Evaluation of Vitamin D and of Some Biomarkers of Bone Remodelling (CTX-1, Osteocalcin, BALP) in Subjects with Periapical Inflammatory Cysts: An Observational Study
Abstract
1. Introduction
2. Materials and Methods
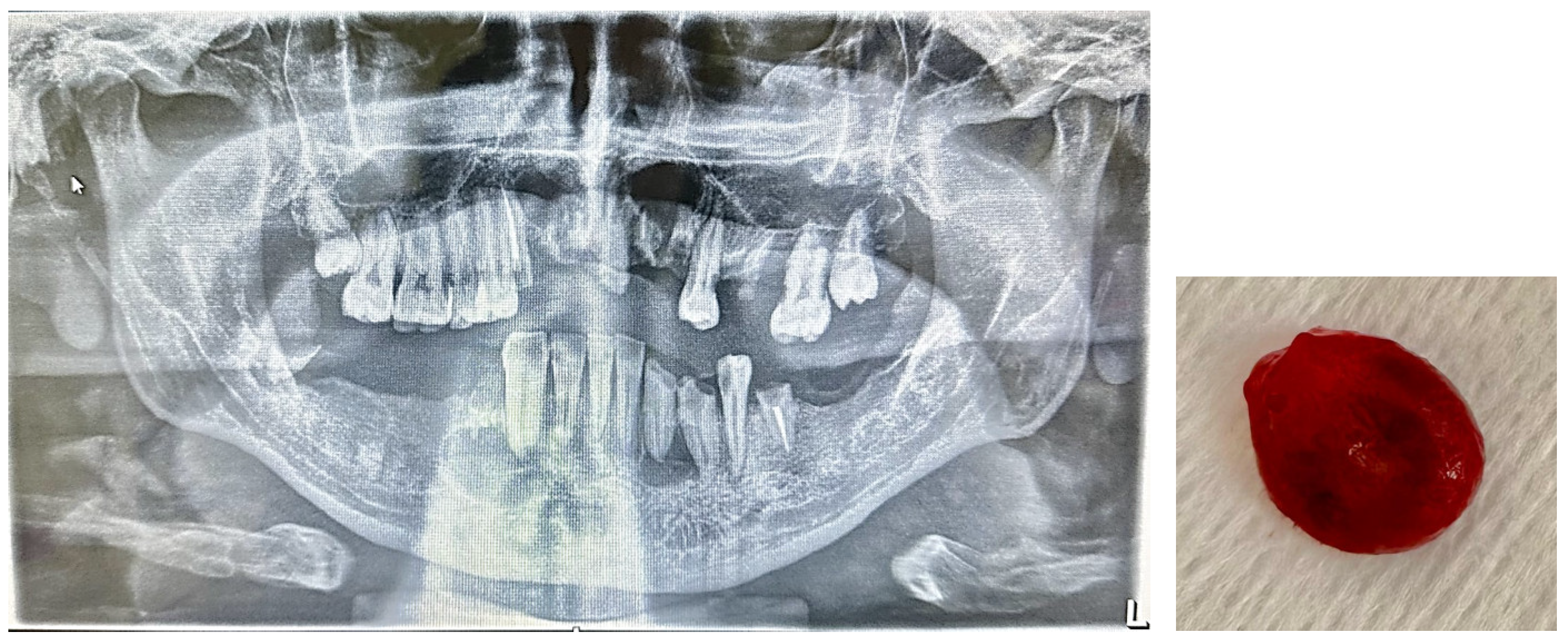
2.1. Study Design and Participants
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Sample Management
2.5. Analytical Determination
2.6. Statistical Analyses
3. Results
4. Discussion
- -
- promote alveolar bone loss by activating an excessive pro-inflammatory response and reversing the OPG/RANKL ratio [46];
- -
- -
- activate T lymphocytes capable of producing cytokines that modulate the nature of the host immune response [49].
