Evaluating the Biocompatibility and Efficacy of Absorbable Three-Dimensional Micro-Nanofiber Scaffolds for Volume Restoration Following Post-Mastectomy Breast Reconstruction: An Experimental Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Scaffold Design and Fabrication
2.2. Experimental Design
2.3. Animal Model
2.4. Histological Analyses
2.5. Statistical Analyses
3. Results
3.1. Cell Infiltration into Scaffolds
3.2. Inflammation at the Interface of Scaffolds
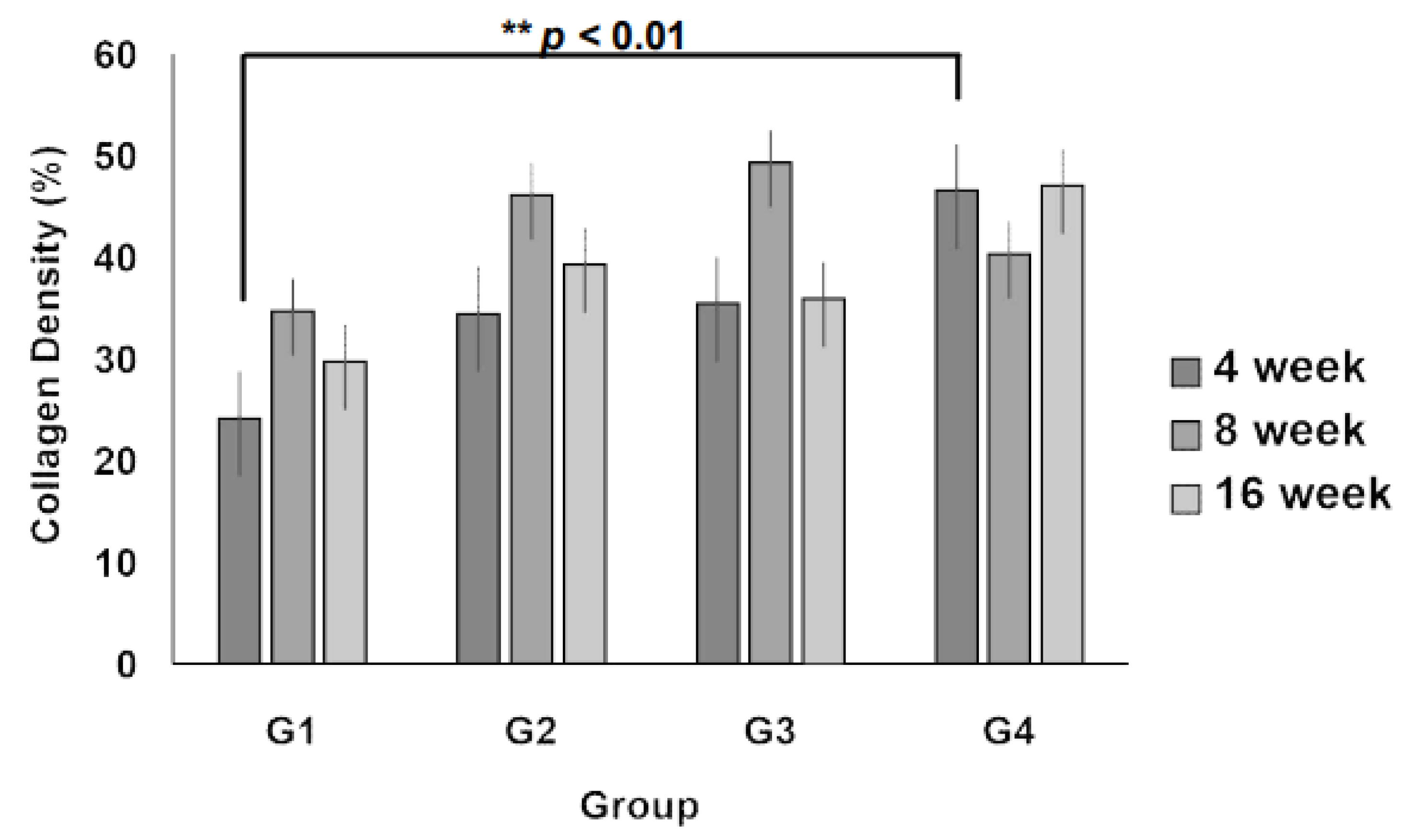
3.3. Collagen Regeneration Within Scaffolds
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 2D | Two-dimensional |
| 3D | Three-dimensional |
| BCS | Breast-conserving surgery |
| ECM | Extracellular matrix |
| H&E | Hematoxylin and eosin |
| MT | Masson’s trichrome |
| PCL | Polycaprolactone |
| SEM | Scanning electron microscopy |
References
- Global Burden of Disease Cancer Collaboration; Fitzmaurice, C.; Allen, C.; Barber, R.M.; Barregard, L.; Bhutta, Z.A.; Brenner, H.; Dicker, D.J.; Chimed-Orchir, O.; Dandona, R.; et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: A systematic analysis for the global burden of disease study. JAMA Oncol. 2017, 3, 524–548. [Google Scholar] [PubMed]
