Comparison of Recurrent and Naïve Keratitis in a Cohort of 1303 Patients
Abstract
1. Introduction
2. Methods
2.1. Study Participants
2.2. Data Collection
- (a)
- Sociodemographic characteristics: age, gender, and ophthalmological history;
- (b)
- The number of previous keratitis episodes and time since the last occurrence;
- (c)
- Time from symptom onset to emergency visit;
- (d)
- Pre-visit treatment (ocular and systemic);
- (e)
- Risk factors for keratitis, including contact lens (CL) use, type of CL, history of eye trauma, post-corneal transplant condition, and CL misuse behaviors (e.g., sleeping, swimming, or bathing with CLs, using tap water for cleaning, or prolonged CL use).
2.3. Definitions
- Naïve keratitis: a single episode of keratitis.
- Recurrent keratitis: a subsequent episode of keratitis occurring at least 3 months after the previous episode or within 3 months of complete healing.
- Primary keratitis: a keratitis that occurs after direct contact with the infectious agent in an individual without latent infection.
- Microbial keratitis: any inflammatory condition of cornea caused by a microorganism or infective agent (bacteria, fungi, protozoa, viruses, or prions), confirmed by positive scrape results or characteristic lesions observed via slit lamp examination with fluorescein staining.
- Therapeutic keratoplasty: a transplant performed for debulking and preserving globe integrity.
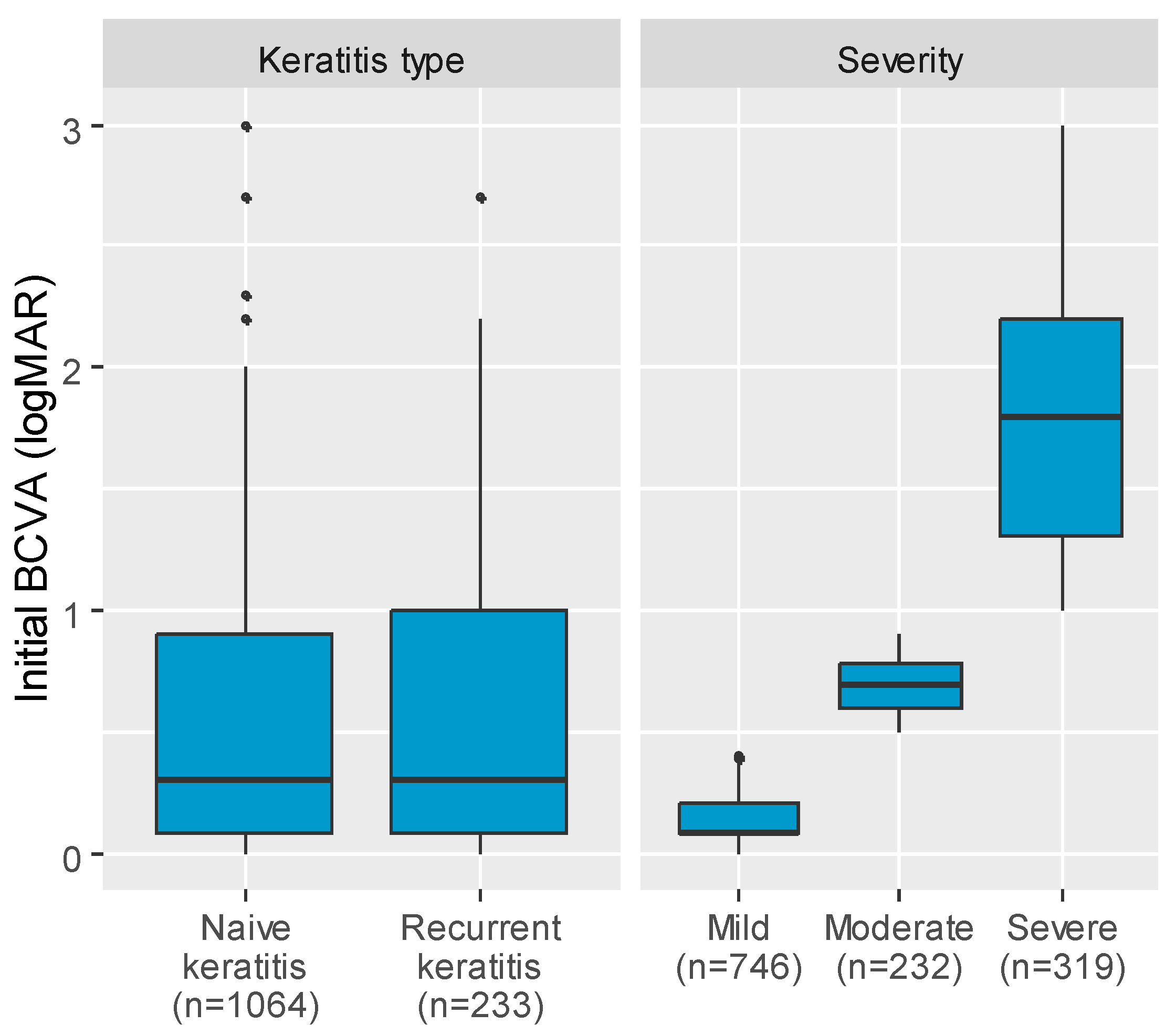
- Mild keratitis: corneal inflammation with reduced best-corrected visual acuity (BCVA) < 0.5 LogMAR.
- Moderate keratitis: corneal inflammation with BCVA ≥ 0.5 and <0.99 LogMAR.
- Severe keratitis: corneal inflammation with central or large peripheral ulcers with BCVA ≥ 1.0 LogMAR.
- CL misuse: defined as sleeping, swimming, or bathing with CLs, cleaning them with tap water, or prolonged use.
- Mixed initial diagnosis: suspected etiology involving at least two pathogens (virus, bacteria, fungus, or amoeba) based solely on clinical examination.
- Systemic treatment: oral or intravenous administration of medication.
