Med. 2011, 31, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Al-Daghri, N.M.; Al-Attas, O.S.; Alokail, M.S.; Alkharfy, K.M.; Yousef, M.; Sabico, S.L.; Chrousos, G.P. Diabetes mellitus type 2 and other chronic non-communicable diseases in the central region, Saudi Arabia (Riyadh cohort 2): A decade of an epidemic. BMC Med. 2011, 9, 76. [Google Scholar] [CrossRef] [PubMed]
- Al Mansour, M.A. The Prevalence and Risk Factors of Type 2 Diabetes Mellitus (DMT2) in a Semi-Urban Saudi Population. Int. J. Environ. Res. Public Health 2020, 17, 7. [Google Scholar] [CrossRef]
- Aljulifi, M.Z. Prevalence and reasons of increased type 2 diabetes in Gulf Cooperation Council Countries. Saudi Med. J. 2021, 42, 481–490. [Google Scholar] [CrossRef]
- Amir, A.; Alasnag, M.; Al-Raddadi, R.; Al-Bassam, T.; Saeed, K.; Yazıcıoğlu, M.; Shabana, A. Patient journey for hypertension and dyslipidemia in Saudi Arabia: Highlighting the evidence gaps. Arch. Public Health 2023, 81, 122. [Google Scholar] [CrossRef]
- Williams, M.F. Sialolithiasis. Otolaryngol. Clin. N. Am. 1999, 32, 819–834. [Google Scholar] [CrossRef]
- Zhang, Z. Multiple imputation with multivariate imputation by chained equation (MICE) package. Ann. Transl. Med. 2016, 4, 30. [Google Scholar] [CrossRef]
- Hammett, J.T.; Walker, C. Sialolithiasis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Algabbani, A.M.; Almubark, R.; Althumiri, N.; Alqahtani, A.; BinDhim, N. The prevalence of cigarette smoking in Saudi Arabia in 2018. Food Drug Regul. Sci. J. 2018, 1, 1. [Google Scholar] [CrossRef]
- Qattan, A.M.N.; Boachie, M.K.; Immurana, M.; Al-Hanawi, M.K. Socioeconomic Determinants of Smoking in the Kingdom of Saudi Arabia. Int. J. Environ. Res. Public Health 2021, 18, 5665. [Google Scholar] [CrossRef]
