The Hidden Threat of Microplastics in Traditional Cigarettes: A Narrative Review of Health and Environmental Risks
Abstract
1. Introduction
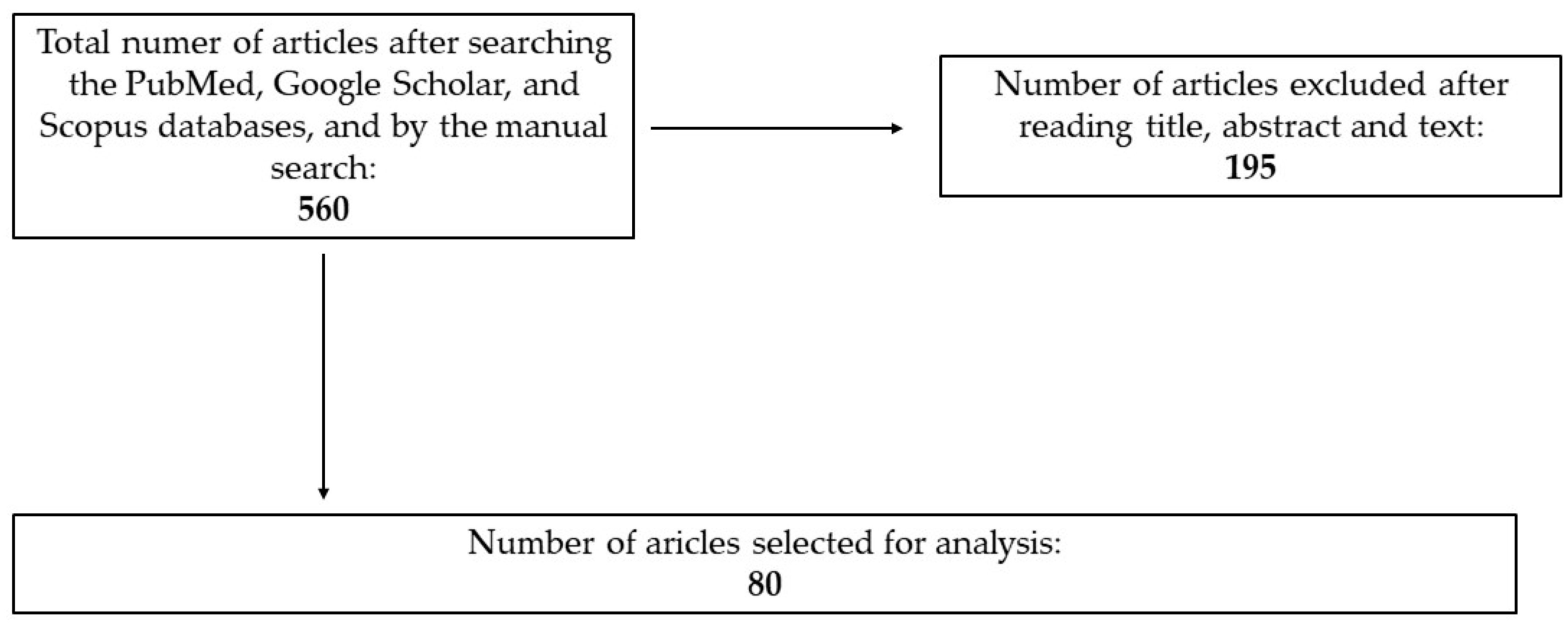
2. Materials and Methods
2.1. Inclusion Criteria
- -
- Timeframe: studies published between 2010 and 2025 to reflect recent scientific findings.
- -
- Relevance: studies addressing microplastics in cigarette filters, their health effects, or their biochemical interactions with human tissues.
- -
- Biological impact: research focusing on oxidative stress, inflammation, and toxicity due to microplastic exposure.
- -
- Regulatory aspects: articles discussing policy and legal measures aimed at mitigating microplastic pollution from smoking products.
- -
- Study design: both human and animal studies investigating microplastic accumulation in tissues (e.g., lungs, blood, placenta).
2.2. Exclusion Criteria
- -
- Non-peer-reviewed sources, editorials, and opinion-based papers.
- -
- Studies focusing solely on general environmental microplastic pollution without human health implications.
- -
- Laboratory-based in vitro studies lacking direct relevance to human exposure.
- -
- Articles with insufficient methodological transparency or those flagged for a high risk of bias.