2.4. Statistical Methods
3. Results
3.1. Demographics
3.2. Group Characteristics
3.3. Initial Diagnosis
3.4. Treatment Approaches
3.5. Visual Outcomes
3.6. Prognostic Factors
4. Discussion
5. Study Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moon, J.; Yoon, C.H.; Kim, M.K.; Oh, J.Y. The Incidence and Outcomes of Recurrence of Infection after Therapeutic Penetrating Keratoplasty for Medically-Uncontrolled Infectious Keratitis. J. Clin. Med. 2020, 9, 3696. [Google Scholar] [CrossRef] [PubMed]
- Vajpayee, R.B.; Boral, S.K.; Dada, T.; Murthy, G.V.S.; Pandey, R.M.; Satpathy, G. Risk factors for graft infection in India: A case-control study. Br. J. Ophthalmol. 2002, 86, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Arunga, S.; Wiafe, G.; Habtamu, E.; Onyango, J.; Gichuhi, S.; Leck, A.; Burton, M. The impact of microbial keratitis on quality of life in Uganda. BMJ Open Ophthalmol. 2019, 4, e000351. [Google Scholar] [CrossRef]
- Reynaud, C.; Rousseau, A.; Kaswin, G.; M’Garrech, M.; Barreau, E.; Labetoulle, M. Persistent Impairment of Quality of Life in Patients with Herpes Simplex Keratitis. Ophthalmology 2016, 124, 160–169. [Google Scholar] [CrossRef]
- Amponin, D.E.; Przybek-Skrzypecka, J.; Zyablitskaya, M.; Takaoka, A.; Suh, L.H.; Nagasaki, T.; Trokel, S.L.; Paik, D.C. Ex vivo anti-microbial efficacy of various formaldehyde releasers against antibiotic resistant and antibiotic sensitive microorganisms involved in infectious keratitis. BMC Ophthalmol. 2020, 20, 1–10. [Google Scholar] [CrossRef]
- Jian, H.-J.; Wu, R.-S.; Lin, T.-Y.; Li, Y.-J.; Lin, H.-J.; Harroun, S.G.; Lai, J.-Y.; Huang, C.-C. Super-Cationic Carbon Quantum Dots Synthesized from Spermidine as an Eye Drop Formulation for Topical Treatment of Bacterial Keratitis. ACS Nano 2017, 11, 6703–6716. [Google Scholar] [CrossRef]
- Han, W.; Li, H.; Chen, B. Research Progress and Potential Applications of Spermidine in Ocular Diseases. Pharmaceutics 2022, 14, 1500. [Google Scholar] [CrossRef]
- Singh, P.; Gupta, A.; Tripathy, K. Keratitis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Erie, J.C.; Nevitt, M.P.; Hodge, D.O.; Ballard, D.J. Incidence of Ulcerative Keratitis in a Defined Population From 1950 Through 1988. Arch. Ophthalmol. 1993, 111, 1665–1671. [Google Scholar] [CrossRef]
- Jeng, B.H.; Gritz, D.C.; Kumar, A.B.; Holsclaw, D.S.; Porco, T.C.; Smith, S.D.; Whitcher, J.P.; Margolis, T.P.; Wong, I.G. Epidemiology of Ulcerative Keratitis in Northern California. Arch. Ophthalmol. 2010, 128, 1022–1028. [Google Scholar] [CrossRef]
- Seal, D.; Kirkness, C.; Bennett, H.; Peterson, M. Population-based cohort study of microbial keratitis in Scotland: Incidence and features. Contact Lens Anterior Eye 1999, 22, 49–57. [Google Scholar] [CrossRef]
- Ibrahim, Y.W.; Boase, D.L.; Cree, I.A. Epidemiological characteristics, predisposing factors and microbiological profiles of infectious corneal ulcers: The Portsmouth corneal ulcer study. Br. J. Ophthalmol. 2009, 93, 1319–1324. [Google Scholar] [CrossRef] [PubMed]
- Ting, D.S.J.; Ho, C.S.; Cairns, J.; Elsahn, A.; Al-Aqaba, M.; Boswell, T.; Said, D.G.; Dua, H.S. 12-year analysis of incidence, microbiological profiles and in vitro antimicrobial susceptibility of infectious keratitis: The Nottingham Infectious Keratitis Study. Br. J. Ophthalmol. 2020, 105, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Green, M.; Carnt, N.; Apel, A.; Stapleton, F. Queensland Microbial Keratitis Database: 2005–2015. Br. J. Ophthalmol. 2019, 103, 1481–1486. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, C.A.; Srinivasan, M.; Whitcher, J.P.; Smolin, G. Incidence of corneal ulceration in Madurai District, South India. Ophthalmic Epidemiol. 1996, 3, 159–166. [Google Scholar] [CrossRef]