Limit of Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Braz-Silva, P.H.; Bergamini, M.L.; Mardegan, A.P.; De Rosa, C.S.; Hasseus, B.; Jonasson, P. Inflammatory profile of chronic apical periodontitis: A literature review. Acta Odontol. Scand. 2019, 77, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Arias, Z.; Nizami, M.Z.I.; Chen, X.; Chai, X.; Xu, B.; Kuang, C.; Omori, K.; Takashiba, S. Recent Advances in Apical Periodontitis Treatment: A Narrative Review. Bioengineering 2023, 10, 488. [Google Scholar] [CrossRef] [PubMed]
- Lu, E.M. The role of vitamin D in periodontal health and disease. J. Periodontal Res. 2023, 58, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Rios Osorio, N.; Caviedes-Bucheli, J.; Mosquera-Guevara, L.; Adames-Martinez, J.S.; Gomez-Pinto, D.; Jimenez-Jimenez, K.; Avendano Maz, H.; Bornacelly-Mendoza, S. The Paradigm of the Inflammatory Radicular Cyst: Biological Aspects to be Considered. Eur. Endod. J. 2023, 8, 20–36. [Google Scholar] [CrossRef]
- Roi, A.; Roi, C.; Negrutiu, M.L.; Rusu, L.C.; Rivis, M. Mesenchymal Stem Cells Derived from Human Periapical Cysts and Their Implications in Regenerative Medicine. Biomedicines 2023, 11, 2436. [Google Scholar] [CrossRef]
- Brown, S.J.; Conn, B.I. Odontogenic cysts: Classification, histological features and a practical approach to common diagnostic problems. Diagn. Histopathol. 2022, 28, 253–266. [Google Scholar] [CrossRef]
- Avelar, R.L.; Antunes, A.A.; Carvalho, R.W.; Bezerra, P.G.; Oliveira Neto, P.J.; Andrade, E.S. Odontogenic cysts: A clinicopathological study of 507 cases. J. Oral. Sci. 2009, 51, 581–586. [Google Scholar] [CrossRef]
- Bhaskar, S.N. Nonsurgical resolution of radicular cysts. Oral. Surg. Oral. Med. Oral. Pathol. 1972, 34, 458–468. [Google Scholar] [CrossRef]
- Loushine, R.J.; Weller, R.N.; Bellizzi, R.; Kulild, J.C. A 2-day decompression: A case report of a maxillary first molar. J. Endod. 1991, 17, 85–87. [Google Scholar] [CrossRef]
- Martin, S.A. Conventional endodontic therapy of upper central incisor combined with cyst decompression: A case report. J. Endod. 2007, 33, 753–757. [Google Scholar] [CrossRef]
- Hoen, M.M.; LaBounty, G.L.; Strittmatter, E.J. Conservative treatment of persistent periradicular lesions using aspiration and irrigation. J. Endod. 1990, 16, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Sjögren, U.; Figdor, D.; Spångberg, L.; Sundqvist, G. The antimicrobial effect of calcium hydroxide as a short-term intracanal dressing. Int. Endod. J. 1991, 24, 119–125. [Google Scholar] [CrossRef]
- Leonardo, M.R.; da Silva, L.A.; Leonardo Rde, T.; Utrilla, L.S.; Assed, S. Histological evaluation of therapy using a calcium hydroxide dressing for teeth with incompletely formed apices and periapical lesions. J. Endod. 1993, 19, 348–352. [Google Scholar] [CrossRef]
- Hoshino, E. LSTR 3Mix-MP method-better and efficient clinical procedures of lesion sterilization and tissue repair (LSTR) therapy. Dent. Rev. 1998, 666, 57–106. [Google Scholar]