2.3. Article Selection and Bias Minimization
3. Sources of Microplastic Exposure and Health Implications
3.1. The Gastrointestinal Route
3.2. Inhalation Route
3.3. Cutaneous Route
3.4. Potential Carcinogenic Risks of Microplastic
3.5. Effects of Microplastics on Reproductive Health
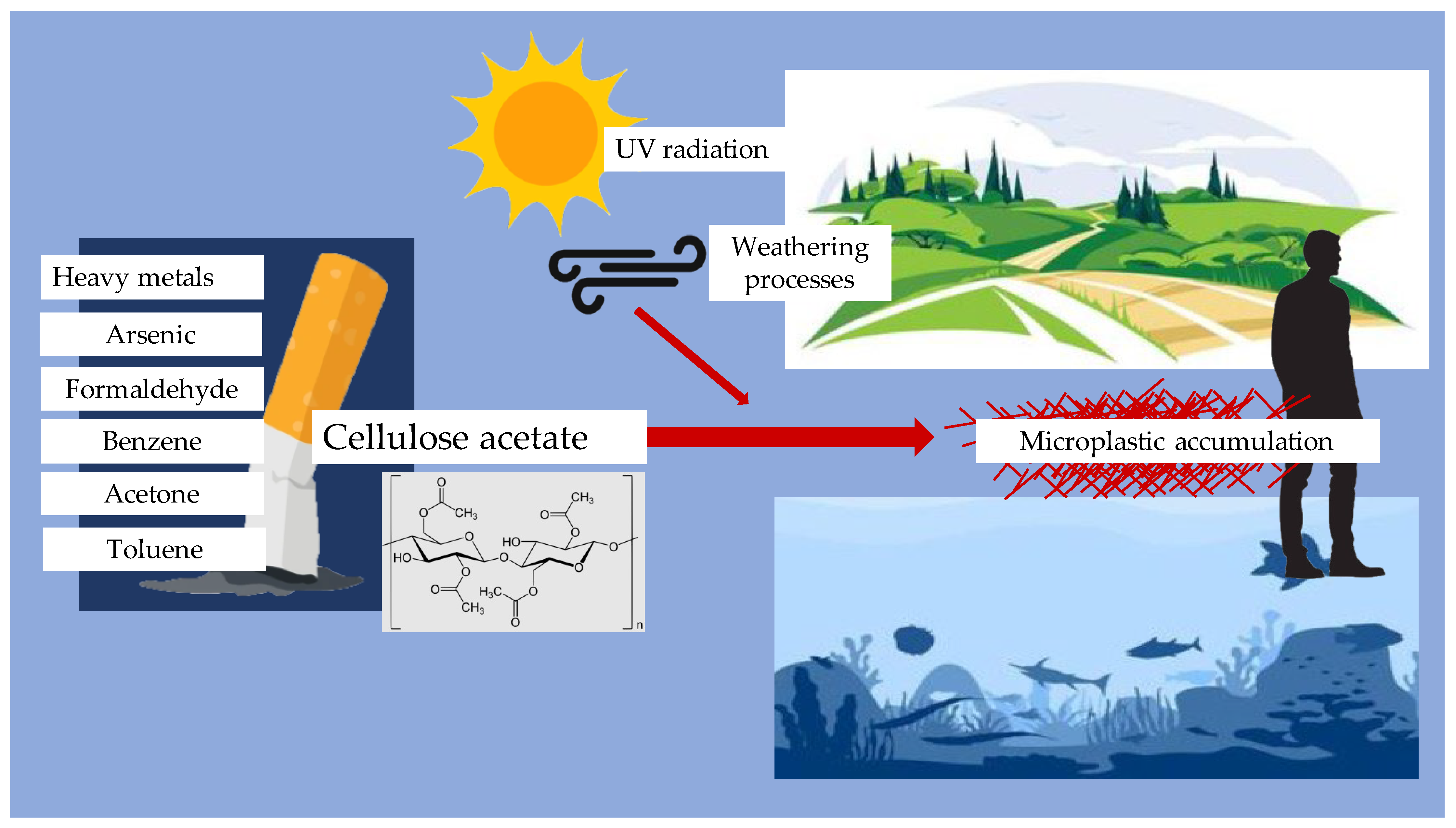
4. Cigarette Filters as Microplastic Sources
5. Effect of Microplastics on Inflammatory Cytokines
6. Effect of Microplastics on Oxidative Stress Parameters
7. Effect of Microplastics on Cells Viability
8. Biomonitoring of Microplastic Exposure
- -
- Flow cytometry, a laser-based method, has been increasingly used for identifying MPs in human fluids. By applying fluorescent staining agents such as Nile red, this approach enables rapid classification of MP particles based on their optical properties [64].
- -
- Pyrolysis-GC/MS, which facilitates detailed compositional analysis of MPs in biological samples such as urine and plasma [65].
- -
- Raman and Fourier-transform infrared (FTIR) spectroscopy, commonly utilized for determining polymer composition in human tissues [66].
- -
- Microscopy-based techniques, including fluorescence and electron microscopy, have been applied to visualize MP accumulation in placenta and breast milk, raising concerns about maternal and fetal exposure [67].
9. Policy and Regulation
- Banning or restricting the use of non-biodegradable cigarette filters: Encouraging the development and adoption of biodegradable alternatives could significantly reduce the amount of plastic waste generated by cigarette butts.
- Implementing Extended Producer Responsibility (EPR) programs: Manufacturers of smoking products could be held accountable for the environmental impact of their products through EPR schemes. This could involve funding waste management programs, recycling initiatives, and public education campaigns.
- Strengthening waste management infrastructure: Improving the availability and accessibility of disposal options for smoking-related waste can help prevent littering and reduce plastic pollution.
- Raising public awareness: Public health campaigns can educate smokers about the environmental and health risks associated with microplastics in smoking products, encouraging responsible disposal practices and reducing tobacco use [70].