- Witt, R.L. Surgery of the Salivary Glands E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Mumtaz, M.; Abdullah Alsuwaiket, A.; Raza, S.; Kazmi, F.; Shaikh, Q.; Tabassum, A. Prevalence of Xerostomia and Associated Systemic Risk Factors in Riyadh, Saudi Arabia: A Cross-sectional Study. J. Pharm. Res. Int. 2021, 33, 186–194. [Google Scholar] [CrossRef]
- Al-Maweri, S.A.; Altayyar, M.O.; AlQahtani, K.W.; Bamasud, M.S.; AlGhamdi, O.Y.; Ashraf, S.; Eshky, R.; Ba-Hattab, R.; Kassim, S. Xerostomia, Salivary Flow, and Oral Health Status Among Saudi Diabetic Patients: A Comparative Cross-Sectional Study. Clin. Cosmet. Investig. Dent. 2021, 13, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Alblowi, S.; Safdar, O.; Aboulola, N.; Alharazy, D.; Najem, N. Renal stone prevalence and risk factors in Jeddah and Riyadh. J. Fam. Med. Prim. Care 2022, 11, 2839–2845. [Google Scholar] [CrossRef]
- Albasheer, O.B.; Hakami, A.; Al Faqih, A.A.; Akkam, I.; Soraihy, S.K.; Mathary, A.; Alharbi, A.A.; Yaqoub, M.; Alotayfi, M.A. Awareness of dehydration state and fluid intake practice among adults population in the Jazan Region of Saudi Arabia, 2019. J. Nutr. Sci. 2021, 10, e84. [Google Scholar] [CrossRef]
- Hung, S.H.; Lin, H.C.; Su, C.H.; Chung, S.D. Association of sialolithiasis with cholelithiasis: A population-based study. Head Neck 2016, 38, 560–563. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.J.; Han, Y.E.; Choi, H.G. The association between sialolithiasis and smoking, alcohol drinking and obesity in Korea: A nested case-control study. BMC Public Health 2020, 20, 516. [Google Scholar] [CrossRef]