- Metzger, Z.; Huber, R.; Slavescu, D.; Dragomirescu, D.; Tobis, I.; Better, H. Healing kinetics of periapical lesions enhanced by the apexum procedure: A clinical trial. J. Endod. 2009, 35, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Takushige, T.; Cruz, E.V.; Asgor Moral, A.; Hoshino, E. Endodontic treatment of primary teeth using a combination of antibacterial drugs. Int. Endod. J. 2004, 37, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.; de Ataide, I. Nonsurgical management of periapical lesions. J. Conserv. Dent. 2010, 13, 240–245. [Google Scholar] [CrossRef]
- Teixeira-Salum, T.B.; Rodrigues, D.B.; Gervasio, A.M.; Souza, C.J.; Rodrigues, V., Jr.; Loyola, A.M. Distinct Th1, Th2 and Treg cytokines balance in chronic periapical granulomas and radicular cysts. J. Oral. Pathol. Med. 2010, 39, 250–256. [Google Scholar] [CrossRef]
- Brescia, V.; Cazzolla, A.P.; Fontana, A.; Varraso, L.; Capobianco, C.; Lovero, R.; Lo Muzio, L.; Dioguardi, M.; Faienza, M.F.; Crincoli, V. Bone Biomarkers Measured on Salivary Matrix: Study of Biological Variability in a Cohort of Young Subjects. Appl. Sci. 2023, 13, 10234. [Google Scholar] [CrossRef]
- Kuo, T.R.; Chen, C.H. Bone biomarker for the clinical assessment of osteoporosis: Recent developments and future perspectives. Biomark. Res. 2017, 5, 18. [Google Scholar] [CrossRef]
- Gaudio, A.; Xourafa, A.; Rapisarda, R.; Zanoli, L.; Signorelli, S.S.; Castellino, P. Hematological Diseases and Osteoporosis. Int. J. Mol. Sci. 2020, 21, 3538. [Google Scholar] [CrossRef] [PubMed]
- Cannalire, G.; Pilloni, S.; Esposito, S.; Biasucci, G.; Di Franco, A.; Street, M.E. Alkaline phosphatase in clinical practice in childhood: Focus on rickets. Front. Endocrinol. 2023, 14, 1111445. [Google Scholar] [CrossRef] [PubMed]
- Nizet, A.; Cavalier, E.; Stenvinkel, P.; Haarhaus, M.; Magnusson, P. Bone alkaline phosphatase: An important biomarker in chronic kidney disease—Mineral and bone disorder. Clin. Chim. Acta 2020, 501, 198–206. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Chen, L.R.; Chen, K.H. Osteoporosis in Patients with Chronic Kidney Diseases: A Systemic Review. Int. J. Mol. Sci. 2020, 21, 6846. [Google Scholar] [CrossRef]
- Nakamura, M.; Slots, J. Salivary enzymes. Origin and relationship to periodontal disease. J. Periodontal Res. 1983, 18, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Beklen, A.; Al-Samadi, A.; Konttinen, Y. Expression of cathepsin K in periodontitis and in gingival fibroblasts. Oral. Dis. 2015, 21, 163–169. [Google Scholar] [CrossRef]
- Chavassieux, P.; Portero-Muzy, N.; Roux, J.P.; Garnero, P.; Chapurlat, R. Are Biochemical Markers of Bone Turnover Representative of Bone Histomorphometry in 370 Postmenopausal Women? J. Clin. Endocrinol. Metab. 2015, 100, 4662–4668. [Google Scholar] [CrossRef]
- Zarei, A.; Morovat, A.; Javaid, K.; Brown, C.P. Vitamin D receptor expression in human bone tissue and dose-dependent activation in resorbing osteoclasts. Bone Res. 2016, 4, 16030. [Google Scholar] [CrossRef]