- Upadhyay, M.P.; Karmacharya, P.C.; Koirala, S.; Shah, D.N.; Shakya, S.; Shrestha, J.K.; Bajracharya, H.; Gurung, C.K.; Whitcher, J.P. The Bhaktapur eye study: Ocular trauma and antibiotic prophylaxis for the prevention of corneal ulceration in Nepal. Br. J. Ophthalmol. 2001, 85, 388–392. [Google Scholar] [CrossRef]
- Roozbahani, M.; Hammersmith, K.M. Management of herpes simplex virus epithelial keratitis. Curr. Opin. Ophthalmol. 2018, 29, 360–364. [Google Scholar] [CrossRef]
- Lakhundi, S.; Siddiqui, R.; Khan, N.A. Pathogenesis of microbial keratitis. Microb. Pathog. 2017, 104, 97–109. [Google Scholar] [CrossRef]
- Gurnani, B.; Kaur, K. Bacterial Keratitis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Hassell, J.R.; Birk, D.E. The molecular basis of corneal transparency. Exp. Eye Res. 2010, 91, 326–335. [Google Scholar] [CrossRef]
- Collaborators GBaVI, Study VLEGotGBoD. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: The Right to Sight: An analysis for the Global Burden of Disease Study. Lancet Glob. Health 2021, 9, e144–e160. [Google Scholar] [CrossRef]
- Ichikawa, K.; Ono, T.; Chen, L.; Kitamoto, K.; Taketatni, Y.; Toyono, T.; Yoshida, J.; Aihara, M.; Miyai, T. Quantitative evaluation of corneal irregularity and scarring after infectious keratitis using anterior segment optical coherence tomography. Graefe’s Arch. Clin. Exp. Ophthalmol. 2024, 262, 133–141. [Google Scholar] [CrossRef]
- Ting, D.S.J.; Ho, C.S.; Deshmukh, R.; Said, D.G.; Dua, H.S. Infectious keratitis: An update on epidemiology, causative microorganisms, risk factors, and antimicrobial resistance. Eye 2021, 35, 1084–1101. [Google Scholar] [CrossRef] [PubMed]
- Grubešić, P.; Jurak, I.; Čaljkušić-Mance, T.; Belančić, A.; Grubešić, A. Clinical and Demographic Characteristics of Herpetic Keratitis Patients—Tertiary Centre Experience. Medicina 2024, 60, 577. [Google Scholar] [CrossRef] [PubMed]
- Labetoulle, M.; Auquier, P.; Conrad, H.; Crochard, A.; Daniloski, M.; Bouee, S.; El Hasnaoui, A.; Colin, J. Incidence of Herpes Simplex Virus Keratitis in France. Ophthalmology 2005, 112, 888–895.e1. [Google Scholar] [CrossRef]
- Kaye, S.; Choudhary, A. Herpes simplex keratitis. Prog. Retin. Eye Res. 2006, 25, 355–380. [Google Scholar] [CrossRef]
- Shuster, J.J.; Kaufman, H.E.; Nesburn, A.B. Statistical Analysis of the Rate of Recurrence of Herpesvirus Ocular Epithelial Disease. Arch. Ophthalmol. 1981, 91, 328–331. [Google Scholar] [CrossRef]
- Kaye, R.; Kaye, A.; Sueke, H.; Neal, T.; Winstanley, C.; Horsburgh, M.; Kaye, S. Recurrent Bacterial Keratitis. Investig. Ophthalmol. Vis. Sci. 2013, 54, 4136–4139. [Google Scholar] [CrossRef]
- Cunningham, A.L.; Diefenbach, R.J.; Miranda-Saksena, M.; Bosnjak, L.; Kim, M.; Jones, C.; Douglas, M.W. The Cycle of Human Herpes Simplex Virus Infection: Virus Transport and Immune Control. J. Infect. Dis. 2006, 194 (Suppl. S1), S11–S18. [Google Scholar] [CrossRef]
- Tuft, S.; Somerville, T.F.; Li, J.-P.O.; Neal, T.; De, S.; Horsburgh, M.J.; Fothergill, J.L.; Foulkes, D.; Kaye, S. Bacterial keratitis: Identifying the areas of clinical uncertainty. Prog. Retin. Eye Res. 2022, 89, 101031. [Google Scholar] [CrossRef]
- Proctor, R.A.; van Langevelde, P.; Kristjansson, M.; Maslow, J.N.; Arbeit, R.D. Persistent and Relapsing Infections Associated with Small-Colony Variants of Staphylococcus aureus. Clin. Infect. Dis. 1995, 20, 95–102. [Google Scholar] [CrossRef]
- Proctor, R.A.; Von Eiff, C.; Kahl, B.C.; Becker, K.; McNamara, P.; Herrmann, M.; Peters, G. Small colony variants: A pathogenic form of bacteria that facilitates persistent and recurrent infections. Nat. Rev. Microbiol. 2006, 4, 295–305. [Google Scholar] [CrossRef]