- Moghe, S.; Pillai, A.; Thomas, S.; Nair, P.P. Parotid sialolithiasis. BMJ Case Rep. 2012, 2012, bcr2012007480. [Google Scholar] [CrossRef]
- Stelmach, R.; Pawłowski, M.; Klimek, L.; Janas, A. Biochemical structure, symptoms, location and treatment of sialoliths. J. Dent. Sci. 2016, 11, 299–303. [Google Scholar] [CrossRef]
- Diep, M.T.; Jensen, J.L.; Skudutyte-Rysstad, R.; Young, A.; Sødal, A.T.T.; Petrovski, B.; Hove, L.H. Xerostomia and hyposalivation among a 65-yr-old population living in Oslo, Norway. Eur. J. Oral Sci. 2021, 129, e12757. [Google Scholar] [CrossRef]
- Österberg, T.; Landahl, S.; Hedegård, B. Salivary flow, saliva, pH and buffering capacity in 70-year-old men and women: Correlation to dental health, dryness in the mouth, disease and drug treatment. J. Oral Rehabil. 1984, 11, 157–170. [Google Scholar] [CrossRef]
- Iro, H.; Zenk, J.; Escudier, M.P.; Nahlieli, O.; Capaccio, P.; Katz, P.; Brown, J.; McGurk, M. Outcome of minimally invasive management of salivary calculi in 4,691 patients. Laryngoscope 2009, 119, 263–268. [Google Scholar] [CrossRef]
- Salam, A.A. Ageing in Saudi Arabia: New dimensions and intervention strategies. Sci. Rep. 2023, 13, 4035. [Google Scholar] [CrossRef] [PubMed]
- Seidell, J.C.; Flegal, K.M. Assessing obesity: Classification and epidemiology. Br. Med. Bull. 1997, 53, 238–252. [Google Scholar] [CrossRef] [PubMed]
- Issrani, R.; Reddy, J.; Bader, A.K.; Albalawi, R.F.H.; Alserhani, E.D.M.; Alruwaili, D.S.R.; Alanazi, G.R.A.; Alruwaili, N.S.R.; Sghaireen, M.G.; Rao, K. Exploring an Association between Body Mass Index and Oral Health-A Scoping Review. Diagnostics 2023, 13, 902. [Google Scholar] [CrossRef]
