Improving Prediction of Postoperative Atrial Fibrillation After Cardiac Surgery Using Multiple Pathophysiological Biomarkers: A Prospective Double-Centre Study
Abstract
1. Background
2. Methods
2.1. Study Design and Participants
2.2. Data Collection
2.3. Sample Size
2.4. POAF Score
2.5. Selection of Biomarkers
2.6. Outcome
2.7. Missing Data
2.8. Statistical Analysis
3. Results
3.1. Patient Population and Outcomes
3.2. Biomarker Selection
3.3. Predictive Performance
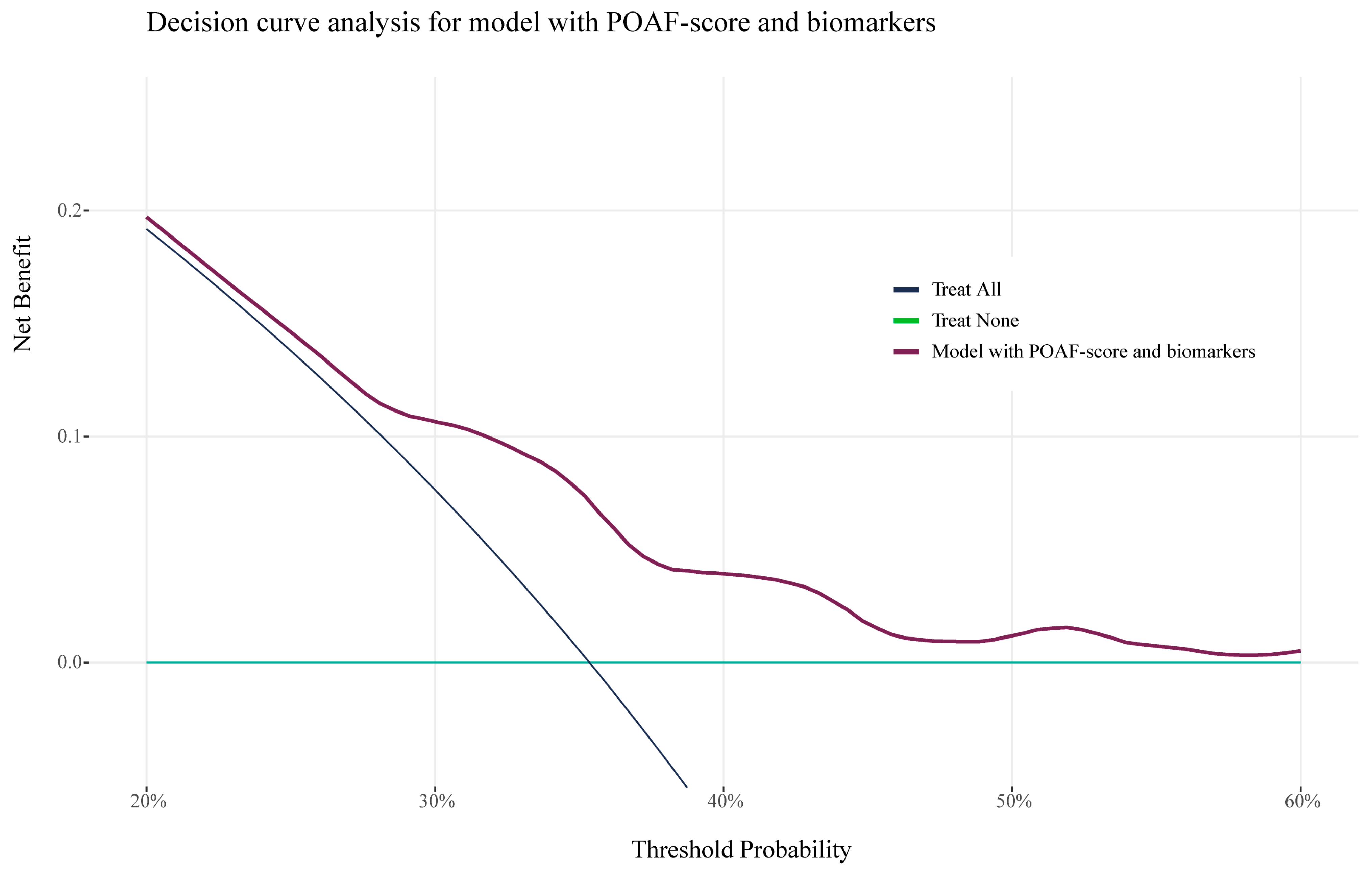
3.4. Risk Stratification and Clinical Benefit
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mariscalco, G.; Biancari, F.; Zanobini, M.; Cottini, M.; Piffaretti, G.; Saccocci, M.; Banach, M.; Beghi, C.; Angelini, G.D. Bedside Tool for Predicting the Risk of Postoperative Atrial Fibrillation After Cardiac Surgery: The POAF Score. J. Am. Heart Assoc. 2014, 3, e000752. [Google Scholar] [CrossRef] [PubMed]
- Cameron, M.J.; Tran, D.T.T.; Abboud, J.; Newton, E.K.; Rashidian, H.; Dupuis, J.-Y. Prospective External Validation of Three Preoperative Risk Scores for Prediction of New Onset Atrial Fibrillation After Cardiac Surgery. Anesth. Analg. 2018, 126, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Maisel, W.H.; Rawn, J.D.; Stevenson, W.G. Atrial fibrillation after cardiac surgery. Ann. Intern Med. 2001, 135, 1061–1073. [Google Scholar] [CrossRef] [PubMed]
- Mathew, J.P.; Fontes, M.L.; Tudor, I.C.; Ramsay, J.; Duke, P.; Mazer, C.D.; Barash, P.G.; Hsu, P.H.; Mangano, D.T.; Investigators of the Ischemia Research and Education Foundation; et al. A multicenter risk index for atrial fibrillation after cardiac surgery. JAMA 2004, 291, 1720–1729. [Google Scholar] [CrossRef] [PubMed]
- Villareal, R.P.; Hariharan, R.; Liu, B.C.; Kar, B.; Lee, V.V.; Elayda, M.; Lopez, J.A.; Rasekh, A.; Wilson, J.M.; Massumi, A. Postoperative atrial fibrillation and mortality after coronary artery bypass surgery. J. Am. Coll. Cardiol. 2004, 43, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Lahtinen, J.; Biancari, F.; Salmela, E.; Mosorin, M.; Satta, J.; Rainio, P.; Rimpiläinen, J.; Lepojärvi, M.; Juvonen, T. Postoperative atrial fibrillation is a major cause of stroke after on-pump coronary artery bypass surgery. Ann. Thorac. Surg. 2004, 77, 1241–1244. [Google Scholar] [CrossRef] [PubMed]