- Grant, W.B. Vitamin D, periodontal disease, tooth loss, and cancer risk. Lancet Oncol. 2008, 9, 612–613. [Google Scholar] [CrossRef]
- Scardina, G.A.; Messina, P. Good oral health and diet. J. Biomed. Biotechnol. 2012, 2012, 720692. [Google Scholar] [CrossRef]
- Chauss, D.; Freiwald, T.; McGregor, R.; Yan, B.; Wang, L.; Nova-Lamperti, E.; Kumar, D.; Zhang, Z.; Teague, H.; West, E.E.; et al. Autocrine vitamin D signaling switches off pro-inflammatory programs of T(H)1 cells. Nat. Immunol. 2022, 23, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Pavlesen, S.; Mai, X.; Wactawski-Wende, J.; LaMonte, M.J.; Hovey, K.M.; Genco, R.J.; Millen, A.E. Vitamin D Status and Tooth Loss in Postmenopausal Females: The Buffalo Osteoporosis and Periodontal Disease (OsteoPerio) Study. J. Periodontol. 2016, 87, 852–863. [Google Scholar] [CrossRef]
- Brunvoll, S.H.; Nygaard, A.B.; Ellingjord-Dale, M.; Holland, P.; Istre, M.S.; Kalleberg, K.T.; Soraas, C.L.; Holven, K.B.; Ulven, S.M.; Hjartaker, A.; et al. Prevention of COVID-19 and other acute respiratory infections with cod liver oil supplementation, a low dose vitamin D supplement: Quadruple blinded, randomised placebo controlled trial. BMJ 2022, 378, e071245. [Google Scholar] [CrossRef]
- Schirinzi, A.; Cazzolla, A.P.; Lovero, R.; Lo Muzio, L.; Testa, N.F.; Ciavarella, D.; Palmieri, G.; Pozzessere, P.; Procacci, V.; Di Serio, F.; et al. New Insights in Laboratory Testing for COVID-19 Patients: Looking for the Role and Predictive Value of Human epididymis secretory protein 4 (HE4) and the Innate Immunity of the Oral Cavity and Respiratory Tract. Microorganisms 2020, 8, 1718. [Google Scholar] [CrossRef] [PubMed]
- Demay, M.B.; Pittas, A.G.; Bikle, D.D.; Diab, D.L.; Kiely, M.E.; Lazaretti-Castro, M.; Lips, P.; Mitchell, D.M.; Murad, M.H.; Powers, S.; et al. Vitamin D for the Prevention of Disease: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2024, 109, 1907–1947. [Google Scholar] [CrossRef]
- Nair, P.N.; Sundqvist, G.; Sjogren, U. Experimental evidence supports the abscess theory of development of radicular cysts. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 2008, 106, 294–303. [Google Scholar] [CrossRef]
- Muglali, M.; Komerik, N.; Bulut, E.; Yarim, G.F.; Celebi, N.; Sumer, M. Cytokine and chemokine levels in radicular and residual cyst fluids. J. Oral. Pathol. Med. 2008, 37, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Graunaite, I.; Lodiene, G.; Maciulskiene, V. Pathogenesis of apical periodontitis: A literature review. J. Oral. Maxillofac. Res. 2012, 2, e1. [Google Scholar] [CrossRef]
- Rotstein, I.; Katz, J. Prevalence of periapical abscesses in vitamin D deficient patients. Am. J. Dent. 2021, 34, 163–165. [Google Scholar]
- Menezes, R.; Bramante, C.M.; da Silva Paiva, K.B.; Letra, A.; Carneiro, E.; Fernando Zambuzzi, W.; Granjeiro, J.M. Receptor activator NFkappaB-ligand and osteoprotegerin protein expression in human periapical cysts and granulomas. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 2006, 102, 404–409. [Google Scholar] [CrossRef]