- Wang, L.; Wang, R.; Xu, C.; Zhou, H. Pathogenesis of Herpes Stromal Keratitis: Immune Inflammatory Response Mediated by Inflammatory Regulators. Front. Immunol. 2020, 11, 766. [Google Scholar] [CrossRef] [PubMed]
- Maycock, N.J.; Jayaswal, R. Update on Acanthamoeba Keratitis: Diagnosis, Treatment, and Outcomes. Cornea 2016, 35, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Dart, J.K.; Saw, V.P.; Kilvington, S. Acanthamoeba Keratitis: Diagnosis and Treatment Update 2009. Arch. Ophthalmol. 2009, 148, 487–499.e2. [Google Scholar] [CrossRef] [PubMed]
- Lobo, A.-M.; Agelidis, A.M.; Shukla, D. Pathogenesis of herpes simplex keratitis: The host cell response and ocular surface sequelae to infection and inflammation. Ocul. Surf. 2019, 17, 40–49. [Google Scholar] [CrossRef]
- Zimmerman, A.; Nixon, A.; Rueff, E. Contact lens associated microbial keratitis: Practical considerations for the optometrist. Clin. Optom. 2016, 8, 1–12. [Google Scholar] [CrossRef]
- Chaudhry, N.L.; Przybek, J.; Hamilton, A.; Carley, F. Unique case of palytoxin-related keratitis. Clin. Exp. Ophthalmol. 2016, 44, 853–854. [Google Scholar] [CrossRef]
- Prajna, N.V.; Srinivasan, M.; Lalitha, P.; Krishnan, T.; Rajaraman, R.; Ravindran, M.; Mascarenhas, J.; Oldenburg, C.E.; Ray, K.J.; McLeod, S.D.; et al. Differences in Clinical Outcomes in Keratitis Due to Fungus and Bacteria. JAMA Ophthalmol 2013, 131, 1088–1089. [Google Scholar] [CrossRef]
- Tanure, M.A.G.; Cohen, E.J.; Sudesh, S.; Rapuano, C.J.; Laibson, P.R. Spectrum of Fungal Keratitis at Wills Eye Hospital, Philadelphia, Pennsylvania. Cornea 2000, 19, 307–312. [Google Scholar] [CrossRef]
- Maier, P.; Betancor, P.K.; Reinhard, T. Contact-lens-associated keratitis—An often underestimated risk. Dtsch. Aerzteblatt Online 2022, 119, 669–674. [Google Scholar] [CrossRef]
- Mauger, T.F.; Hill, R.M. Corneal epithelial healing under contact lenses. Acta Ophthalmol. 1992, 70, 361–365. [Google Scholar] [CrossRef]
- Ladage, P.M.; Ren, D.H.; Petroll, W.M.; Jester, J.V.; Bergmanson, J.P.G.; Cavanagh, H.D. Effects of eyelid closure and disposable and silicone hydrogel extended contact lens wear on rabbit corneal epithelial proliferation. Investig. Opthalmology Vis. Sci. 2003, 44, 1843–1849. [Google Scholar] [CrossRef] [PubMed]
- Ladage, P.M.; Jester, J.V.; Petroll, W.M.; Bergmanson, J.P.G.; Cavanagh, H.D. Vertical Movement of Epithelial Basal Cells toward the Corneal Surface during Use of Extended-Wear Contact Lenses. Investig. Opthalmology Vis. Sci. 2003, 44, 1056–1063. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, F.; Keay, L.; Edwards, K.; Naduvilath, T.; Dart, J.K.; Brian, G.; Holden, B.A. The incidence of contact lens-related microbial keratitis in Australia. Ophthalmology 2008, 115, 1655–1662. [Google Scholar] [CrossRef]
- Szczotka-Flynn, L.B.; Shovlin, J.P.; Schnider, C.M.; Caffery, B.E.; Alfonso, E.C.; Carnt, N.A.; Willcox, M.D. American Academy of Optometry Microbial Keratitis Think Tank. Optom. Vis. Sci. 2021, 98, 182–198. [Google Scholar] [CrossRef]
- Keay, L.; Harmis, N.; Corrigan, K.; Sweeney, D.; Willcox, M. Infiltrative keratitis associated with extended wear of hydrogel lenses and Abiotrophia defective. Cornea 2000, 19, 864–869. [Google Scholar] [CrossRef]
- Sankaridurg, P.R.; Sharma, S.; Willcox, M.; Sweeney, D.F.; Naduvilath, T.J.; Holden, B.A.; Rao, G.N. Colonization of hydrogel lenses with Streptococcus pneumoniae: Risk of development of corneal infiltrates. Cornea 1999, 18, 289–295. [Google Scholar] [CrossRef]