- Ambrosetti, M.; Tramarin, R.; Griffo, R.; De Feo, S.; Fattirolli, F.; Vestri, A.; Riccio, C.; Temporelli, P.L.; ISYDE and ICAROS Investigators of the Italian Society for Cardiovascular Prevention, Rehabilitation and Epidemiology (IACPR-GICR). Late postoperative atrial fibrillation after cardiac surgery: A national survey within the cardiac rehabilitation setting. J. Cardiovasc. Med. 2011, 12, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Gaudino, M.; Di Franco, A.; Rong, L.Q.; Piccini, J.; Mack, M. Postoperative atrial fibrillation: From mechanisms to treatment. Eur. Heart J. 2023, 44, 1020–1039. [Google Scholar] [CrossRef]
- Echahidi, N.; Pibarot, P.; O’Hara, G.; Mathieu, P. Mechanisms, prevention, and treatment of atrial fibrillation after cardiac surgery. J. Am. Coll. Cardiol. 2008, 51, 793–801. [Google Scholar] [CrossRef]
- Noordzij, P.G.; Ruven, H.J.T.; Reniers, T.; Idema, R.N.; Thio, M.S.; Cremer, O.L.; Hollema, N.; Smit, K.N.; Vernooij, L.M.; Dijkstra, I.M.; et al. Cohort profile of BIGPROMISE: A perioperative biobank of a high-risk surgical population. BMJ Open 2024, 14, e078307. [Google Scholar] [CrossRef]
- Collins, G.S.; Reitsma, J.B.; Altman, D.G.; Moons, K.G.M. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): The TRIPOD Statement. Ann. Intern Med. 2015, 162, 55–63. [Google Scholar] [CrossRef]
- Riley, R.D.; Snell, K.I.E.; Ensor, J.; Burke, D.L.; Harrell, F.E., Jr.; Moons, K.G.M.; Collins, G.S. Minimum sample size for developing a multivariable prediction model: Part II—Binary and time-to-event outcomes. Stat. Med. 2019, 38, 1276–1296. [Google Scholar] [CrossRef] [PubMed]
- Beattie, W.S.; Lalu, M.; Feng, S.; Wijeysundera, D.N.; Nagele, P.; Fleisher, L.A.; Kurz, A.; Biccard, B.; Leslie, K.; Howell, S.; et al. Systematic review and consensus definitions for the Standardised Endpoints in Perioperative Medicine (StEP) initiative: Cardiovascular outcomes. Br. J. Anaesth. 2021, 126, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Heymans, M.W. psfmi: Prediction Model Pooling, Selection and Performance Evaluation Across Multiply Imputed Datasets, R package Version 1.4.0. 2023.