- Fisher, B.; Anderson, S.; Bryant, J.; Margolese, R.G.; Deutsch, M.; Fisher, E.R.; Jeong, J.H.; Wolmark, N. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N. Engl. J. Med. 2022, 347, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Pappas, L.; Neumayer, L.; Kokeny, K.; Agarwal, J. Effect of breast conservation therapy vs mastectomy on disease-specific survival for early-stage breast cancer. JAMA Surg. 2014, 149, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Delay, E.; Garson, S.; Tousson, G.; Sinna, R. Fat injection to the breast: Technique, results, and indications based on 880 procedures over 10 years. Aesthetic. Surg. J. 2009, 29, 360–376. [Google Scholar] [CrossRef] [PubMed]
- Bayram, Y.; Sezgic, M.; Karakol, P.; Bozkurt, M.; Filinte, G.T. The use of autologous fat grafts in breast surgery: A literature review. Arch. Plast. Surg. 2019, 46, 498–510. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- Dash, T.K.; Konkimalla, V.B. Poly-ε-caprolactone based formulations for drug delivery and tissue engineering: A review. J. Control. Release 2012, 158, 15–33. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.X.F.; Mo, X.M.; Teoh, S.H.; Hutmacher, D.W. Scaffold development using 3D printing with a starch-based polymer. Mater. Sci. Eng. C 2002, 20, 49–56. [Google Scholar] [CrossRef]