- Mucci, J.J.; Utz, V.M.; Galor, A.; Feuer, W.; Jeng, B.H. Recurrence rates of herpes simplex virus keratitis in contact lens and non-contact lens wearers. Eye Contact Lens 2009, 35, 185–187. [Google Scholar] [CrossRef]
- World Health Organization. World Report on Vision; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Efron, N.; Morgan, P.B. Rethinking contact lens associated keratitis. Clin. Exp. Optom. 2006, 89, 280–298. [Google Scholar] [CrossRef]
- Alzahrani, O.; Alshehri, F.A.; Alali, A.O.; Alzahrani, O.H.; Alzahrani, Z.A.; AlZahrani, A.; Almazrou, A.A. Contact Lens Practices and Knowledge of Complications and its Association With Refractive Error in Saudi Arabia. Cureus 2021, 13, e12786. [Google Scholar] [CrossRef]
- Stapleton, F.; Edwards, K.; Keay, L.; Naduvilath, T.; Dart, J.K.; Brian, G.; Holden, B. Risk Factors for Moderate and Severe Microbial Keratitis in Daily Wear Contact Lens Users. Ophthalmology 2012, 119, 1516–1521. [Google Scholar] [CrossRef]
- Prajna, N.V.; Krishnan, T.; Mascarenhas, J.; Srinivasan, M.; Oldenburg, C.E.; Toutain-Kidd, C.M.; Sy, A.; McLeod, S.D.; Zegans, M.E.; Acharya, N.R.; et al. Predictors of outcome in fungal keratitis. Eye 2012, 26, 1226–1231. [Google Scholar] [CrossRef] [PubMed]
- Ting, D.S.J.; Henein, C.; Said, D.G.; Dua, H.S. Amniotic membrane transplantation for infectious keratitis: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Arunga, S.; Kintoki, G.M.; Gichuhi, S.; Onyango, J.; Newton, R.; Leck, A.; Macleod, D.; Hu, V.H.; Burton, M.J. Delay Along the Care Seeking Journey of Patients with Microbial Keratitis in Uganda. Ophthalmic Epidemiology 2019, 26, 311–320. [Google Scholar] [CrossRef]
- Hoffman, J.J.; Yadav, R.; Das Sanyam, S.; Chaudhary, P.; Roshan, A.; Singh, S.K.; Mishra, S.K.; Arunga, S.; Hu, V.H.; Macleod, D.; et al. Delay in accessing definitive care for patients with microbial keratitis in Nepal. Front. Med. 2022, 9, 915293. [Google Scholar] [CrossRef]
- Faragher, R.G.A.; Mulholland, B.; Tuft, S.J.; Sandeman, S.; Khaw, P.T. Aging and the cornea. Br. J. Ophthalmol. 1997, 81, 814–817. [Google Scholar] [CrossRef]
- Chang, S.-W.; Hu, F.-R. Changes in Corneal Autofluorescence and Corneal Epithelial Barrier Function With Aging. Cornea 1993, 12, 493–499. [Google Scholar] [CrossRef]
- Kim, C.K.; Karslioglu, M.Z.; Zhao, S.H.; Lee, O.L. Infectious Keratitis in Patients Over 65: A Review on Treatment and Preserving Eyesight. Clin. Interv. Aging 2024, ume 19, 1393–1405. [Google Scholar] [CrossRef]
- Kelly, D.S.; Sabharwal, S.; Ramsey, D.J.; Morkin, M.I. The effects of female sex hormones on the human cornea across a woman’s life cycle. BMC Ophthalmol. 2023, 23, 1–10. [Google Scholar] [CrossRef]
- Nuzzi, R.; Caselgrandi, P. Sex Hormones and Their Effects on Ocular Disorders and Pathophysiology: Current Aspects and Our Experience. Int. J. Mol. Sci. 2022, 23, 3269. [Google Scholar] [CrossRef]
- Zhao, X.; Yuan, Y.; Sun, T.; Zhang, Y.; Chen, Y. Associations Between Keratoconus and the Level of Sex Hormones: A Cross-Sectional Study. Front. Med. 2022, 9, 828233. [Google Scholar] [CrossRef]
- Chen, K.-J.; Chen, Y.-P.; Chao, A.-N.; Wang, N.-K.; Wu, W.-C.; Lai, C.-C.; Chen, T.-L. Prevention of Evisceration or Enucleation in Endogenous Bacterial Panophthalmitis with No Light Perception and Scleral Abscess. PLoS ONE 2017, 12, e0169603. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Agrawal, D. Recurrence of Infection in Corneal Grafts After Therapeutic Penetrating Keratoplasty for Microbial Keratitis. Cornea 2020, 39, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Koçluk, Y.; Sukgen, E.A. Results of therapeutic penetrating keratoplasty for bacterial and fungal keratitis. Int. Ophthalmol. 2016, 37, 1085–1093. [Google Scholar] [CrossRef]