| No POAF | POAF | p-Value | |
|---|---|---|---|
| N = 620 | N = 339 | ||
| Female, n (%) | 126 (20.3) | 76 (22.4) | 0.498 |
| Age, y (IQR) | 64 [59–69] | 66 [62–72] | <0.001 |
| BMI | 27 [25–30] | 27 [25–29] | 0.009 |
| Surgery type, n (%) | <0.001 | ||
| AVR | 88 (14.2) | 54 (15.9) | |
| Bentall | 10 (1.6) | 13 (3.8) | |
| CABG | 393 (63.4) | 167 (49.3) | |
| CABG + AVR | 51 (8.2) | 48 (14.2) | |
| MVR | 42 (6.8) | 20 (5.9) | |
| Other | 36 (5.8) | 34 (10.0) | |
| Urgent surgery | 134 (21.6) | 66 (19.5) | 0.485 |
| Diabetes, n (%) | 154 (24.8) | 70 (20.6) | 0.541 |
| COPD, n (%) | 0.504 | ||
| None | 571 (92.1) | 314 (92.6) | |
| GOLD I | 3 (0.5) | 4 (1.2) | |
| GOLD II | 12 (1.9) | 8 (2.4) | |
| GOLD III | 6 (1.0) | 4 (1.2) | |
| Unknown | 28 (4.6) | 9 (2.7) | |
| Hypertension, n (%) | 344 (55.7) | 187 (55.2) | 0.935 |
| Heart failure, n (%) | 43 (7.0) | 26 (7.7) | 0.787 |
| History of ischemic heart disease, n (%) | 418 (67.6) | 214 (63.3) | 0.201 |
| Previous myocardial infarction, n (%) | 194 (31.3) | 90 (26.5) | 0.139 |
| Myocardial infarction within 90 days prior to surgery, n (%) | 128 (20.7) | 58 (17.1) | 0.207 |
| Peripheral artery disease, n (%) | 91 (14.7) | 64 (18.9) | 0.115 |
| Pulmonary hypertension, n (%) | 0.332 | ||
| No | 614 (99.0) | 335 (99.1) | |
| Moderate | 6 (1.0) | 2 (0.6) | |
| Severe | 0 (0.0) | 1 (0.3) | |
| LVEF, n (%) | 0.204 | ||
| >50 | 460 (74.2) | 260 (77.2) | |
| 31–50 | 116 (18.7) | 62 (18.4) | |
| 21–30 | 14 (2.3) | 8 (2.4) | |
| <20 | 7 (1.1) | 0 (0.0) | |
| Unknown | 23 (3.7) | 7 (2.1) | |
| NYHA, n (%) | 0.737 | ||
| Class 1 | 132 (21.3) | 82 (24.2) | |
| Class 2 | 270 (43.6) | 150 (44.2) | |
| Class 3 | 66 (10.7) | 29 (8.6) | |
| Class 4 | 11 (1.8) | 6 (1.8) | |
| Unknown | 140 (22.6) | 72 (21.2) | |
| CCS IV, n (%) | 0.256 | ||
| No | 509 (82.2) | 287 (84.7) | |
| Yes | 55 (8.9) | 20 (5.9) | |
| Unknown | 55 (8.9) | 32 (9.4) | |
| Previous cardiac surgery, n (%) | 75 (12.1) | 34 (10.0) | 0.386 |
| Previous CVA or TIA, n (%) | 70 (11.3) | 39 (11.5) | 1.000 |
| Kidney function, n (%) | 0.299 | ||
| CC > 85 | 290 (46.8) | 154 (45.4) | |
| CC 50–85 | 298 (48.1) | 160 (47.2) | |
| CC < 50 | 30 (4.8) | 25 (7.4) | |
| Dialysis | 2 (0.3) | 0 (0.0) |
| No POAF | POAF | p-Value | |
|---|---|---|---|
| N = 620 | N = 339 | ||
| SHBG (nmol/L) | 32.00 [23.7–42.0] | 35.8 [27.8–47.1] | <0.001 |
| NT-proBNP (pg/mL) | 176.8 [76.1–430.2] | 228.3 [96.0–508.2] | 0.012 |
| Cholesterol (mmol/L) | 3.6 [3.0–4.2] | 3.7 [3.2–4.5] | 0.016 |