- ISO 10993-6; Biological Evaluation of Medical Devices—Part 6: Tests for Local Effects after Implantation. International Organization for Standardization: Geneva, Switzerland, 2016.
- Simonacci, F.; Bertozzi, N.; Grieco, M.P.; Grignaffini, E.; Raposio, E. Procedure, applications, and outcomes of autologous fat grafting. Ann. Med. Surg. 2017, 24, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Colaris, M.J.L.; Ruhl, T.; Beier, J.P. Effects of silicone breast implants on human cell types in vitro: A closer look on host and implant. Aesthetic. Plast. Surg. 2022, 46, 2208–2217. [Google Scholar] [CrossRef] [PubMed]
- Aktas, E.; Chamberlain, C.S.; Saether, E.E.; Duenwald-Kuehl, S.E.; Kondratko-Mittnacht, J.; Stitgen, M.; Lee, J.S.; Clements, A.E.; Murphy, W.L.; Vanderby, R. Immune modulation with primed mesenchymal stem cells delivered via biodegradable scaffold to repair an Achilles tendon segmental defect. J. Orthop. Res. 2017, 35, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Kim, D.S.; Hwang, G.Y.; Lee, J.K.; Lee, H.L.; Jung, J.W.; Hwang, S.Y.; Baek, S.W.; Yoon, S.L.; Ha, Y.; et al. Multi-modulation of immune-inflammatory response using bioactive molecule-integrated PLGA composite for spinal fusion. Mater. Today Bio 2023, 19, 100611. [Google Scholar] [CrossRef] [PubMed]