- Raj, A.; Bahadur, H.; Dhasmana, R. Outcome of therapeutic penetrating keratoplasty in advanced infectious keratitis. J. Curr. Ophthalmol. 2018, 30, 315–320. [Google Scholar] [CrossRef]
- Sharma, N.; Sachdev, R.; Jhanji, V.; Titiyal, J.S.; Vajpayee, R.B. Therapeutic keratoplasty for microbial keratitis. Curr. Opin. Ophthalmol. 2010, 21, 293–300. [Google Scholar] [CrossRef]
- Ti, S.-E.; Scott, J.A.; Janardhanan, P.; Tan, D.T. Therapeutic Keratoplasty for Advanced Suppurative Keratitis. Arch. Ophthalmol. 2007, 143, 755–762.e2. [Google Scholar] [CrossRef]
- Shi, W.; Wang, T.; Xie, L.; Li, S.; Gao, H.; Liu, J.; Li, H. Risk Factors, Clinical Features, and Outcomes of Recurrent Fungal Keratitis after Corneal Transplantation. Ophthalmology 2010, 117, 890–896. [Google Scholar] [CrossRef]
- Lyall, D.A.M.; Tarafdar, S.; Gilhooly, M.J.; Roberts, F.; Ramaesh, K. Long term visual outcomes, graft survival and complications of deep anterior lamellar keratoplasty in patients with herpes simplex related corneal scarring. Br. J. Ophthalmol. 2012, 96, 1200–1203. [Google Scholar] [CrossRef]
- Wu, S.-Q.; Zhou, P.; Zhang, B.; Qiu, W.-Y.; Yao, Y.-F. Long-term Comparison of Full-Bed Deep Lamellar Keratoplasty With Penetrating Keratoplasty in Treating Corneal Leucoma Caused by Herpes Simplex Keratitis. Am. J. Ophthalmol. 2012, 153, 291–299.e2. [Google Scholar] [CrossRef]
- Maier, A.-K.B.; Özlügedik, S.; Rottler, J.; Heussen, F.M.A.; Klamann, M.K.J.; Huber, K.K.; Joussen, A.M.; Winterhalter, S. Efficacy of Postoperative Immunosuppression After Keratoplasty in Herpetic Keratitis. Cornea 2011, 30, 1398–1405. [Google Scholar] [CrossRef]
- Przybek-Skrzypecka, J.; Ryk-Adamska, M.; Szewczuk, A.; Skrzypecki, J.; Izdebska, J.; Udziela, M.; Rypniewska, A.; Suh, L.H.; Szaflik, J.P. Severe Microbial Keratitis in Virgin and Transplanted Cornea—Probability of Visual Acuity Improvement. J. Clin. Med. 2024, 14, 124. [Google Scholar] [CrossRef] [PubMed]
- Khor, W.-B.; Prajna, V.N.; Garg, P.; Mehta, J.S.; Xie, L.; Liu, Z.; Padilla, M.D.B.; Joo, C.-K.; Inoue, Y.; Goseyarakwong, P.; et al. The Asia Cornea Society Infectious Keratitis Study: A Prospective Multicenter Study of Infectious Keratitis in Asia. Am. J. Ophthalmol. 2018, 195, 161–170. [Google Scholar] [CrossRef]
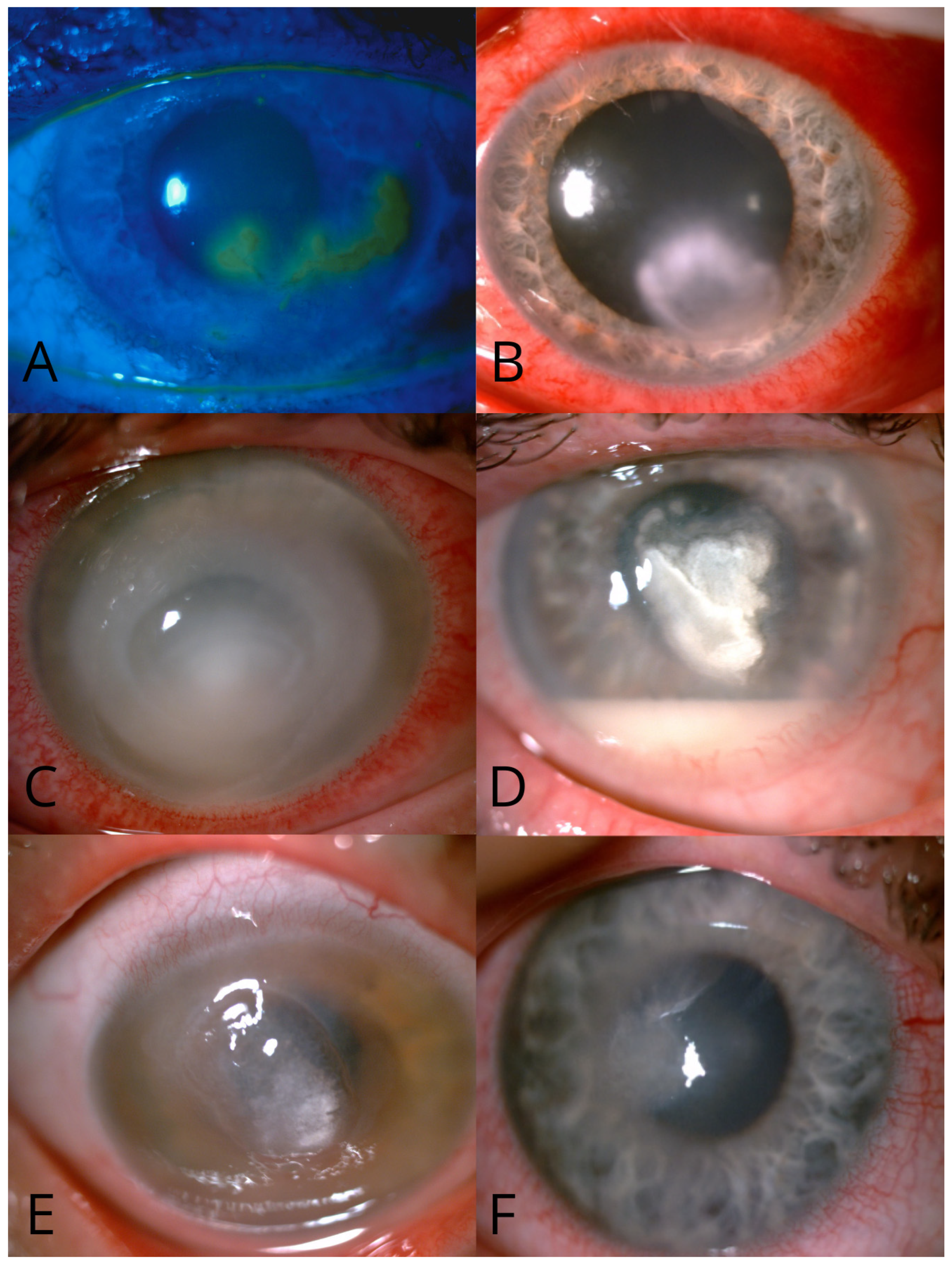
| Variable | Group 1 (Recurrent Keratitis) | Group 2 (Non-Recurrent Keratitis) | p |
|---|---|---|---|
| N | 233 | 1070 | |
| Age, years, median (IQR) | 51.00 (36.00; 68.00) | 45.00 (31.00; 67.00) | 0.989 2 |
| Sex, female, n (%) | 144 (61.80) | 619 (57.85) | 0.267 1 |
| Ethnicity, n (%) | |||
| Caucasian | 233 (100.00) | 1066 (97.83) | 0.584 1 |
| African | - | 2 (0.19) | 1.00 1 |
| Asian | - | 2 (0.19) | 1.00 1 |
| Eye condition, (n %) | 2 (0.86) | 13 (1.21) | 0.644 1 |
| Cataract, n (%) | 11 (4.72) | 125 (11.68) | 0.001 1 |
| Refractive error, n (%) | 18 (7.73) | 45 (4.21) | 0.023 1 |
| Post-corneal transplant, n (%) | |||
| PK | 3 (0.01) | 31 (0.03) | 0.242 1 |
| DALK | 1 (0.004) | 4 (0.004) | 1.00 1 |
| DSAEK | 1 (0.004) | 2 (0.002) | 1.00 1 |
| Initial BCVA (logMAR), median (IQR) | 0.30 (0.08; 1.00) | 0.30 (0.08; 0.90) | 0.502 2 |
| Management type, n (%) | |||
| Inpatient, n (%) | 9 (3.86) | 188 (17.57) | 0.000 1 |
| Outpatient, n (%) | 224 (96.14) | 882 (82.43) | |
| CL use, n (%) | 28 (12.02) | 324 (30.28) | 0.000 1 |
| CL misuse *, n (%) | 5 (2.15) | 85 (7.94) | 0.002 1 |
| History of ocular trauma, n (%) | 10 (4.29) | 127 (11.87) | 0.001 1 |
| Variable | Group 1 (Recurrent Keratitis) | Group 2 (Non-Recurrent Keratitis) | p |
|---|---|---|---|
| N | 233 | 1070 | |
| Time from the symptoms to the visit, days, mean (SD) | 17.01 (±124.56) | 10.79 (±27.42) | 0.759 2 |