10. Discussion
10.1. Inflammatory Response: Conflicting Findings on Cytokine Modulation
10.2. Oxidative Stress Paradox: Increased ROS Production vs. Adaptive Responses
10.3. Cytotoxicity Discrepancies: Material-Dependent Toxicity
10.4. Study Quality Assessment: Standardization Challenges
11. Future Perspectives
12. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AGS cells | Human gastric adenocarcinoma cell line; |
| AGS | Human gastric adenocarcinoma cell line; |
| Caco-2 | Human colorectal adenocarcinoma cell line; |
| GSSG | Glutathione disulfide; |
| GST | Glutathione reductase; |
| H2O2 | Hydrogen peroxide; |
| HaCaT | Spontaneously immortalized human keratinocyte cell line; |
| HEK293 | Human embryonic kidney cell line; |
| HeLa | Epithelial cervical cancer cell line; |
| HMOX1 | Heme oxygenase 1; |
| IL-10 | Interleukin 10; |
| IL-1β | Interleukin beta 1; |
| IL-6 | Interleukin 6; |
| IL-8 | Interleukin 8; |
| INF-y | Interferon gamma; |
| MP | Microplastic; |
| MPO | Myeloperoxidase; |
| NF-κB | Nuclear Factor-Kappa B; |
| PE | Polyethylene; |
| PMMA | Polymethylmethacrylate; |
| RedOx | Reduction–oxidation; |
| ROS | Reactive oxygen species; |
| SOD | Superoxide dismutase; |
| T98G | Glioblastoma cell line; |
| TGF-β1 | Transforming growth factor beta 1; |
| THP-1 | Human leukemia monocytic cell line; |
| TNF-α | Tumor necrosis factor α; |
| U937 | Human pro-monocytic leukemia cell line |
References
- Turner, A.; Scott, J.W.; Backshall-Kennedy, T.; Dabrowski, M.C. Deconstructing contemporary disposable vapes: A material and elemental analysis. Sci. Total Environ. 2024, 954, 176292. [Google Scholar] [CrossRef]
- George, M.; Khadtar, R. Review on Recycling of Microplastics in Cigarette Butts. IOP Conf. Ser. Earth Environ. Sci. 2022, 1084, 012027. [Google Scholar] [CrossRef]
- Soltani, M.; Shahsavani, A.; Hopke, P.K.; Bakhtiarvand, N.A.; Abtahi, M.; Rahmatinia, M.; Kermani, M. Investigating the Inflammatory Effect of Microplastics in Cigarette Butts on Peripheral Blood Mononuclear Cells. Sci. Rep. 2025, 15, 458. [Google Scholar] [CrossRef]
- De Granda-Orive, J.I.; Solano-Reina, S.; Jiménez-Ruiz, C.A. Tobacco as a Source of Microplastics. Tobacco and Environment: World No Tobacco Day 2022. Arch. Bronconeumol. 2022, 58, 395–397. [Google Scholar] [CrossRef]
- Thompson, R.C.; Courtene-Jones, W.; Boucher, J.; Pahl, S.; Raubenheimer, K.; Koelmans, A.A. Twenty Years of Microplastic Pollution Research—What Have We Learned? Science 2024, 386, eadl2746. [Google Scholar] [CrossRef] [PubMed]
- Sapru, S.; Vardhan, M.; Li, Q.; Guo, Y.; Li, X.; Saxena, D. E-Cigarettes Use in the United States: Reasons for Use, Perceptions, and Effects on Health. BMC Public Health 2020, 20, 1518. [Google Scholar] [CrossRef]
- Rom, O.; Pecorelli, A.; Valacchi, G.; Reznick, A.Z. Are E-cigarettes a Safe and Good Alternative to Cigarette Smoking? Ann. N. Y. Acad. Sci. 2015, 1340, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Fadus, M.C.; Smith, T.T.; Squeglia, L.M. The Rise of E-Cigarettes, Pod Mod Devices, and JUUL among Youth: Factors Influencing Use, Health Implications, and Downstream Effects. Drug Alcohol. Depend. 2019, 201, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Znyk, M.; Jurewicz, J.; Kaleta, D. Exposure to Heated Tobacco Products and Adverse Health Effects, a Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 6651. [Google Scholar] [CrossRef]
- Green, D.S.; Tongue, A.D.W.; Boots, B. The Ecological Impacts of Discarded Cigarette Butts. Trends Ecol. Evol. 2022, 37, 183–192. [Google Scholar] [CrossRef]
- De Deus, B.C.T.; Costa, T.C.; Altomari, L.N.; Brovini, E.M.; De Brito, P.S.D.; Cardoso, S.J. Coastal Plastic Pollution: A Global Perspective. Mar. Pollut. Bull. 2024, 203, 116478. [Google Scholar] [CrossRef] [PubMed]
- Belzagui, F.; Buscio, V.; Gutiérrez-Bouzán, C.; Vilaseca, M. Cigarette Butts as a Microfiber Source with a Microplastic Level of Concern. Sci. Total Environ. 2021, 762, 144165. [Google Scholar] [CrossRef]
- Prata, J.C.; Da Costa, J.P.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Environmental Exposure to Microplastics: An Overview on Possible Human Health Effects. Sci. Total Environ. 2020, 702, 134455. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Rehati, P.; Yang, Z.; Cai, Z.; Guo, C.; Li, Y. The Potential Toxicity of Microplastics on Human Health. Sci. Total Environ. 2024, 912, 168946. [Google Scholar] [CrossRef]