- Tay, J.Y.; Bay, B.H.; Yeo, J.F.; Harris, M.; Meghji, S.; Dheen, S.T. Identification of RANKL in osteolytic lesions of the facial skeleton. J. Dent. Res. 2004, 83, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Robling, A.G.; Castillo, A.B.; Turner, C.H. Biomechanical and molecular regulation of bone remodeling. Annu. Rev. Biomed. Eng. 2006, 8, 455–498. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.C.; Choi, Y. Biology of the RANKL-RANK-OPG System in Immunity, Bone, and Beyond. Front. Immunol. 2014, 5, 511. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, T.; Shinzawa, M.; Akiyama, N. RANKL-RANK interaction in immune regulatory systems. World J. Orthop. 2012, 3, 142–150. [Google Scholar] [CrossRef]
- Hayden, M.S.; West, A.P.; Ghosh, S. NF-kappaB and the immune response. Oncogene 2006, 25, 6758–6780. [Google Scholar] [CrossRef]
- Cafferata, E.A.; Terraza-Aguirre, C.; Barrera, R.; Faundez, N.; Gonzalez, N.; Rojas, C.; Melgar-Rodriguez, S.; Hernandez, M.; Carvajal, P.; Cortez, C.; et al. Interleukin-35 inhibits alveolar bone resorption by modulating the Th17/Treg imbalance during periodontitis. J. Clin. Periodontol. 2020, 47, 676–688. [Google Scholar] [CrossRef]
- Jurisic, V.; Terzic, T.; Colic, S.; Jurisic, M. The concentration of TNF-alpha correlate with number of inflammatory cells and degree of vascularization in radicular cysts. Oral. Dis. 2008, 14, 600–605. [Google Scholar] [CrossRef]
- Hayashi, M.; Ohshima, T.; Ohshima, M.; Yamaguchi, Y.; Miyata, H.; Takeichi, O.; Ogiso, B.; Ito, K.; Ostman, A.; Otsuka, K. Profiling of radicular cyst and odontogenic keratocyst cytokine production suggests common growth mechanisms. J. Endod. 2008, 34, 14–21. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhao, Y.; Shui, Y.; Zhou, X.; Cheng, L.; Ren, B.; Chen, Z.; Li, M. Interactions Between Neutrophils and Periodontal Pathogens in Late-Onset Periodontitis. Front. Cell Infect. Microbiol. 2021, 11, 627328. [Google Scholar] [CrossRef]
- Meghil, M.M.; Cutler, C.W. Influence of Vitamin D on Periodontal Inflammation: A Review. Pathogens 2023, 12, 1180. [Google Scholar] [CrossRef]
- Aranow, C. Vitamin D and the immune system. J. Investig. Med. 2011, 59, 881–886. [Google Scholar] [CrossRef]
- Stein, S.H.; Livada, R.; Tipton, D.A. Re-evaluating the role of vitamin D in the periodontium. J. Periodontal Res. 2014, 49, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Samietz, S.; Holtfreter, B.; Hannemann, A.; Meisel, P.; Nauck, M.; Völzke, H.; Wallaschofski, H.; Dietrich, T.; Kocher, T. Prospective Study of Serum 25-hydroxy Vitamin D and Tooth Loss. J. Dent. Res. 2014, 93, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Wacker, M.; Holick, M.F. Vitamin D—Effects on skeletal and extraskeletal health and the need for supplementation. Nutrients 2013, 5, 111–148. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Mahon, B.D.; Froicu, M.; Cantorna, M.T. Calcium and 1 alpha,25-dihydroxyvitamin D3 target the TNF-alpha pathway to suppress experimental inflammatory bowel disease. Eur. J. Immunol. 2005, 35, 217–224. [Google Scholar] [CrossRef]