- Geronatsios, K. Sialolithiasis. In Otolaryngology Study Guide: A Case-Based Approach; Tsetsos, N., Stavrakas, M., Garefis, K., Eds.; Springer Nature Switzerland: Cham, Switzerland, 2025; pp. 267–270. [Google Scholar]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef]
- Dybiec, J.; Baran, W.; Dąbek, B.; Fularski, P.; Młynarska, E.; Radzioch, E.; Rysz, J.; Franczyk, B. Advances in treatment of dyslipidemia. Int. J. Mol. Sci. 2023, 24, 13288. [Google Scholar] [CrossRef]
- Al Rasadi, K.; Almahmeed, W.; AlHabib, K.F.; Abifadel, M.; Farhan, H.A.; AlSifri, S.; Jambart, S.; Zubaid, M.; Awan, Z.; Al-Waili, K.; et al. Dyslipidaemia in the Middle East: Current status and a call for action. Atherosclerosis 2016, 252, 182–187. [Google Scholar] [CrossRef]
- Al-Rubeaan, K.; Bawazeer, N.; Al Farsi, Y.; Youssef, A.M.; Al-Yahya, A.A.; AlQumaidi, H.; Al-Malki, B.M.; Naji, K.A.; Al-Shehri, K.; Al Rumaih, F.I. Prevalence of metabolic syndrome in Saudi Arabia-a cross sectional study. BMC Endocr. Disord. 2018, 18, 16. [Google Scholar] [CrossRef]
- Ahmed, A.E.; Alsamghan, A.; Momenah, M.A.; Alqhtani, H.A.; Aldawood, N.A.; Alshehri, M.A.; Ali Alshehri, A.M.; Alhag, S.K.; Mosaad, Y.O.; Ahmed, H. Metabolic Syndrome and Cardiometabolic Risk Factors in the Mixed Hypercholesterolemic Populations with Respect to Gender, Age, and Obesity in Asir, Saudi Arabia. Int. J. Environ. Res. Public Health 2022, 19, 14985. [Google Scholar] [CrossRef]
- Aldubikhi, A. Obesity management in the Saudi population. Saudi Med. J. 2023, 44, 725–731. [Google Scholar] [CrossRef]