- Yang, X.; Man, Y.B.; Wong, M.H.; Owen, R.B.; Chow, K.L. Environmental Health Impacts of Microplastics Exposure on Structural Organization Levels in the Human Body. Sci. Total Environ. 2022, 825, 154025. [Google Scholar] [CrossRef] [PubMed]
- Inam, Ö. Impact of Microplastics on Female Reproductive Health: Insights from Animal and Human Experimental Studies: A Systematic Review. Arch. Gynecol. Obstet. 2025. [CrossRef]
- Pletz, M. Ingested Microplastics: Do Humans Eat One Credit Card per Week? J. Hazard. Mater. Lett. 2022, 3, 100071. [Google Scholar] [CrossRef]
- Horvatits, T.; Tamminga, M.; Liu, B.; Sebode, M.; Carambia, A.; Fischer, L.; Püschel, K.; Huber, S.; Fischer, E.K. Microplastics Detected in Cirrhotic Liver Tissue. eBioMedicine 2022, 82, 104147. [Google Scholar] [CrossRef]
- Waszkiewicz, N.; Jelski, W.; Zalewska, A.; Szulc, A.; Szmitkowski, M.; Zwierz, K.; Szajda, S.D. Salivary Alcohol Dehydrogenase in Non-Smoking and Smoking Alcohol-Dependent Persons. Alcohol 2014, 48, 611–616. [Google Scholar] [CrossRef]
- Wang, Y.-F.; Wang, X.-Y.; Chen, B.-J.; Yang, Y.-P.; Li, H.; Wang, F. Impact of Microplastics on the Human Digestive System: From Basic to Clinical. World J. Gastroenterol. 2025, 31. [Google Scholar] [CrossRef]
- Wang, X.; Deng, K.; Zhang, P.; Chen, Q.; Magnuson, J.T.; Qiu, W.; Zhou, Y. Microplastic-Mediated New Mechanism of Liver Damage: From the Perspective of the Gut-Liver Axis. Sci. Total Environ. 2024, 919, 170962. [Google Scholar] [CrossRef]
- Saha, S.C.; Saha, G. Effect of Microplastics Deposition on Human Lung Airways: A Review with Computational Benefits and Challenges. Heliyon 2024, 10, e24355. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Jia, Z. Recent Insights into Uptake, Toxicity, and Molecular Targets of Microplastics and Nanoplastics Relevant to Human Health Impacts. iScience 2023, 26, 106061. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Zhan, D.; Fang, Y.; Li, L.; Chen, G.; Chen, S.; Wang, L. Microplastics, Potential Threat to Patients with Lung Diseases. Front. Toxicol. 2022, 4, 958414. [Google Scholar] [CrossRef]
- Goodman, K.E.; Hare, J.T.; Khamis, Z.I.; Hua, T.; Sang, Q.-X.A. Exposure of Human Lung Cells to Polystyrene Microplastics Significantly Retards Cell Proliferation and Triggers Morphological Changes. Chem. Res. Toxicol. 2021, 34, 1069–1081. [Google Scholar] [CrossRef] [PubMed]
- Jenner, L.C.; Rotchell, J.M.; Bennett, R.T.; Cowen, M.; Tentzeris, V.; Sadofsky, L.R. Detection of Microplastics in Human Lung Tissue Using μFTIR Spectroscopy. Sci. Total Environ. 2022, 831, 154907. [Google Scholar] [CrossRef]
- Tomonaga, T.; Higashi, H.; Izumi, H.; Nishida, C.; Kawai, N.; Sato, K.; Morimoto, T.; Higashi, Y.; Yatera, K.; Morimoto, Y. Investigation of Pulmonary Inflammatory Responses Following Intratracheal Instillation of and Inhalation Exposure to Polypropylene Microplastics. Part Fibre Toxicol 2024, 21, 29. [Google Scholar] [CrossRef]
- Aristizabal, M.; Jiménez-Orrego, K.V.; Caicedo-León, M.D.; Páez-Cárdenas, L.S.; Castellanos-García, I.; Villalba-Moreno, D.L.; Ramírez-Zuluaga, L.V.; Hsu, J.T.S.; Jaller, J.; Gold, M. Microplastics in Dermatology: Potential Effects on Skin Homeostasis. J. Cosmet. Dermatol. 2024, 23, 766–772. [Google Scholar] [CrossRef]
- Amado Enrique, N.-F.; Marelis, P.-L.; Paula Montserrat, C.-B. Human Skin and Micro- and Nanoplastics: A Mini-Review. MOJ Eco Environ. Sci. 2024, 9, 122–125. [Google Scholar] [CrossRef]
- Dzierżyński, E.; Gawlik, P.J.; Puźniak, D.; Flieger, W.; Jóźwik, K.; Teresiński, G.; Forma, A.; Wdowiak, P.; Baj, J.; Flieger, J. Microplastics in the Human Body: Exposure, Detection, and Risk of Carcinogenesis: A State-of-the-Art Review. Cancers 2024, 16, 3703. [Google Scholar] [CrossRef]
- Kumar, N.; Lamba, M.; Pachar, A.K.; Yadav, S.; Acharya, A. Microplastics—A Growing Concern as Carcinogens in Cancer Etiology: Emphasis on Biochemical and Molecular Mechanisms. Cell Biochem. Biophys. 2024, 82, 3109–3121. [Google Scholar] [CrossRef]
- Deng, X.; Gui, Y.; Zhao, L. The Micro(Nano)Plastics Perspective: Exploring Cancer Development and Therapy. Mol. Cancer 2025, 24, 30. [Google Scholar] [CrossRef]
- Wang, M.; Wu, Y.; Li, G.; Xiong, Y.; Zhang, Y.; Zhang, M. The Hidden Threat: Unraveling the Impact of Microplastics on Reproductive Health. Sci. Total Environ. 2024, 935, 173177. [Google Scholar] [CrossRef] [PubMed]
- Zurub, R.E.; Cariaco, Y.; Wade, M.G.; Bainbridge, S.A. Microplastics Exposure: Implications for Human Fertility, Pregnancy and Child Health. Front. Endocrinol. 2024, 14, 1330396. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, J.; Ma, S.; Sun, Z.; Wang, Z. Microplastics May Be a Significant Cause of Male Infertility. Am. J. Mens. Health 2022, 16, 15579883221096549. [Google Scholar] [CrossRef]