| Vitamin D (nmol/L) | 45.0 [31.5–60.9] | 49.0 [34.9–63.8] | 0.024 |
| Thrombocytes (×109/L) | 205.0 [173.0–237.0] | 198.0 [169.0–226.0] | 0.038 |
| IGF-1 (nmol/L) | 15.4 [12.0–19.1] | 14.5 [11.7–18.0] | 0.044 |
| Glucose (mmol/L) | 5.9 [5.5–6.8] | 5.81 [5.40, 6.40] | 0.044 |
| IL-6 (pg/mL) | 3.1 [1.9–4.1] | 2.9 [1.9–3.6] | 0.328 |
| Red cell distribution width (%) | 12.9 [12.4–13.4] | 13.0 [12.5–13.5] | 0.070 |
| Reticulocytes (×109/L) | 61.0 [49.0–74.0] | 58.3 [48.5–72.0] | 0.088 |
| Potassium (mmol/L) | 3.9 [3.7–4.1] | 3.9 [3.7–4.1] | 0.181 |
| Sodium (mmol/L) | 139.5 [138.0–141.0] | 139.9 [138.0–141.0] | 0.200 |
| GDF-15 (pg/mL) | 1076.5 [779.5–1660.0] | 1164.0 [858.5–1626.0] | 0.186 |
| Biomarker-Enhanced Model | ||
|---|---|---|
| POAF Score | <0.4 | ≥0.4 |
| <0.4 | 196 | 56 |
| ≥0.4 | 0 | 87 |
| Biomarker-Enhanced Model | ||
|---|---|---|
| POAF Score | <0.4 | ≥0.4 |
| <0.4 | 454 | 82 |
| ≥0.4 | 9 | 75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noordzij, P.G.; Thio, M.S.Y.; Reniers, T.; Dijkstra, I.; Mondelli, G.; Langelaan, M.; Ruven, H.J.T.; Rettig, T.C.D. Improving Prediction of Postoperative Atrial Fibrillation After Cardiac Surgery Using Multiple Pathophysiological Biomarkers: A Prospective Double-Centre Study. J. Clin. Med. 2025, 14, 3737. https://doi.org/10.3390/jcm14113737
Noordzij PG, Thio MSY, Reniers T, Dijkstra I, Mondelli G, Langelaan M, Ruven HJT, Rettig TCD. Improving Prediction of Postoperative Atrial Fibrillation After Cardiac Surgery Using Multiple Pathophysiological Biomarkers: A Prospective Double-Centre Study. Journal of Clinical Medicine. 2025; 14(11):3737. https://doi.org/10.3390/jcm14113737
Chicago/Turabian StyleNoordzij, Peter G., Maaike S. Y. Thio, Ted Reniers, Ineke Dijkstra, Gabriele Mondelli, Marloes Langelaan, Henk J. T. Ruven, and Thijs C. D. Rettig. 2025. "Improving Prediction of Postoperative Atrial Fibrillation After Cardiac Surgery Using Multiple Pathophysiological Biomarkers: A Prospective Double-Centre Study" Journal of Clinical Medicine 14, no. 11: 3737. https://doi.org/10.3390/jcm14113737
APA StyleNoordzij, P. G., Thio, M. S. Y., Reniers, T., Dijkstra, I., Mondelli, G., Langelaan, M., Ruven, H. J. T., & Rettig, T. C. D. (2025). Improving Prediction of Postoperative Atrial Fibrillation After Cardiac Surgery Using Multiple Pathophysiological Biomarkers: A Prospective Double-Centre Study. Journal of Clinical Medicine, 14(11), 3737. https://doi.org/10.3390/jcm14113737