- Mizoguchi, N.; Nohno, K.; Yoshihara, A.; Ito, K.; Funayama, S.; Ogawa, H. Association of Hyper-Low-Density Lipoprotein and Hypo-High-Density Lipoprotein Cholesterolemia with Low Saliva Flow Rates in Japanese Community-Dwelling Elders. Int. Arch. Otorhinolaryngol. 2023, 27, e24–e31. [Google Scholar] [CrossRef]
- Izumi, M.; Hida, A.; Takagi, Y.; Kawabe, Y.; Eguchi, K.; Nakamura, T. MR imaging of the salivary glands in sicca syndrome: Comparison of lipid profiles and imaging in patients with hyperlipidemia and patients with Sjögren’s syndrome. AJR Am. J. Roentgenol. 2000, 175, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Sánchez Barrueco, A.; López-Acevedo Cornejo, M.V.; Alcalá Rueda, I.; López Andrés, S.; González Galán, F.; Díaz Tapia, G.; Mahillo Fernández, I.; Cenjor Español, C.; Aubá, J.M.V. Sialolithiasis: Mineralogical composition, crystalline structure, calculus site, and epidemiological features. Br. J. Oral Maxillofac. Surg. 2022, 60, 1385–1390. [Google Scholar] [CrossRef] [PubMed]
- Teymoortash, A.; Buck, P.; Jepsen, H.; Werner, J.A. Sialolith crystals localized intraglandularly and in the Wharton’s duct of the human submandibular gland: An X-ray diffraction analysis. Arch. Oral Biol. 2003, 48, 233–236. [Google Scholar] [CrossRef]
- Musiał, N.; Bogucka, A.; Tretiakow, D.; Skorek, A.; Ryl, J.; Czaplewska, P. Proteomic analysis of sialoliths from calcified, lipid and mixed groups as a source of potential biomarkers of deposit formation in the salivary glands. Clin. Proteom. 2023, 20, 11. [Google Scholar] [CrossRef]
- Boskey, A.L.; Boyan-Salyers, B.D.; Burstein, L.S.; Mandel, I.D. Lipids associated with mineralization of human submandibular gland sialoliths. Arch. Oral Biol. 1981, 26, 779–785. [Google Scholar] [CrossRef]