- Hong, Y.; Wu, S.; Wei, G. Adverse Effects of Microplastics and Nanoplastics on the Reproductive System: A Comprehensive Review of Fertility and Potential Harmful Interactions. Sci. Total Environ. 2023, 903, 166258. [Google Scholar] [CrossRef] [PubMed]
- Balali, H.; Morabbi, A.; Karimian, M. Concerning Influences of Micro/Nano Plastics on Female Reproductive Health: Focusing on Cellular and Molecular Pathways from Animal Models to Human Studies. Reprod. Biol. Endocrinol. 2024, 22, 141. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, S.; Meccariello, R. Microplastics: A Threat for Male Fertility. Int. J. Environ. Res. Public Health 2021, 18, 2392. [Google Scholar] [CrossRef]
- Liu, W.; Qu, Z.; Wang, X.; Feng, H.; Ma, S.; Zheng, Y.; Lin, G.; Huang, S.; Yang, Q.; Feng, X.; et al. Microplastic Exposure Is Associated with Male Reproductive Health. Med. Rev. 2024, 4, 549–552. [Google Scholar] [CrossRef]
- Dobaradaran, S.; Nabipour, I.; Saeedi, R.; Ostovar, A.; Khorsand, M.; Khajeahmadi, N.; Hayati, R.; Keshtkar, M. Association of Metals (Cd, Fe, As, Ni, Cu, Zn and Mn) with Cigarette Butts in Northern Part of the Persian Gulf. Tob. Control 2017, 26, 461–463. [Google Scholar] [CrossRef]
- Green, D.S.; Kregting, L.; Boots, B. Smoked Cigarette Butt Leachate Impacts Survival and Behaviour of Freshwater Invertebrates. Environ. Pollut. 2020, 266, 115286. [Google Scholar] [CrossRef] [PubMed]
- Rebischung, F.; Chabot, L.; Biaudet, H.; Pandard, P. Cigarette Butts: A Small but Hazardous Waste, According to European Regulation. Waste Manag. 2018, 82, 9–14. [Google Scholar] [CrossRef]
- Branka, B.; Sever Škapin, A. Weathering Effects on Cellulose Acetate Microplastics from Discarded Cigarette Butts. In Proceedings of the Socratic Lectures 10—Part I, Ljubljana, Slovenia, 9 December 2023; University of Lubljana Press: Ljubljana, Slovenia, 2024; pp. 168–174. [Google Scholar]
- Chevalier, Q.; El Hadri, H.; Petitjean, P.; Bouhnik-Le Coz, M.; Reynaud, S.; Grassl, B.; Gigault, J. Nano-Litter from Cigarette Butts: Environmental Implications and Urgent Consideration. Chemosphere 2018, 194, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Kasza, K.A.; Edwards, K.C.; Anesetti-Rothermel, A.; Creamer, M.R.; Cummings, K.M.; Niaura, R.S.; Sharma, A.; Pitts, S.R.; Head, S.K.; Everard, C.D.; et al. E-Cigarette Use and Change in Plans to Quit Cigarette Smoking among Adult Smokers in the United States: Longitudinal Findings from the PATH Study 2014–2019. Addict. Behav. 2022, 124, 107124. [Google Scholar] [CrossRef]
- Weber, A.; Schwiebs, A.; Solhaug, H.; Stenvik, J.; Nilsen, A.M.; Wagner, M.; Relja, B.; Radeke, H.H. Nanoplastics Affect the Inflammatory Cytokine Release by Primary Human Monocytes and Dendritic Cells. Environ. Int. 2022, 163, 107173. [Google Scholar] [CrossRef]
- Prietl, B.; Meindl, C.; Roblegg, E.; Pieber, T.; Lanzer, G.; Fröhlich, E. Nano-sized and micro-sized polystyrene particles affect phagocyte function. Cell Biol. Toxicol. 2014, 30, 1–16. [Google Scholar] [CrossRef]
- Kwon, W.; Kim, D.; Kim, H.-Y.; Jeong, S.W.; Lee, S.-G.; Kim, H.-C.; Lee, Y.-J.; Kwon, M.K.; Hwang, J.-S.; Han, J.E. Microglial phagocytosis of polystyrene microplastics results in immune alteration and apoptosis in vitro and in vivo. Sci. Total Environ. 2022, 807, 150817. [Google Scholar] [CrossRef]
- Zhang, M.; Shi, J.; Huang, Q.; Xie, Y.; Wu, R.; Zhong, J.; Deng, H. Multi-omics analysis reveals size-dependent toxicity and vascular endothelial cell injury induced by microplastic exposure in vivo and in vitro. Environ. Sci. Nano 2022, 9, 663–683. [Google Scholar] [CrossRef]
- da Silva Brito, W.A.; Singer, D.; Miebach, L.; Saadati, F.; Wende, K.; Schmidt, A.; Bekeschus, S. Comprehensive in vitro polymer type, concentration, and size correlation analysis to microplastic toxicity and inflammation. Sci. Total Environ. 2023, 854, 158731. [Google Scholar] [CrossRef]
- KC, P.B.; Maharjan, A.; Acharya, M.; Lee, D.; Kusma, S.; Gautam, R.; Kwon, J.-T.; Kim, C.; Kim, K.; Kim, H. Polytetrafluorethylene microplastic particles mediated oxidative stress, inflammation, and intracellular signaling pathway alteration in human derived cell lines. Sci. Total Environ. 2023, 897, 165295. [Google Scholar]