- Overbergh, L.; Decallonne, B.; Valckx, D.; Verstuyf, A.; Depovere, J.; Laureys, J.; Rutgeerts, O.; Saint-Arnaud, R.; Bouillon, R.; Mathieu, C. Identification and immune regulation of 25-hydroxyvitamin D-1-alpha-hydroxylase in murine macrophages. Clin. Exp. Immunol. 2000, 120, 139–146. [Google Scholar] [CrossRef]
- Sadeghi, K.; Wessner, B.; Laggner, U.; Ploder, M.; Tamandl, D.; Friedl, J.; Zügel, U.; Steinmeyer, A.; Pollak, A.; Roth, E.; et al. Vitamin D3 down-regulates monocyte TLR expression and triggers hyporesponsiveness to pathogen-associated molecular patterns. Eur. J. Immunol. 2006, 36, 361–370. [Google Scholar] [CrossRef]
- Walsh, M.C.; Kim, N.; Kadono, Y.; Rho, J.; Lee, S.Y.; Lorenzo, J.; Choi, Y. Osteoimmunology: Interplay between the immune system and bone metabolism. Annu. Rev. Immunol. 2006, 24, 33–63. [Google Scholar] [CrossRef]
- Grenier, D.; Morin, M.P.; Fournier-Larente, J.; Chen, H. Vitamin D inhibits the growth of and virulence factor gene expression by Porphyromonas gingivalis and blocks activation of the nuclear factor kappa B transcription factor in monocytes. J. Periodontal Res. 2016, 51, 359–365. [Google Scholar] [CrossRef]
- Gatera, V.A.; Lesmana, R.; Musfiroh, I.; Judistiani, R.T.D.; Setiabudiawan, B.; Abdulah, R. Vitamin D Inhibits Lipopolysaccharide (LPS)-Induced Inflammation in A549 Cells by Downregulating Inflammatory Cytokines. Med. Sci. Monit. Basic. Res. 2021, 27, e931481. [Google Scholar] [CrossRef]
- Oh, C.; Kim, H.J.; Kim, H.M. Vitamin D maintains E-cadherin intercellular junctions by downregulating MMP-9 production in human gingival keratinocytes treated by TNF-alpha. J. Periodontal Implant. Sci. 2019, 49, 270–286. [Google Scholar] [CrossRef] [PubMed]
- Schroth, R.J.; Lavelle, C.; Tate, R.; Bruce, S.; Billings, R.J.; Moffatt, M.E. Prenatal vitamin D and dental caries in infants. Pediatrics 2014, 133, e1277–e1284. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Revisiting Vitamin D Guidelines: A Critical Appraisal of the Literature. Endocr. Pract. 2024, 30, 1227–1241. [Google Scholar] [CrossRef] [PubMed]
- Gong, A.; Liu, Y.; Xu, F.; Chu, Y.; Wu, J.; Goltzman, D.; Miao, D. Role of 1,25-dihydroxyvitamin D in alleviating alveolar bone loss and gingival inflammation in ligature-induced periodontitis. Am. J. Transl. Res. 2022, 14, 3079–3091. [Google Scholar]
- Gong, A.; Chen, J.; Wu, J.; Li, J.; Wang, L.; Goltzman, D.; Miao, D. 1,25-dihydroxyvitamin D deficiency accelerates alveolar bone loss independent of aging and extracellular calcium and phosphorus. J. Periodontol. 2018, 89, 983–994. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Cai, Y.; Chen, H. Association of Serum Vitamin D with Periodontal Disease. Int. Dent. J. 2023, 73, 777–783. [Google Scholar] [CrossRef]
- Liu, X.; Dai, B.; Chuai, Y.; Hu, M.; Zhang, H. Associations between vitamin D levels and periodontal attachment loss. Clin. Oral. Investig. 2023, 27, 4727–4733. [Google Scholar] [CrossRef]