- Salthouse, D.; Novakovic, K.; Hilkens, C.M.U.; Ferreira, A.M. Interplay between biomaterials and the immune system: Challenges and opportunities in regenerative medicine. Acta Biomater. 2023, 155, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Liu, S.; Zhang, H.; Zhu, B.; Su, Y.; Zheng, C.; Tian, R.; Wang, M.; Kuang, H.; Zhao, X.; et al. Mesenchymal stem cells and extracellular matrix scaffold promote muscle regeneration by synergistically regulating macrophage polarization toward the M2 phenotype. Stem Cell Res. Ther. 2018, 9, 88. [Google Scholar] [CrossRef] [PubMed]
- Chhaya, M.P.; Melchels, F.P.W.; Holzapfel, B.M.; Baldwin, J.G.; Hutmacher, D.W. Sustained regeneration of high-volume adipose tissue for breast reconstruction using an in vivo bioreactor. Biomaterials 2015, 52, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Qin, C.; Wu, C. 3D printing of cell-delivery scaffolds for tissue regeneration. Regen. Biomater. 2023, 10, rbad032. [Google Scholar] [CrossRef] [PubMed]
- Dzobo, K.; Thomford, N.E.; Senthebane, D.A.; Shipanga, H.; Rowe, A.; Dandara, C.; Pillay, M.; Motaung, K.S.C.M. Advances in regenerative medicine and tissue engineering: Innovation and transformation of medicine. Stem Cells Int. 2018, 2018, 2495848. [Google Scholar] [CrossRef] [PubMed]
- Sorotos, M.; Paolini, G.; D'Orsi, G.; Firmani, G.; Santanelli di Pompeo, F. Long-term clinical and aesthetic results of a systematic fat transfer protocol for total breast reconstruction after nipple-sparing mastectomy. Plast. Reconstr. Surg. 2022, 150, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Sorotos, M.; Paolini, G.; D'Orsi, G.; Firmani, G.; Timmermans, F.W.; Santanelli di Pompeo, F. Oncologic outcome of 1000 postmastectomy breast reconstructions with fat transfer: A single-center, matched case-control study. Plast. Reconstr. Surg 2022, 150, 4S–12S. [Google Scholar] [CrossRef] [PubMed]
- Scaffold-Guided Breast Surgery (SGBS). ClinicalTrials.gov Identifier: NCT05437757. U.S. National Library of Medicine. Available online: https://clinicaltrials.gov/study/NCT05437757 (accessed on 23 March 2025).
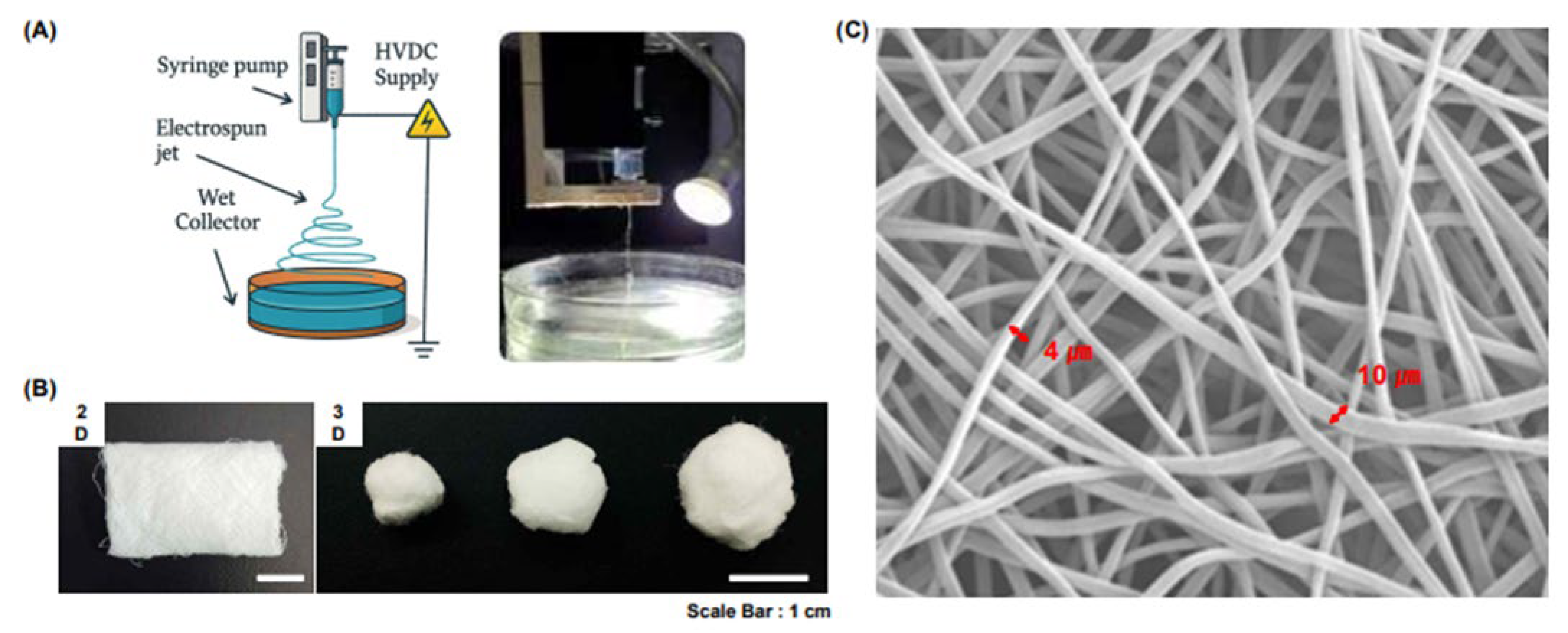


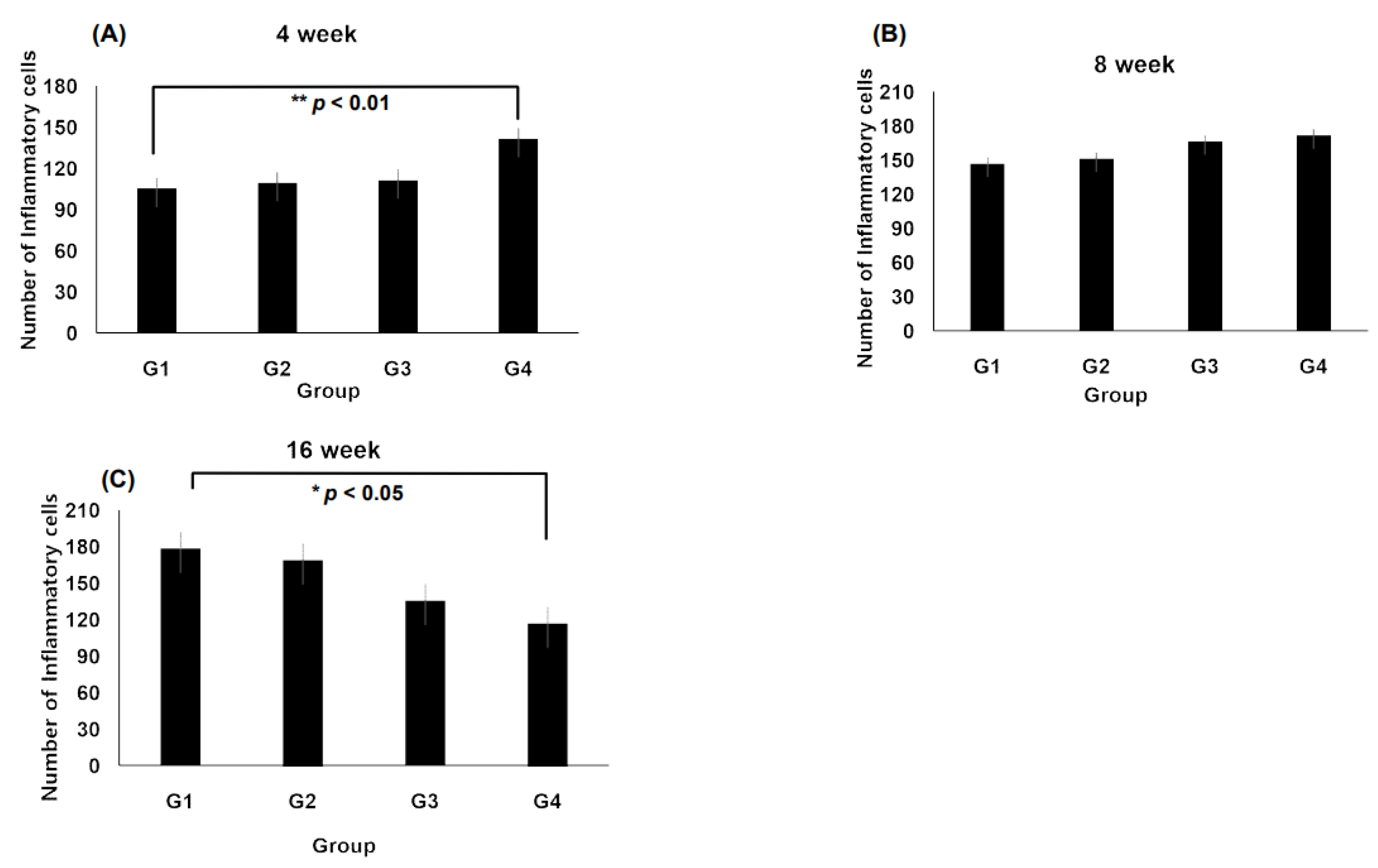
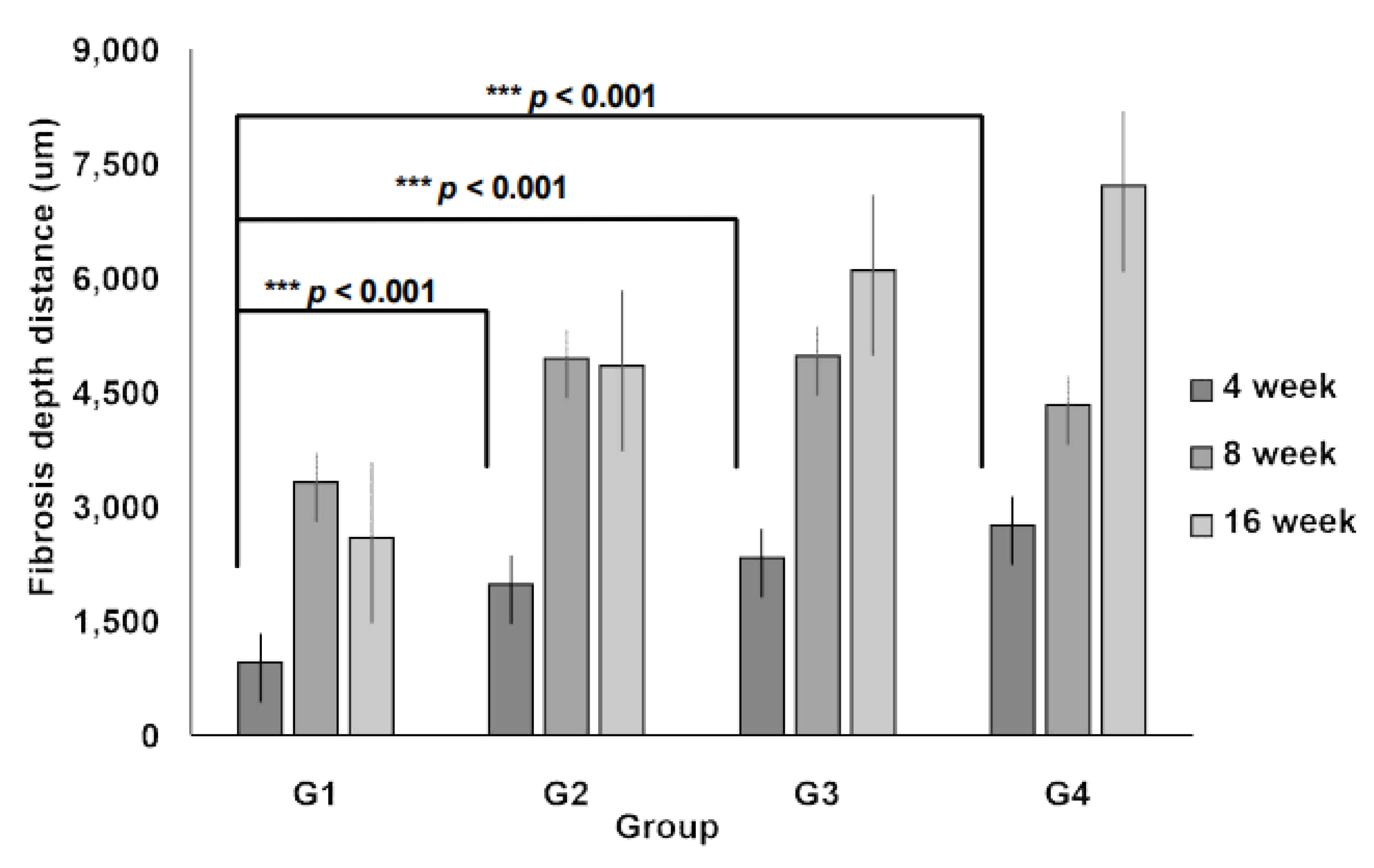
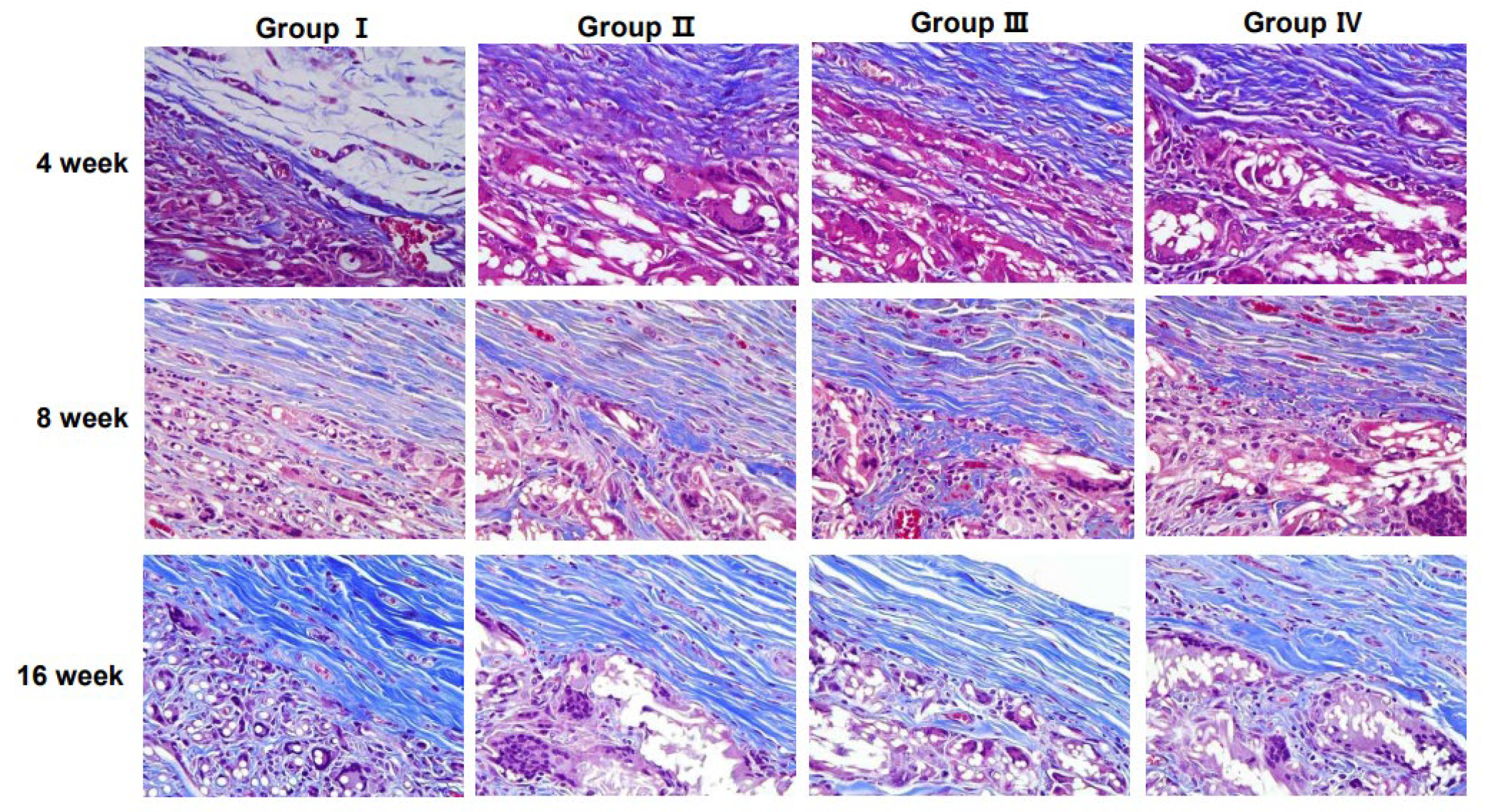
| Group | Type of Electrospinning | Weight (g) |
|---|---|---|