- Forte, M.; Iachetta, G.; Tussellino, M.; Carotenuto, R.; Prisco, M.; De Falco, M.; Laforgia, V.; Valiante, S. Polystyrene nanoparticles internalization in human gastric adenocarcinoma cells. Toxicology 2016, 31, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Shi, W.; Hu, F.; Song, X.; Cheng, Z.; Zhou, J. Prolonged Oral Ingestion of Microplastics Induced Inflammation in the Liver Tissues of C57BL/6J Mice through Polarization of Macrophages and Increased Infiltration of Natural Killer Cells. Ecotoxicol. Environ. Saf. 2021, 227, 112882. [Google Scholar] [CrossRef]
- Wang, Q.; Bai, J.; Ning, B.; Fan, L.; Sun, T.; Fang, Y.; Wu, J.; Li, S.; Duan, C.; Zhang, Y.; et al. Effects of Bisphenol A and Nanoscale and Microscale Polystyrene Plastic Exposure on Particle Uptake and Toxicity in Human Caco-2 Cells. Chemosphere 2020, 254, 126788. [Google Scholar] [CrossRef]
- Wu, B.; Wu, X.; Liu, S.; Wang, Z.; Chen, L. Size-Dependent Effects of Polystyrene Microplastics on Cytotoxicity and Efflux Pump Inhibition in Human Caco-2 Cells. Chemosphere 2019, 221, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Schirinzi, G.F.; Pérez-Pomeda, I.; Sanchís, J.; Rossini, C.; Farré, M.; Barceló, D. Cytotoxic Effects of Commonly Used Nanomaterials and Microplastics on Cerebral and Epithelial Human Cells. Environ. Res. 2017, 159, 579–587. [Google Scholar] [CrossRef]
- Saenen, N.D.; Witters, M.S.; Hantoro, I.; Tejeda, I.; Ethirajan, A.; Van Belleghem, F.; Smeets, K. Polystyrene Microplastics of Varying Sizes and Shapes Induce Distinct Redox and Mitochondrial Stress Responses in a Caco-2 Monolayer. Antioxidants 2023, 12, 739. [Google Scholar] [CrossRef]
- Salimi, A.; Alavehzadeh, A.; Ramezani, M.; Pourahmad, J. Differences in Sensitivity of Human Lymphocytes and Fish Lymphocytes to Polyvinyl Chloride Microplastic Toxicity. Toxicol. Ind. Health 2022, 38, 100–111. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Chen, K.-F.; Lin, K.-Y.A.; Chen, J.-K.; Jiang, X.-Y.; Lin, C.-H. The Nephrotoxic Potential of Polystyrene Microplastics at Realistic Environmental Concentrations. J. Hazard. Mater. 2022, 427, 127871. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Li, X.; Zhou, Y.; Yu, H.; Xie, Y.; Guo, H.; Wang, H.; Li, Y.; Feng, Y.; Wang, Y. Polystyrene microplastics induce hepatotoxicity and disrupt lipid metabolism in the liver organoids. Sci. Total Environ. 2022, 806, 150328. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, Q.; Li, Y.; Feng, Y.; Wang, Y.; Cheng, W. Low-Dose of Polystyrene Microplastics Induce Cardiotoxicity in Mice and Human-Originated Cardiac Organoids. Environ. Int. 2023, 179, 108171. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, S.; Olga, V.; Xue, Y.; Lv, S.; Diao, X.; Zhang, Y.; Han, Q.; Zhou, H. The Potential Effects of Microplastic Pollution on Human Digestive Tract Cells. Chemosphere 2022, 291, 132714. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.; Love, D.C.; Rochman, C.M.; Neff, R.A. Microplastics in Seafood and the Implications for Human Health. Curr. Envir Health Rep. 2018, 5, 375–386. [Google Scholar] [CrossRef]
- Li, J.; Huang, F.; Zhang, G.; Zhang, Z.; Zhang, X. Separation and Flow Cytometry Analysis of Microplastics and Nanoplastics. Front. Chem. 2023, 11, 1201734. [Google Scholar] [CrossRef]
- Schwabl, P.; Köppel, S.; Königshofer, P.; Bucsics, T.; Trauner, M.; Reiberger, T.; Liebmann, B. Detection of Various Microplastics in Human Stool: A Prospective Case Series. Ann. Intern. Med. 2019, 171, 453–457. [Google Scholar] [CrossRef]
- Jenner, L.C.; Sadofsky, L.R.; Danopoulos, E.; Chapman, E.; White, D.; Jenkins, R.L.; Rotchell, J.M. Outdoor Atmospheric Microplastics within the Humber Region (United Kingdom): Quantification and Chemical Characterisation of Deposited Particles Present. Atmosphere 2022, 13, 265. [Google Scholar] [CrossRef]
- Ragusa, A.; Svelato, A.; Santacroce, C.; Catalano, P.; Notarstefano, V.; Carnevali, O.; Papa, F.; Rongioletti, M.C.A.; Baiocco, F.; Draghi, S.; et al. Plasticenta: First Evidence of Microplastics in Human Placenta. Environ. Int. 2021, 146, 106274. [Google Scholar] [CrossRef]
- Borgatta, M.; Breider, F. Inhalation of Microplastics—A Toxicological Complexity. Toxics 2024, 12, 358. [Google Scholar] [CrossRef] [PubMed]
- Leslie, H.A.; Van Velzen, M.J.M.; Brandsma, S.H.; Vethaak, A.D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and Quantification of Plastic Particle Pollution in Human Blood. Environ. Int. 2022, 163, 107199. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.who.int/news-room/fact-sheets/detail/tobacco (accessed on 19 May 2025).
