Screening for Cardiac Amyloidosis When Conducting Carpal Tunnel Surgery
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
4.1. The Role of Advanced Echocardiography in the Diagnosis of Cardiac Amyloidosis
4.2. Comparison of ECG and Imaging Findings
4.3. Cost-Effectiveness and Potential Long-Term Clinical Benefits of Routine Screening
5. Conclusions and Recommendations
- Consider screening older patients (≥60 years) undergoing CTS surgery, particularly those with bilateral idiopathic CTS, for CA. This age group has a higher prevalence of ATTRwt CA, and bilateral CTS is a recognized red flag.
- Incorporate a thorough assessment of cardiovascular risk factors and symptoms for patients presenting CTS surgery. The presence of additional red flags, such as a history of HF, AF, conduction abnormalities, unexplained LVH detected via prior imaging, or elevated levels of cardiac biomarkers, should raise suspicion for underlying CA.
- Consider obtaining a tenosynovial biopsy at the time of carpal tunnel release surgery from patients meeting the criteria given above or those for whom there is a strong clinical suspicion for amyloidosis. While the immediate yield of detecting cardiac involvement may be low, amyloid in the carpal tunnel tissue warrants further cardiac evaluation and long-term follow-ups.
- Implement a screening pathway for patients with amyloid deposits in their carpal tunnel tissue but no initial cardiac involvement. This pathway should include regular cardiac evaluations, including echocardiography and biomarker assessment, to monitor the potential development of cardiac amyloidosis over time.
- Further research is needed to establish the cost-effectiveness of various screening strategies for CA in the context of CTS surgery. Long-term follow-up studies on patients with localized amyloid in the carpal tunnel and comprehensive cost–benefit analyses are essential to refine screening guidelines.
6. Limitations
- Tenosynovial or surgical specimen biopsies were not performed systematically. This was due to several factors, including the primary focus of the case series on cardiac findings, patient refusal, or the absence of surgical specimens in some cases.
- Genetic testing was not performed for all patients with positive scintigraphy. Genetic testing was prioritized for patients in whom the presence of amyloid deposits had been histologically confirmed. This approach was employed due to resource constraints and the diagnostic algorithm typically followed at our center.
- The prevalence of subclinical CA in our case series may have been high, which might reflect a selection bias due to the recruitment of patients with bilateral CTS at a specialized referral center. Patients with bilateral CTS are more likely to be referred for further investigation, potentially enriching our sample with individuals at higher risk for underlying conditions like amyloidosis.
- This is a pilot case series within the larger CarPoS project, and larger validation studies are ongoing.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AF | Atrial fibrillation |
| ATTR | Transthyretin-associated amyloidosis |
| AV | Atrioventricular |
| CA | Cardiac amyloidosis |
| CMR | Cardiac magnetic resonance |
| CTS | Carpal tunnel syndrome |
| CV | Cardiovascular |
| ECG | Electrocardiogram |
| GLS | Global longitudinal strain |
| HBP | High blood pressure |
| HCM | Hypertrophic cardiomyopathy |
| HF | Heart failure |
| LGE | Late gadolinium enhancement |
| LV | Left ventricle |
| LVEF | Left-ventricular ejection fraction |
| LVH | Left-ventricle hypertrophy |
| PW | Posterior wall |
| Tc-DPD | Tc-3,3-diphosphonate-1,2-propanodicarboxylic acid |
References
- Gertz, M.A.; Benson, M.D.; Dyck, P.J.; Grogan, M.; Coelho, T.; Cruz, M.; Berk, J.L.; Plante-Bordeneuve, V.; Schmidt, H.H.J.; Merlini, G. Diagnosis, Prognosis, and Therapy of Transthyretin Amyloidosis. J. Am. Coll. Cardiol. 2015, 66, 2451–2466. [Google Scholar] [CrossRef] [PubMed]
- Milandri, A.; Farioli, A.; Gagliardi, C.; Longhi, S.; Salvi, F.; Curti, S.; Foffi, S.; Caponetti, A.G.; Lorenzini, M.; Ferlini, A.; et al. Carpal tunnel syndrome in cardiac amyloidosis: Implications for early diagnosis and prognostic role across the spectrum of aetiologies. Eur. J. Heart Fail. 2020, 22, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, M.; Sekijima, Y.; Yazaki, M.; Tojo, K.; Yoshinaga, T.; Doden, T.; Koyama, J.; Yanagisawa, S.; Ikeda, S. Carpal tunnel syndrome: A common initial symptom of systemic wild-type ATTR (ATTRwt) amyloidosis. Amyloid 2016, 23, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Ruberg, F.L.; Berk, J.L. Transthyretin (TTR) cardiac amyloidosis. Circulation 2012, 126, 1286–1300. [Google Scholar] [CrossRef]