- Dietrich, T.; Nunn, M.; Dawson-Hughes, B.; Bischoff-Ferrari, H.A. Association between serum concentrations of 25-hydroxyvitamin D and gingival inflammation. Am. J. Clin. Nutr. 2005, 82, 575–580. [Google Scholar] [CrossRef]
- Liu, K.; Meng, H.; Hou, J. Activity of 25-hydroxylase in human gingival fibroblasts and periodontal ligament cells. PLoS ONE 2012, 7, e52053. [Google Scholar] [CrossRef]
- Schini, M.; Vilaca, T.; Gossiel, F.; Salam, S.; Eastell, R. Bone Turnover Markers: Basic Biology to Clinical Applications. Endocr. Rev. 2023, 44, 417–473. [Google Scholar] [CrossRef]
- Alshouibi, E.N.; Kaye, E.K.; Cabral, H.J.; Leone, C.W.; Garcia, R.I. Vitamin D and periodontal health in older men. J. Dent. Res. 2013, 92, 689–693. [Google Scholar] [CrossRef] [PubMed]
- Bertoldo, F.; Cianferotti, L.; Di Monaco, M.; Falchetti, A.; Fassio, A.; Gatti, D.; Gennari, L.; Giannini, S.; Girasole, G.; Gonnelli, S.; et al. Definition, Assessment, and Management of Vitamin D Inadequacy: Suggestions, Recommendations, and Warnings from the Italian Society for Osteoporosis, Mineral Metabolism and Bone Diseases (SIOMMMS). Nutrients 2022, 14, 4148. [Google Scholar] [CrossRef] [PubMed]
| 25OHD ng/mL GroupH | 25OHD ng/mL Group P | 25OHD ng/mL Group P1 | 25OHD ng/mL Group P2 | BALP µg/L Group H | BALP µg/L Group P | BALP µg/L Group P1 | BALP µg/L Group P2 | CTX ng/mL Group H | CTX ng/mL Group_P | CTX ng/mL Group P1 | CTX ng/mL Group P2 | OC ng/mL Group_H | OC ng/mL Group P | OC ng/mL Group P1 | OC ng/mL Group P2 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
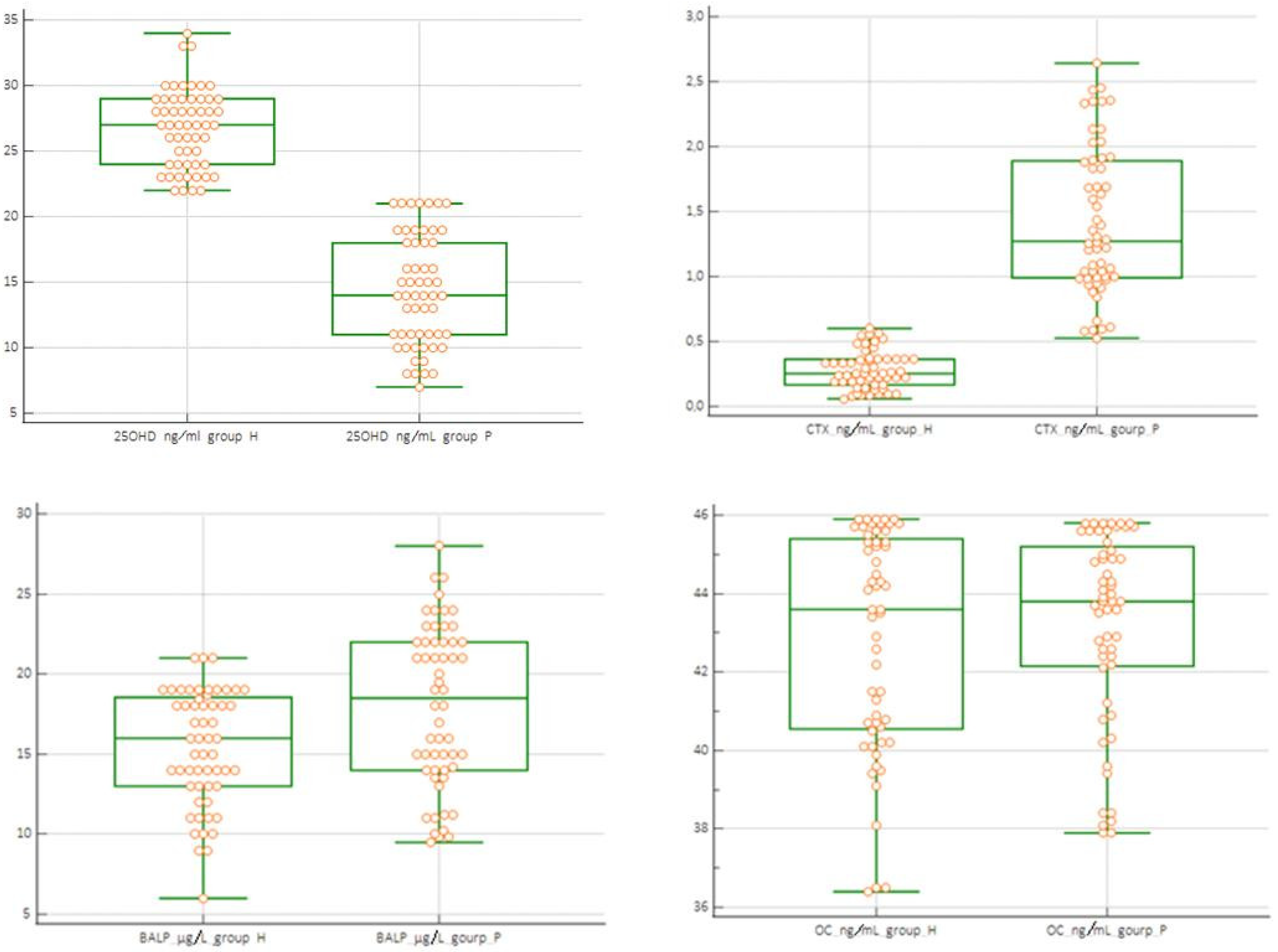
| N | 56 | 56 | 42 | 14 | 56 | 56 | 42 | 14 | 56 | 56 | 42 | 14 | 56 | 56 | 42 | 14 |
| Minimum | 22 | 7 | 10 | 7 | 6 | 9.5 | 9.5 | 9.8 | 0.059 | 0.526 | 0.526 | 1.541 | 36.40 | 37.90 | 37.90 | 43.80 |
| Maximum | 34 | 21 | 21 | 11 | 21 | 28 | 26 | 28 | 0.60 | 2.64 | 2.34 | 2.64 | 45.9 | 45.8 | 45.7 | 45.8 |
| Mean | 26.7 | 14.32 | 15.9 | 9.214 | 15.39 | 18.02 | 17.58 | 19.34 | 0.278 | 1.423 | 1.199 | 2.097 | 42.83 | 43.18 | 42.50 | 45.43 |
| 95% CI | 25.9 to 27.5 | 13.19 to 15.8 | 14.93 to 17.02 | 8.49 to 9.93 | 14.44 to 16.34 | 16.67 to 19.37 | 16.13 to 19.03 | 15.85 to 22.83 | 0.24 to 0.31 | 1.27 to 1.57 | 1.06 to 1.33 | 1.89 to 2.29 | 42.07 to 43.58 | 42.53 to 43.84 | 41.74 to 43.26 | 45.09 to 45.77 |
| Median | 27 | 14.0 | 15.5 | 9.5 | 16 | 18.5 | 16.5 | 21.5 | 0.254 | 1.27 | 1.07 | 2.09 | 43.6 | 43.8 | 43.20 | 45.75 |
| 95% CI | 26 to 28 | 13 to 15 | 14.18 to 18.0 | 8. to 10 | 14 to 18 | 15 to 21 | 15 to 20.81 | 13.60 to 23.00 | 0.22 to 0.33 | 1.07 to 1.62 | 0.99 to 1.25 | 1.85 to 2.36 | 41.50 to 44.74 | 42.90 to 44.46 | 42.23 to 43.88 | 44.99 to 45.80 |
| SD | 2.95 | 4.2 | 3.35 | 1.25 | 3.56 | 5.03 | 4.655 | 6.045 | 0.142 | 0.571 | 0.438 | 0.349 | 2.816 | 2.454 | 2.436 | 0.587 |
| RSD | 0.11 | 0.56 | 0.20 | 0.13 | 0.23 | 0.27 | 0.264 | 0.312 | 0.513 | 0.401 | 0.366 | 0.166 | 0.065 | 0.056 | 0.057 | 0.012 |