| Clinical features in slit lamp test, n (%) | |||
| Central location, n (%) | 47 (20.17) | 248 (23.17) | 0.356 1 |
| Paracentral location, n (%) | 32 (13.73) | 172 (16.07) | |
| Peripheral location, n (%) | 101 (43.35) | 461 (43.08) | |
| Total corneal surface area, n (%) | 42 (18.03) | 144 (13.46) | |
| Surface area, n (%) | |||
| Singular lesion, n (%) | 148 (63.52) | 802 (74.95) | 0.000 1 |
| Multiple lesions, n (%) | 80 (34.33) | 252 (23.55) | |
| Initial diagnosis, n (%) | |||
| Direct bacteria, n (%) | 90 (38.63) | 503 (47.0) | 0.001 1 |
| Direct virus, n (%) | 73 (31.33) | 213 (19.91) | |
| Direct fungi, n (%) | 17 (7.3) | 37 (3.46) | |
| Direct amoeba, n (%) | 2 (0.86) | 20 (1.87) | |
| Mix diagnosis, n (%) | 30 (12.88) | 101 (9.44) | |
| Initial treatment, n (%) * | |||
| Antibiotic, n (%) | 200 (85.84) | 975 (91.12) | 0.024 1 |
| Antiviral, n (%) | 82 (35.19) | 230 (21.5) | 0.000 1 |
| Steroid, n (%) | 51 (21.89) | 152 (14.21) | 0.003 1 |
| Antifungal, n (%) | 26 (11.16) | 168 (15.70) | 0.078 1 |
| General treatment, n (%) | 117 (50.21) | 491 (45.89) | 0.162 1 |
| Amniotic membrane transplant, n (%) | 2 (0.86) | 51 (4.77) | 0.006 1 |
| Treatment before the visit, started by another clinician, n (%) | 71 (30.47) | 264 (24.67) | 0.066 1 |
| Need for a corneal transplant, n (%) | 1 (0.43) | 47 (4.39) | 0.509 1 |
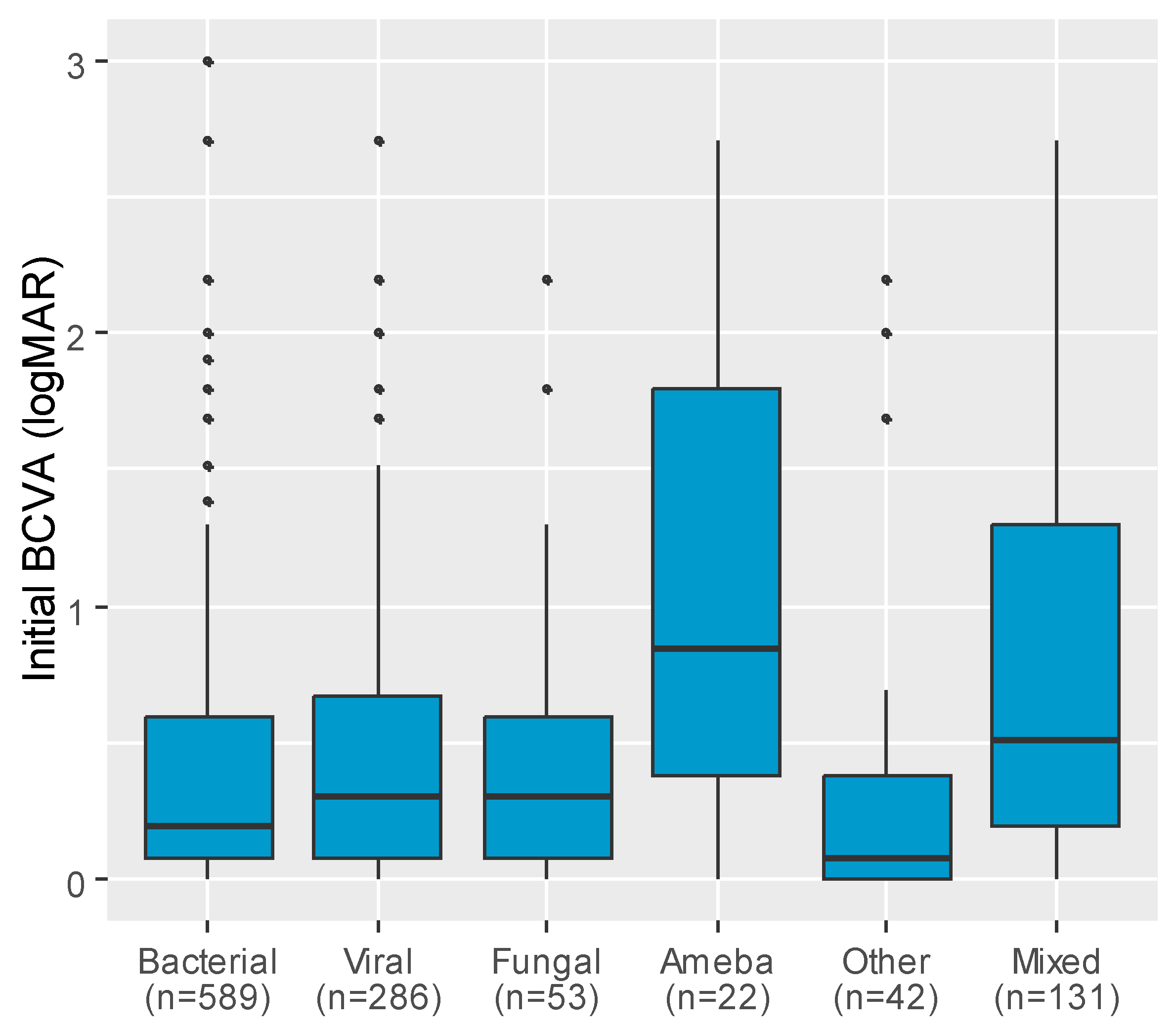
| Variable | n | Initial BCVA (logMAR), Median (IQR) | p |
|---|---|---|---|
| Etiology | <0.001 | ||
| Bacterial | 589 | 0.20 (0.08; 0.60) abg | |
| Viral | 286 | 0.30 (0.08; 0.67) aceh | |
| Fungal | 53 | 0.30 (0.08; 0.60) d | |
| Ameba | 22 | 0.85 (0.38; 1.80) bcdf | |
| Other | 42 | 0.08 (0.00; 0.38) efi | |
| Mixed | 131 | 0.51 (0.20; 1.30) ghi |
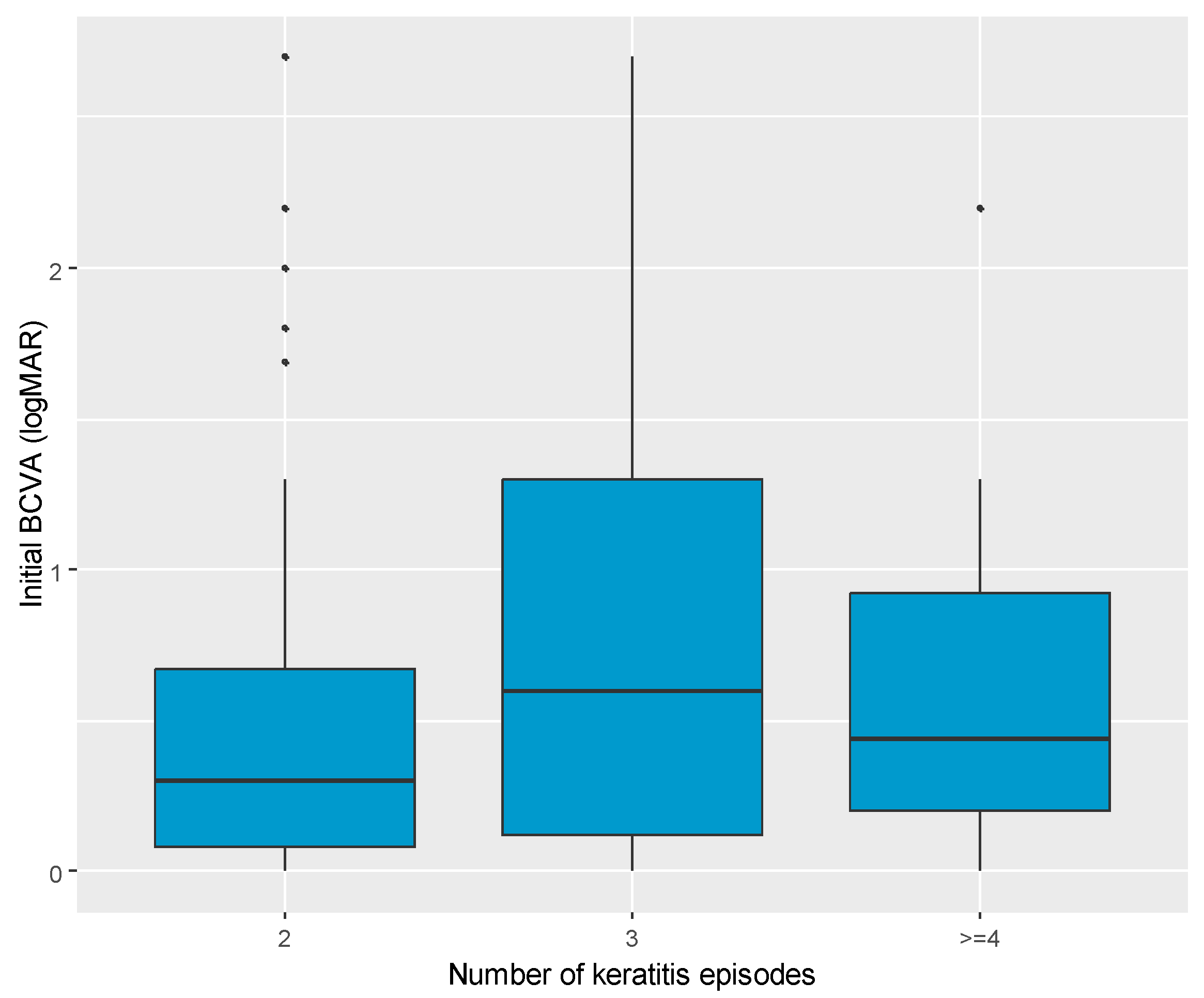
| Variable | Number of Keratitis Episodes | p | ||
|---|---|---|---|---|
| 2 | 3 | ≥4 | ||
| N | 126 | 27 | 18 | - |
| Age, years, median (IQR) | 50.00 (33.25; 67.00) | 57.00 (36.50; 68.50) | 50.50 (43.25; 68.75) | 0.397 |
| Initial BCVA (logMAR), median (IQR) | 0.30 (0.08; 0.67) | 0.60 (0.12; 1.30) | 0.44 (0.20; 0.92) | 0.049 |
| CL use, n (%) | 21 (16.7) | 1 (3.7) | 1 (5.6) | 0.141 |
| CL misuse, n (%) | 3 (2.4) | 1 (3.7) | 0 (0.0) | 0.709 |
| Refractive error, n (%) | 3 (2.4) | 3 (11.1) | 0 (0.0) | 0.080 |
| Post-corneal transplant, n (%) | 8 (6.3) | 3 (11.1) | 1 (5.6) | 0.544 |
| Time from the last keratitis, days, median (IQR) | 382.00 (183.00; 1095.00) | 730.00 (365.00; 1095.00) | 776.50 (379.75; 2828.75) | 0.117 |
| Time from the symptoms to the visit, days, median (IQR) | 3.00 (1.00; 5.00) | 3.00 (2.00; 5.50) | 3.00 (1.00; 4.75) | 0.876 |
| Treatment prior to emergency department visit, n (%) | 37 (29.4) | 4 (14.8) | 5 (27.8) | 0.301 1 |
| Initial treatment, n (%) * | ||||
| Antibiotic, n (%) | 105 (84.0) | 25 (92.6) | 17 (94.4) | 0.393 |
| Antiviral, n (%) | 48 (38.1) | 11 (40.7) | 3 (16.7) | 0.182 1 |
| Steroid, n (%) | 22 (17.5) | 8 (29.6) | 5 (27.8) | 0.261 1 |
| Antifungal, n (%) | 13 (10.3) | 3 (11.1) | 4 (22.2) | 0.352 |
| Other treatment, n (%) | 65 (51.6) | 14 (51.9) | 12 (66.7) | 0.481 1 |
| General treatment, n (%) | 62 (49.2) | 16 (59.3) | 9 (50.0) | 0.636 1 |
| Clinical features in slit lamp test, n (%) | ||||
| Central location, n (%) | 28 (22.2) | 3 (11.1) | 2 (11.1) | 0.226 |
| Paracentral location, n (%) | 19 (15.1) | 2 (7.4) | 2 (11.1) | |
| Peripheral location, n (%) | 58 (46.0) | 13 (48.1) | 7 (38.9) | |
| Total corneal, n (%) | 17 (13.5) | 8 (29.6) | 5 (27.8) | |
| Not indicated, n (%) | 4 (3.2) | 1 (3.7) | 2 (11.1) | |
| Surface area, n (%) | ||||
| Singular lesion, n (%) | 85 (69.7) | 18 (66.7) | 6 (33.3) | 0.010 1 |