- Einhorn, O.M.; Georgiou, K.; Tompa, A. Salivary dysfunction caused by medication usage. Physiol. Int. 2020, 107, 195–208. [Google Scholar] [CrossRef]
- Prasanthi, B.; Kannan, N.; Patil, R. Effect of Diuretics on Salivary Flow, Composition and Oral Health Status: A Clinico-biochemical Study. Ann. Med. Health Sci. Res. 2014, 4, 549–553. [Google Scholar] [CrossRef]
- Thomas, M.; Parolia, A.; Kundabala, M.; Vikram, M. Asthma and oral health: A review. Aust. Dent. J. 2010, 55, 128–133. [Google Scholar] [CrossRef]
- Slob, E.M.; Richards, L.B.; Vijverberg, S.J.; Longo, C.; Koppelman, G.H.; Pijnenburg, M.W.; Bel, E.H.; Neerincx, A.H.; Herrera Luis, E.; Perez-Garcia, J. Genome-wide association studies of exacerbations in children using long-acting beta2-agonists. Pediatr. Allergy Immunol. 2021, 32, 1197–1207. [Google Scholar] [CrossRef]
- Pacheco-Quito, E.-M.; Jaramillo, J.; Sarmiento-Ordoñez, J.; Cuenca-León, K. Drugs Prescribed for Asthma and Their Adverse Effects on Dental Health. Dent. J. 2023, 11, 113. [Google Scholar] [CrossRef]
- Schulz, R.E.; Bonzanini, L.I.L.; Ortigara, G.B.; Soldera, E.B.; Danesi, C.C.; Antoniazzi, R.P.; Ferrazzo, K.L. Prevalence of hyposalivation and associated factors in survivors of head and neck cancer treated with radiotherapy. J. Appl. Oral Sci. 2021, 29, e20200854. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.B.; Pedersen, A.M.; Vissink, A.; Andersen, E.; Brown, C.G.; Davies, A.N.; Dutilh, J.; Fulton, J.S.; Jankovic, L.; Lopes, N.N.; et al. A systematic review of salivary gland hypofunction and xerostomia induced by cancer therapies: Prevalence, severity and impact on quality of life. Support. Care Cancer 2010, 18, 1039–1060. [Google Scholar] [CrossRef] [PubMed]