| 5-95 P | 21.0 to 32.1 | 7.00 to 21.0 | 11.00 to 19 | 7.20 to 11.00 | 9.30 to 20.40 | 9.86 to 25.7 | 10.6 to 24.4 | 9.80 to 27.6 | 0.08 to 0.55 | 0.58 to 2.41 | 0.58 to 2.00 | 1.55 to 2.60 | 36.9 to 45.9 | 38.1 to 45.8 | 38.0 to 45.6 | 44.0 to 45.8 |
| Normal Distr. | 0.65 | 0.003 | 0.003 | 0.503 | 0.207 | 0.003 | 0.015 | 0.426 | 0.172 | 0.047 | 0.139 | 0.505 | 0.058 | 0.028 | 0.083 | 0.009 |
| Accept normal | Reject norma | Accept normal | Accept normal | Accept normal | Reject normal | Reject norma | Accept normal | Accept normal | Reject normal | Accept normal | Accept normal | Accept normal | Reject normal | Accept normal | Reject normal |
| (a) | ||
|---|---|---|
| Comparison Sample | Mann–Whitney Test | p Value |
| Group H vs. P | ||
| 25OHD | 236 | <0.001 |
| CTX | 7 | <0.001 |
| BALP | 1253 | >0.05 |
| OC | 1235 | >0.05 |
| (b) | ||
| Comparison Sample | Mann–Whitney Test | p Value |
| Group H vs. P1 | ||
| 25OHD | 236 | <0.001 |
| CTX | 7.01 | <0.001 |
| Group H vs. P2 | ||
| 25OHD | 2.5 | <0.001 |
| CTX | 0.01 | <0.001 |
| Group P1 vs. P2 | ||
| 25OHD | 9.5 | <0.001 |
| CTX | 40 | <0.001 |
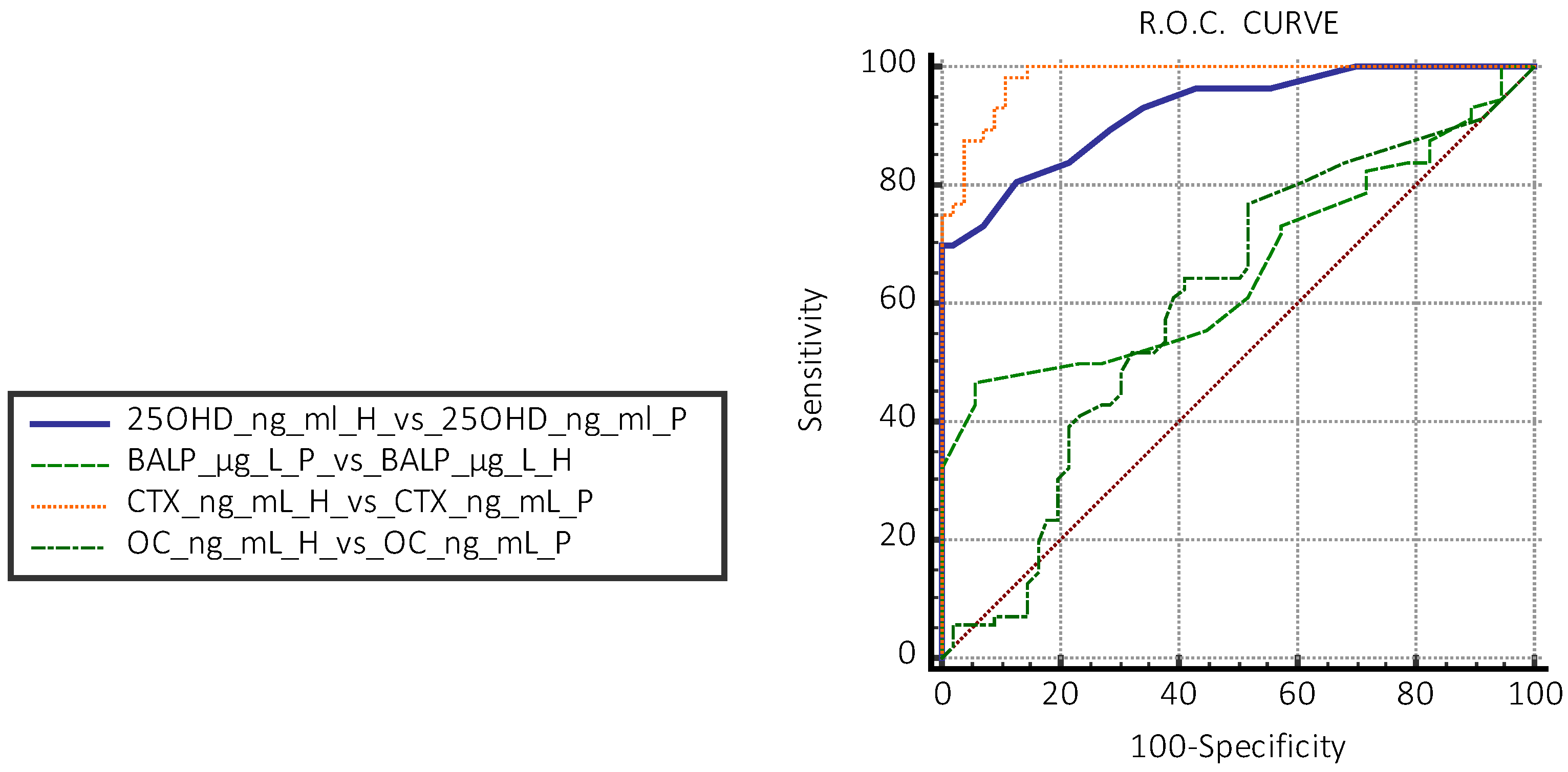
| Group H vs. P | Dosage | AUC | Sensitivity % | Specificity % |
|---|---|---|---|---|
| 25OHD | ≤19 ng/mL | 0.925 | 91 | 99 |
| CTX | 0.561 ng/mL | 0.96 | 92 | 98 |
| BALP | >19 µg/L | 0.660 | 46 | 94 |
| OC | 41.5 ng/mL | 0.512 | 76 | 39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cazzolla, A.P.; Brescia, V.; Lovero, R.; Cardinali, R.; Di Serio, F.; Lorusso, M.; Ciavarella, D.; Testa, N.F.; Dipalma, G.; Di Cosola, M.; et al. Evaluation of Vitamin D and of Some Biomarkers of Bone Remodelling (CTX-1, Osteocalcin, BALP) in Subjects with Periapical Inflammatory Cysts: An Observational Study. J. Clin. Med. 2025, 14, 3712. https://doi.org/10.3390/jcm14113712
Cazzolla AP, Brescia V, Lovero R, Cardinali R, Di Serio F, Lorusso M, Ciavarella D, Testa NF, Dipalma G, Di Cosola M, et al. Evaluation of Vitamin D and of Some Biomarkers of Bone Remodelling (CTX-1, Osteocalcin, BALP) in Subjects with Periapical Inflammatory Cysts: An Observational Study. Journal of Clinical Medicine. 2025; 14(11):3712. https://doi.org/10.3390/jcm14113712
Chicago/Turabian StyleCazzolla, Angela Pia, Vincenzo Brescia, Roberto Lovero, Roberta Cardinali, Francesca Di Serio, Mauro Lorusso, Domenico Ciavarella, Nunzio Francesco Testa, Gianna Dipalma, Michele Di Cosola, and et al. 2025. "Evaluation of Vitamin D and of Some Biomarkers of Bone Remodelling (CTX-1, Osteocalcin, BALP) in Subjects with Periapical Inflammatory Cysts: An Observational Study" Journal of Clinical Medicine 14, no. 11: 3712. https://doi.org/10.3390/jcm14113712
APA StyleCazzolla, A. P., Brescia, V., Lovero, R., Cardinali, R., Di Serio, F., Lorusso, M., Ciavarella, D., Testa, N. F., Dipalma, G., Di Cosola, M., Lo Muzio, L., Crincoli, V., & Di Comite, M. (2025). Evaluation of Vitamin D and of Some Biomarkers of Bone Remodelling (CTX-1, Osteocalcin, BALP) in Subjects with Periapical Inflammatory Cysts: An Observational Study. Journal of Clinical Medicine, 14(11), 3712. https://doi.org/10.3390/jcm14113712