| Multiple lesions, n (%) | 37 (30.3) | 9 (33.3) | 12 (66.7) | |
| Need for a corneal transplant, n (%) | 1 (0.8) | 0 (0.0) | 0 (0.0) | >0.999 |
| Variable | Univariate Models | Multivariate Model | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI for OR | p | OR | 95% CI for OR | p | |
| Age, years | 0.96 | 0.96–0.97 | <0.001 | 0.97 | 0.96–0.97 | <0.001 |
| Sex, male (vs. female) | 1.05 | 0.84–1.31 | 0.672 | - | - | - |
| CL | 2.37 | 1.84–3.08 | <0.001 | - | - | - |
| CL misuse | 2.25 | 1.42–3.66 | 0.001 | - | - | - |
| Recurrent keratitis | 0.98 | 0.74–1.30 | 0.899 | - | - | - |
| Number of keratitis | 0.77 | 0.53–1.03 | 0.120 | - | - | - |
| Time from the last keratitis, days | 1.00 | 1.00–1.00 | 0.023 | - | - | - |
| Treatment prior to emergency department visit | 0.82 | 0.64–1.05 | 0.120 | 0.80 | 0.59–1.09 | 0.158 |
| Time from the symptoms to the visit, days | 0.98 | 0.97–0.99 | <0.001 | 0.99 | 0.98–1.00 | 0.008 |
| Initial diagnosis | ||||||
| Viral (vs. bacterial) | 0.64 | 0.48–0.86 | 0.003 | - | - | - |
| Fungal (vs. bacterial) | 0.78 | 0.44–1.41 | 0.406 | - | - | - |
| Amoeba (vs. bacterial) | 0.15 | 0.05–0.39 | <0.001 | - | - | - |
| Other/Mix (vs. bacterial) | 0.47 | 0.34–0.67 | <0.001 | - | - | - |
| Any treatment (vs. no treatment) | 18.37 | 3.73–332.05 | 0.005 | 8.02 | 1.05–179.39 | 0.089 |
| Antibiotic treatment (vs. no antibiotics) | 0.89 | 0.61–1.30 | 0.555 | - | - | - |
| Antiviral treatment (vs. no antiviral treatment) | 0.97 | 0.75–1.25 | 0.814 | - | - | - |
| Steroid treatment (vs. no steroids) | 0.91 | 0.67–1.23 | 0.526 | - | - | - |
| Antifungal treatment (vs. no antifungal treatment) | 0.35 | 0.25–0.48 | <0.001 | 0.39 | 0.25–0.58 | <0.001 |
| Other treatment (vs. not treated with other type of drugs) | 0.98 | 0.79–1.23 | 0.873 | - | - | - |
| General treatment (vs. no general treatment) | 0.36 | 0.28–0.45 | <0.001 | - | - | - |
| Refractive error | 0.87 | 0.61–1.24 | 0.445 | - | - | - |
| Cataract | 0.14 | 0.02–0.49 | 0.009 | 0.33 | 0.04–1.67 | 0.212 |
| Post-corneal transplant | 0.04 | 0.01–0.11 | <0.001 | 0.11 | 0.02–0.32 | <0.001 |
| Paracentral location (vs. central) | 2.25 | 1.56–3.25 | <0.001 | - | - | - |
| Peripheral location (vs. central) | 4.70 | 3.48–6.39 | <0.001 | - | - | - |
| Total corneal location (vs. central) | 1.48 | 1.01–2.16 | 0.045 | - | - | - |
| Location not indicated (vs. central) | 1.97 | 1.06–3.65 | 0.032 | - | - | - |
| Multiple lesions | 1.33 | 1.04–1.72 | 0.026 | 1.82 | 1.24–2.70 | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwiatkowski, M.; Babula, E.; Sikora, A.; Izdebska, J.; Skrzypecki, J.; Szaflik, J.P.; Przybek-Skrzypecka, J. Comparison of Recurrent and Naïve Keratitis in a Cohort of 1303 Patients. J. Clin. Med. 2025, 14, 3760. https://doi.org/10.3390/jcm14113760
Kwiatkowski M, Babula E, Sikora A, Izdebska J, Skrzypecki J, Szaflik JP, Przybek-Skrzypecka J. Comparison of Recurrent and Naïve Keratitis in a Cohort of 1303 Patients. Journal of Clinical Medicine. 2025; 14(11):3760. https://doi.org/10.3390/jcm14113760
Chicago/Turabian StyleKwiatkowski, Maciej, Emilia Babula, Aleksandra Sikora, Justyna Izdebska, Janusz Skrzypecki, Jacek P. Szaflik, and Joanna Przybek-Skrzypecka. 2025. "Comparison of Recurrent and Naïve Keratitis in a Cohort of 1303 Patients" Journal of Clinical Medicine 14, no. 11: 3760. https://doi.org/10.3390/jcm14113760
APA StyleKwiatkowski, M., Babula, E., Sikora, A., Izdebska, J., Skrzypecki, J., Szaflik, J. P., & Przybek-Skrzypecka, J. (2025). Comparison of Recurrent and Naïve Keratitis in a Cohort of 1303 Patients. Journal of Clinical Medicine, 14(11), 3760. https://doi.org/10.3390/jcm14113760