- Williams, A.T.; Rangel-Buitrago, N. The Past, Present, and Future of Plastic Pollution. Mar. Pollut. Bull. 2022, 176, 113429. [Google Scholar] [CrossRef]
- Wang, Q.-Y.; Wang, Q.-R.; Wang, T.-Y.; Zhang, S.-Q.; Yu, H.-W. Impacts of Polypropylene Microplastics on the Distribution of Cadmium, Enzyme Activities, and Bacterial Community in Black Soil at the Aggregate Level. Sci. Total Environ. 2024, 917, 170541. [Google Scholar] [CrossRef]
- Śniadach, J.; Kicman, A.; Michalska-Falkowska, A.; Jończyk, K.; Waszkiewicz, N. Changes in Concentration of Selected Biomarkers of Exposure in Users of Classic Cigarettes, E-Cigarettes, and Heated Tobacco Products—A Narrative Review. Int. J. Mol. Sci. 2025, 26, 1796. [Google Scholar] [CrossRef] [PubMed]
- Bajt, O. From Plastics to Microplastics and Organisms. FEBS Open Bio 2021, 11, 954–966. [Google Scholar] [CrossRef]
- Guo, K.; Zhou, L.; Shang, X.; Yang, C.; E, F.; Wang, Y.; Xu, M.; Wu, Y.; Li, Y.; Li, M.; et al. Varenicline and Related Interventions on Smoking Cessation: A Systematic Review and Network Meta-Analysis. Drug Alcohol. Depend. 2022, 241, 109672. [Google Scholar] [CrossRef] [PubMed]
- Kolovelonis, A.; Goudas, M.; Theodorakis, Y. Examining the Effectiveness of the Smoking Prevention Program “I Do Not Smoke, I Exercise” in Elementary and Secondary School Settings. Health Promot. Pract. 2016, 17, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Czoli, C.D.; White, C.M.; Reid, J.L.; OConnor, R.J.; Hammond, D. Awareness and Interest in IQOS Heated Tobacco Products among Youth in Canada, England and the USA. Tob. Control 2020, 29, 89–95. [Google Scholar] [CrossRef]
- Bekki, K.; Inaba, Y.; Uchiyama, S.; Kunugita, N. Comparison of Chemicals in Mainstream Smoke in Heat-Not-Burn Tobacco and Combustion Cigarettes. J. UOEH 2017, 39, 201–207. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, Y.; Lemos, B.; Ren, H. Tissue Accumulation of Microplastics in Mice and Biomarker Responses Suggest Widespread Health Risks of Exposure. Sci. Rep. 2017, 7, 46687. [Google Scholar] [CrossRef]
- Durazzo, T.C.; Meyerhoff, D.J.; Nixon, S.J. Chronic cigarette smoking: Implications for neurocognition and brain neurobiology. Int. J. Environ. Res. Public Health 2010, 7, 3760–3791. [Google Scholar] [CrossRef]
- Prüst, M.; Meijer, J.; Westerink, R.H.S. The Plastic Brain: Neurotoxicity of Micro- and Nanoplastics. Part. Fibre Toxicol. 2020, 17, 24. [Google Scholar] [CrossRef]
- Smith, J.; Patel, R.; Brown, K. Microplastic accumulation in the brain and its potential neurotoxic effects. Environ. Res. 2023, 225, 115712. [Google Scholar]
- Garcia, M.; Campen, M. Microplastics in the human brain: Emerging risks and neurotoxic effects. Nat. Med. 2025, 231, 112345. [Google Scholar]

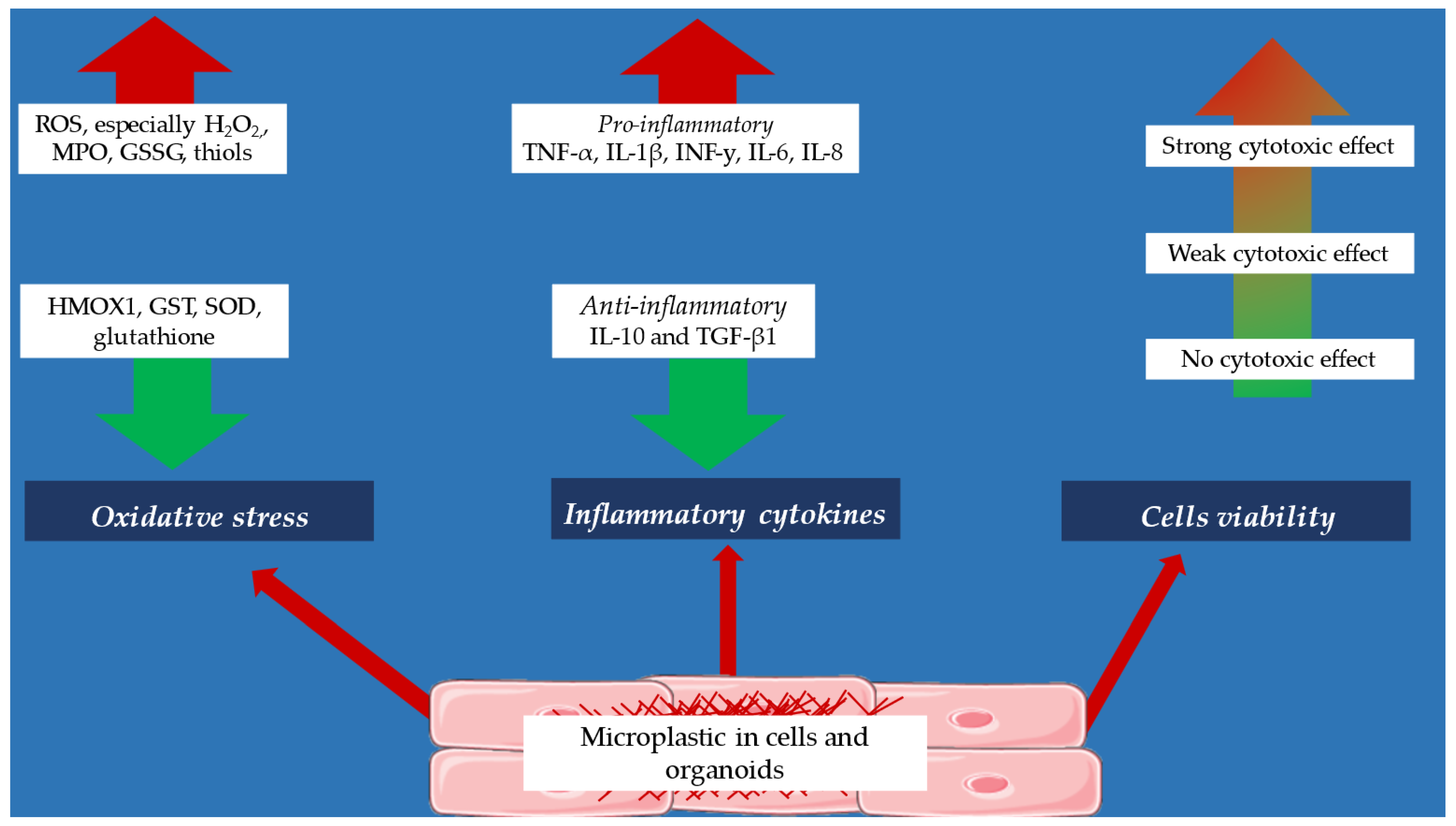
| Effect of micropastics on inflammatory cytokines |