- Khovidhunkit, S.O.; Suwantuntula, T.; Thaweboon, S.; Mitrirattanakul, S.; Chomkhakhai, U.; Khovidhunkit, W. Xerostomia, hyposalivation, and oral microbiota in type 2 diabetic patients: A preliminary study. J. Med. Assoc. Thai. 2009, 92, 1220–1228. [Google Scholar]
- Vesterinen, M.; Ruokonen, H.; Furuholm, J.; Honkanen, E.; Meurman, J.H. Clinical questionnaire study of oral health care and symptoms in diabetic vs. non-diabetic predialysis chronic kidney disease patients. Clin. Oral Investig. 2012, 16, 559–563. [Google Scholar] [CrossRef]
- López-Pintor, R.M.; Casañas, E.; González-Serrano, J.; Serrano, J.; Ramírez, L.; de Arriba, L.; Hernández, G. Xerostomia, Hyposalivation, and Salivary Flow in Diabetes Patients. J. Diabetes Res. 2016, 2016, 4372852. [Google Scholar] [CrossRef]
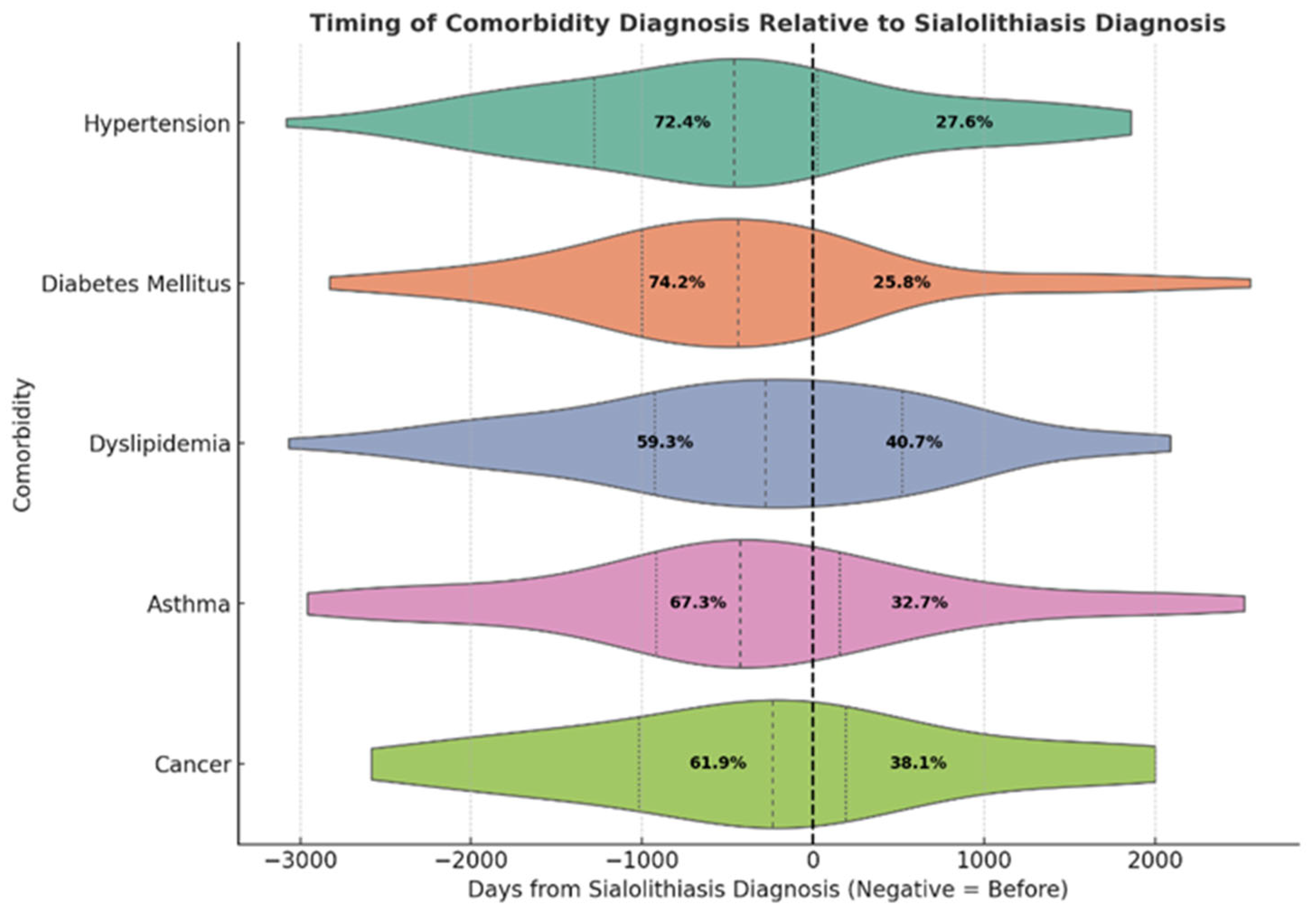
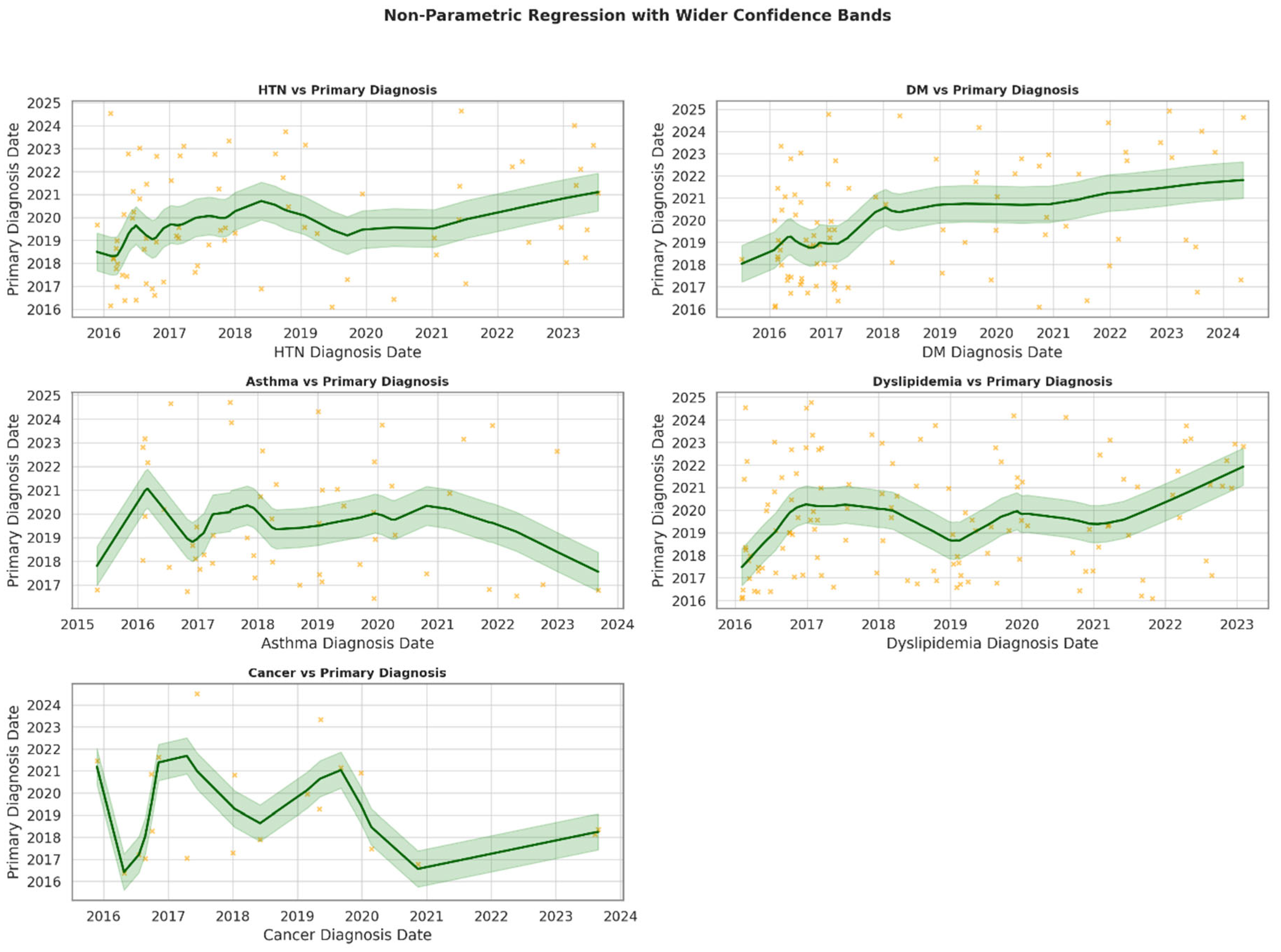
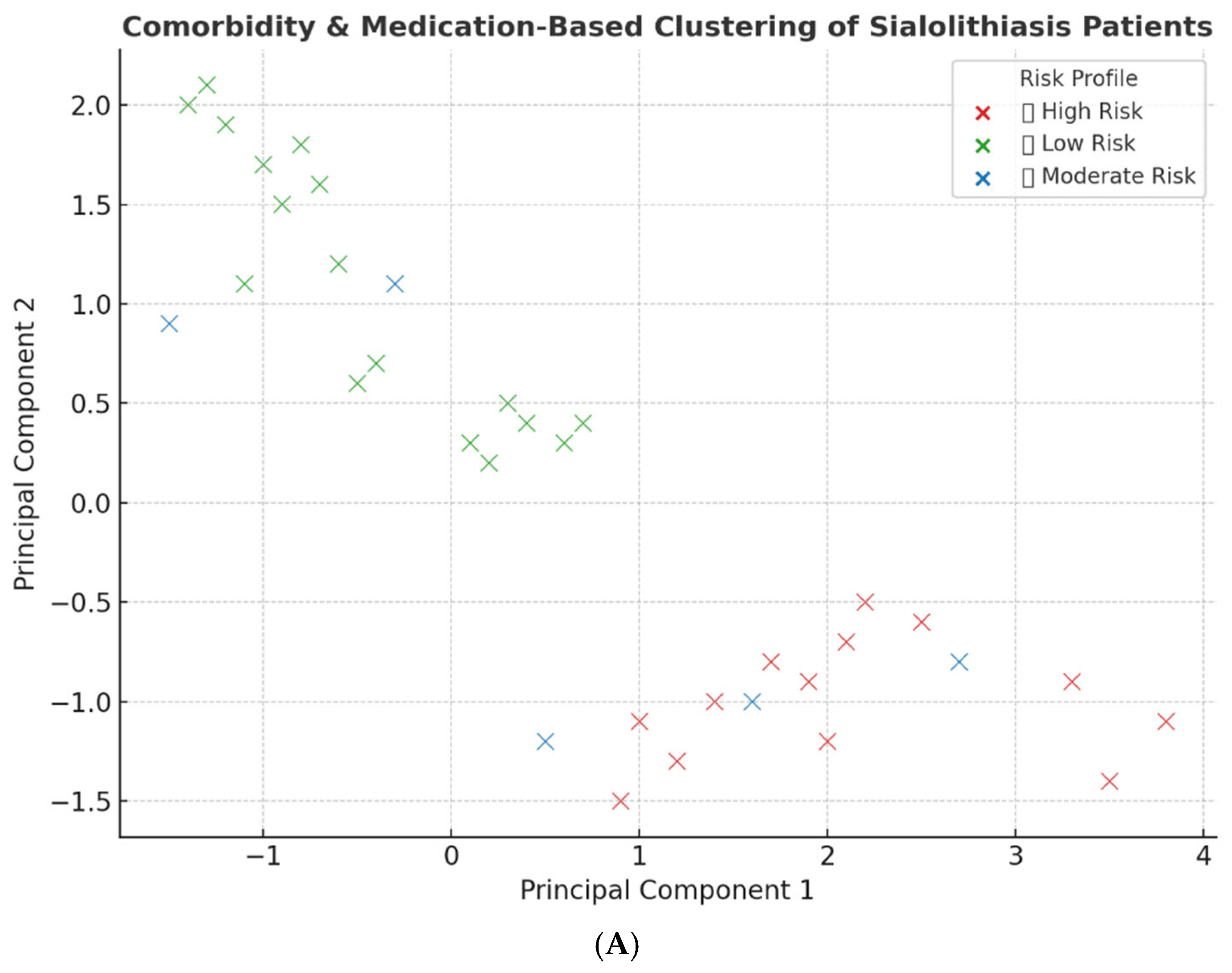
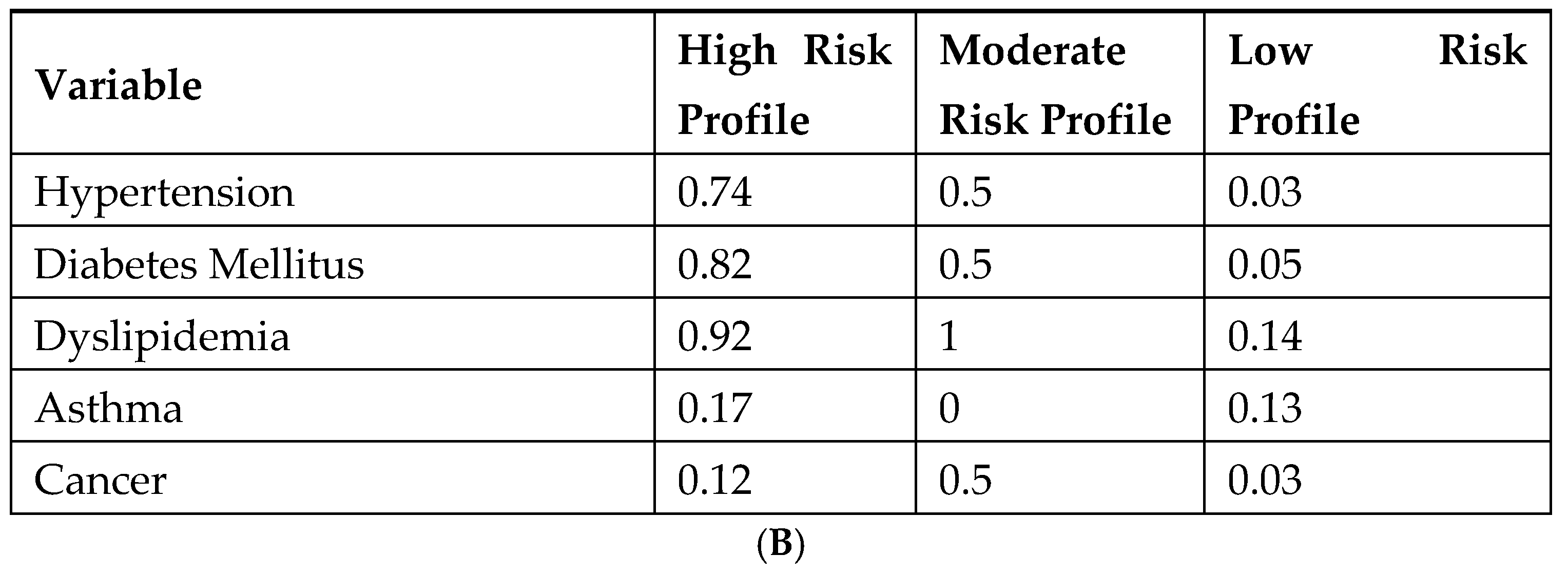
| Variable | Category | N (%) 1 |
|---|---|---|
| Gender | Male | 208 (55.5%) |
| Female | 167 (44.5%) | |
| Marital Status | Married | 226 (60.3%) |
| Single | 114 (30.4%) | |
| Widowed | 15 (4.0%) | |
| Unknown | 12 (2.9%) | |
| Divorced | 8 (2.1%) | |
| Year | ||
| 2016 | 43 (11.5%) | |
| 2017 | 49 (13.1%) | |
| 2018 | 48 (12.8%) | |
| 2019 | 56 (15%) | |
| 2020 | 44 (11.8%) | |
| 2021 | 40 (10.7%) | |
| 2022 | 42 (11.2%) | |
| 2023 | 27 (7.2%) | |
| 2024 | 25 (6.7%) |
| Variable | N 1 | Mean  ± SD | Median [IQR] |
|---|---|---|---|
| Current Age | 375 | 44.7 ± 17.6 | 43.0 [31.0–58.0] |
| Age at Diagnosis | 373 | 39.8 ± 17.4 | 38.0 [27.0–53.0] |