| Group I (G1) | 2-dimensional (2D) micro-nano fiber scaffold | 0.2 |
| Group II (G2) | 3-dimensional (3D) micro-nano fiber scaffold | 0.2 |
| Group III (G3) | 3-dimensional (3D) micro-nano fiber scaffold | 0.3 |
| Group IV (G4) | 3-dimensional (3D) micro-nano fiber scaffold | 0.6 |
| PMNs | Lymphocyte | Plasma Cell | Macrophage | Giant Cell | Necrosis | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 W | 8 W | 16 W | 4 W | 8 W | 16 W | 4 W | 8 W | 16 W | 4 W | 8 W | 16 W | 4 W | 8 W | 16 W | 4 W | 8 W | 16 W | |
| Group I | 0 | 0 | 0 | 1 | 1 | 0.4 | 1 | 1 | 0.8 | 0 | 1 | 1 | 1 | 0.6 | 0.8 | 0 | 0 | 0 |
| Group II | 0 | 0 | 0 | 1 | 1 | 0.6 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 0.4 | 0 | 0 | 0 |
| Group III | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0.2 | 1.2 | 1.4 | 0 | 0 | 0 |
| Group IV | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0.6 | 0.8 | 1 | 0.8 | 1.4 | 1.4 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bae, J.-Y.; Shim, J.; Hwang, S.; Kim, T.; Koo, B.; Lee, Y.J.; Hong, K.Y.; Heo, C.Y. Evaluating the Biocompatibility and Efficacy of Absorbable Three-Dimensional Micro-Nanofiber Scaffolds for Volume Restoration Following Post-Mastectomy Breast Reconstruction: An Experimental Study. J. Clin. Med. 2025, 14, 3754. https://doi.org/10.3390/jcm14113754