| ↑ produce of IL-6, TNF-α, IL-8, IL-10 (monocytes, dendritic cells) [44,45] |
| ↑ produce of IL-6, TNF-α, IL-8, IL-10 (epithelial cells) [47] |
| ↑ produce of TNF-α, IL-1β, INF-y, IL-6, IL-8 (cancer cell lines—HeLa, A549, AGS, THP-1, HaCaT, U937 [49,50] |
| ↑ produce of TNF-α, IL-1β, INF-y, IL-6, IL-8 (kidney of a human embryo) [50] |
| ↑ TNF-α, IL-6, IL-1β expression (microglia) [46] |
| ↓ TGF-β and IL-10 expression (microglia) [46] |
| ↑ IFN-γ, TNF-α, IL-1β, IL-6, and IL-33 expression (liver tissue) [53] |
| ↓ IL-4, IL-5, IL-10, IL-18, and TGF-β1 expression (liver tissue) [53] |
| Effect of micropastics on oxidative stress parameters |
| ↑ produce of ROS (cancer cell lines—U937 cells, THP-1, A549, HaCaT, Caco-2 and HeLa cells [50,53,54,55] |
| ↑↑ produce of H202 (Caco-2 cells) [54] |
| ↑ produce of nitric oxide (monocytes) [45] |
| ↑ amount of thiol groups (kidney of a human embryo) [48] |
| ↓ amount of glutathione (lymphocytes, liver and heart organoids) [57,59,60] |
| ↑ production of myeloperoxidase (monocytes, dendritic cells) [45] |
| ↑ glutathione disulfide activity (lymphocytes) [58] |
| ↓ heme oxygenase activity (HEK293 cells) [58] |
| ↓ glutathione reductase activity (liver organoids) [60] |
| ↑ superoxide dismutase activity (liver and hearts organoids) [59,60] |
| Effect of micropastics on cells viability |
| -induction of apoptosis and necrosis (cancer cell lines, human-originated cardiac organoids) [48,59,60] |
| -weak cytotoxicity effect (cancer cell lines—A549, HEK293, HeLa, AGS) [54,56] |
| -weak cytotoxic effect in concentration (kidney of a human embryo) [50] |
| -no cytotoxic effect (U937 cells, THP-1, A549, HaCaT, Caco-2, T98G and HeLa cells) [55,61] |
| -cytotoxic effect (lymphocytes) [64] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Śniadach, J.; Kicman, A.; Szymkowiak, S.; Waszkiewicz, N. The Hidden Threat of Microplastics in Traditional Cigarettes: A Narrative Review of Health and Environmental Risks. J. Clin. Med. 2025, 14, 3721. https://doi.org/10.3390/jcm14113721
Śniadach J, Kicman A, Szymkowiak S, Waszkiewicz N. The Hidden Threat of Microplastics in Traditional Cigarettes: A Narrative Review of Health and Environmental Risks. Journal of Clinical Medicine. 2025; 14(11):3721. https://doi.org/10.3390/jcm14113721
Chicago/Turabian StyleŚniadach, Justyna, Aleksandra Kicman, Sylwia Szymkowiak, and Napoleon Waszkiewicz. 2025. "The Hidden Threat of Microplastics in Traditional Cigarettes: A Narrative Review of Health and Environmental Risks" Journal of Clinical Medicine 14, no. 11: 3721. https://doi.org/10.3390/jcm14113721
APA StyleŚniadach, J., Kicman, A., Szymkowiak, S., & Waszkiewicz, N. (2025). The Hidden Threat of Microplastics in Traditional Cigarettes: A Narrative Review of Health and Environmental Risks. Journal of Clinical Medicine, 14(11), 3721. https://doi.org/10.3390/jcm14113721