| BMI | 216 | 28.0 ± 10.8 | 27.3 [22.6–31.4] |
| Comorbidity count | 187 | 0.96 ± 1.19 | 5.0 [0.0–5.0] |
| Variable | Category | N (%) 1 |
|---|---|---|
| Hypertension | No | 299 (79.7%) |
| Yes | 76 (20.3%) | |
| Diabetes Mellitus | No | 287 (76.5%) |
| Yes | 88 (23.5%) | |
| Dyslipidemia | No | 252 (67.2%) |
| Yes | 123 (32.8%) | |
| Asthma | No | 323 (86.1%) |
| Yes | 52 (13.9%) | |
| Cancer | No | 342 (91.8%) |
| Yes | 33 (8.8%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saleem, R.A.; Sukkarieh, H.H.; Alkattan, R.K.; Fakhoury, H.M.A.; Aljada, A.; Theyab, A.; Ababneh, K.T. Comorbidity Patterns Among Patients Diagnosed with Sialolithiasis: A Retrospective Analysis. J. Clin. Med. 2025, 14, 3795. https://doi.org/10.3390/jcm14113795
Saleem RA, Sukkarieh HH, Alkattan RK, Fakhoury HMA, Aljada A, Theyab A, Ababneh KT. Comorbidity Patterns Among Patients Diagnosed with Sialolithiasis: A Retrospective Analysis. Journal of Clinical Medicine. 2025; 14(11):3795. https://doi.org/10.3390/jcm14113795
Chicago/Turabian StyleSaleem, Rimah Abdullah, Hatouf Husni Sukkarieh, Rana K. Alkattan, Hana M. A. Fakhoury, Ahmad Aljada, Abdulrahman Theyab, and Khansa Taha Ababneh. 2025. "Comorbidity Patterns Among Patients Diagnosed with Sialolithiasis: A Retrospective Analysis" Journal of Clinical Medicine 14, no. 11: 3795. https://doi.org/10.3390/jcm14113795
APA StyleSaleem, R. A., Sukkarieh, H. H., Alkattan, R. K., Fakhoury, H. M. A., Aljada, A., Theyab, A., & Ababneh, K. T. (2025). Comorbidity Patterns Among Patients Diagnosed with Sialolithiasis: A Retrospective Analysis. Journal of Clinical Medicine, 14(11), 3795. https://doi.org/10.3390/jcm14113795