Bae J-Y, Shim J, Hwang S, Kim T, Koo B, Lee YJ, Hong KY, Heo CY. Evaluating the Biocompatibility and Efficacy of Absorbable Three-Dimensional Micro-Nanofiber Scaffolds for Volume Restoration Following Post-Mastectomy Breast Reconstruction: An Experimental Study. Journal of Clinical Medicine. 2025; 14(11):3754. https://doi.org/10.3390/jcm14113754
Chicago/Turabian StyleBae, Ji-Yeon, JungHee Shim, Sunyoung Hwang, TaeHo Kim, BumMo Koo, Young Jin Lee, Ki Yong Hong, and Chan Yeong Heo. 2025. "Evaluating the Biocompatibility and Efficacy of Absorbable Three-Dimensional Micro-Nanofiber Scaffolds for Volume Restoration Following Post-Mastectomy Breast Reconstruction: An Experimental Study" Journal of Clinical Medicine 14, no. 11: 3754. https://doi.org/10.3390/jcm14113754
APA StyleBae, J.-Y., Shim, J., Hwang, S., Kim, T., Koo, B., Lee, Y. J., Hong, K. Y., & Heo, C. Y. (2025). Evaluating the Biocompatibility and Efficacy of Absorbable Three-Dimensional Micro-Nanofiber Scaffolds for Volume Restoration Following Post-Mastectomy Breast Reconstruction: An Experimental Study. Journal of Clinical Medicine, 14(11), 3754. https://doi.org/10.3390/jcm14113754