- Yamada, T.; Takashio, S.; Arima, Y.; Nishi, M.; Morioka, M.; Hirakawa, K.; Hanatani, S.; Fujisue, K.; Yamanaga, K.; Kanazawa, H.; et al. Clinical characteristics and natural history of wild-type transthyretin amyloid cardiomyopathy in Japan. ESC Heart Fail. 2020, 7, 2829–2837. [Google Scholar] [CrossRef]
- Sperry, B.W.; Reyes, B.A.; Ikram, A.; Donnelly, J.P.; Phelan, D.; Jaber, W.A.; Shapiro, D.; Evans, P.J.; Maschke, S.; Kilpatrick, S.E.; et al. Tenosynovial and Cardiac Amyloidosis in Patients Undergoing Carpal Tunnel Release. J. Am. Coll. Cardiol. 2018, 72, 2040–2050. [Google Scholar] [CrossRef]
- Sekijima, Y.; Uchiyama, S.; Tojo, K.; Sano, K.; Shimizu, Y.; Imaeda, T.; Hoshii, Y.; Kato, H.; Ikeda, S. High prevalence of wild-type transthyretin deposition in patients with idiopathic carpal tunnel syndrome: A common cause of carpal tunnel syndrome in the elderly. Hum. Pathol. 2011, 42, 1785–1791. [Google Scholar] [CrossRef]
- Fosbøl, E.L.; Rørth, R.; Leicht, B.P.; Schou, M.; Maurer, M.S.; Kristensen, S.L.; Kober, L.; Gustafsson, F. Association of Carpal Tunnel Syndrome with Amyloidosis, Heart Failure, and Adverse Cardiovascular Outcomes. J. Am. Coll. Cardiol. 2019, 74, 15–23. [Google Scholar] [CrossRef]
- Donnelly, J.P.; Hanna, M.; Sperry, B.W.; Seitz, W.H., Jr. Carpal Tunnel Syndrome: A Potential Early, Red-Flag Sign of Amyloidosis. J. Hand Surg. Am. 2019, 44, 868–876. [Google Scholar] [CrossRef]
- Takei, Y.; Hattori, T.; Gono, T.; Tokuda, T.; Saitoh, S.; Hoshii, Y.; Ikeda, S. Senile systemic amyloidosis presents as bilateral carpal tunnel syndrome. Amyloid 2002, 9, 252–255. [Google Scholar] [CrossRef]
- Cappelli, F.; Gallini, C.; Di Mario, C.; Costanzo, E.N.; Vaggelli, L.; Tutino, F.; Ciaccio, A.; Bartolini, S.; Angelotti, P.; Frusconi, S.; et al. Accuracy of 99mTc-Hydroxymethylene diphosphonate scintigraphy for diagnosis of transthyretin cardiac amyloidosis. J. Nucl. Cardiol. 2019, 26, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Gillmore, J.D.; Maurer, M.S.; Falk, R.H.; Merlini, G.; Damy, T.; Dispenzieri, A.; Wechalekar, A.D.; Berk, J.L.; Quarta, C.C.; Grogan, M.; et al. Nonbiopsy Diagnosis of Cardiac Transthyretin Amyloidosis. Circulation 2016, 133, 2404–2412. [Google Scholar] [CrossRef] [PubMed]
- Cappelli, F.; Gallini, C.; Perfetto, F. PYP or DPD and HDP for cardiac amyloidosis one for all, all for one. J. Nucl. Cardiol. 2020, 27, 1041–1042. [Google Scholar] [CrossRef] [PubMed]
- Rapezzi, C.; Quarta, C.C.; Guidalotti, P.L.; Pettinato, C.; Fanti, S.; Leone, O.; Ferlini, A.; Longhi, S.; Lorenzini, M.; Reggiani, L.B.; et al. Role of (99m)Tc-DPD scintigraphy in diagnosis and prognosis of hereditary transthyretin-related cardiac amyloidosis. JACC Cardiovasc. Imaging 2011, 4, 659–670. [Google Scholar] [CrossRef] [PubMed]
- Haq, M.; Pawar, S.; Berk, J.L.; Miller, E.J.; Ruberg, F.L. Can (99m)Tc-Pyrophosphate Aid in Early Detection of Cardiac Involvement in Asymptomatic Variant TTR Amyloidosis? JACC Cardiovasc. Imaging 2017, 10, 713–714. [Google Scholar] [CrossRef]
- Hutt, D.F.; Quigley, A.-M.; Page, J.; Hall, M.L.; Burniston, M.; Gopaul, D.; Lane, T.; Whelan, C.J.; Lachmann, H.J.; Gillmore, J.D.; et al. Utility and limitations of 3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy in systemic amyloidosis. Eur. Heart J. Cardiovasc. Imaging 2014, 15, 1289–1298. [Google Scholar] [CrossRef]
- Liang, S.; Liu, Z.; Li, Q.; He, W.; Huang, H. Advance of echocardiography in cardiac amyloidosis. Heart Fail. Rev. 2023, 28, 1345–1356. [Google Scholar] [CrossRef]
- Stricagnoli, M.; Cameli, M.; Incampo, E.; Lunghetti, S.; Mondillo, S. Speckle tracking echocardiography in cardiac amyloidosis. Heart Fail. Rev. 2019, 24, 701–707. [Google Scholar] [CrossRef]
- Trimarchi, G.; Carerj, S.; Di Bella, G.; Manganaro, R.; Pizzino, F.; Restelli, D.; Pelaggi, G.; Lofrumento, F.; Licordari, R.; Taverna, G.; et al. Clinical Applications of Myocardial Work in Echocardiography: A Comprehensive Review. J. Cardiovasc. Echogr. 2024, 34, 99–113. [Google Scholar] [CrossRef]
- Holcman, K.; Kostkiewicz, M.; Szot, W.; Ćmiel, B.; Mróz, K.; Stępień, A.; Graczyk, K.; Dziewięcka, E.; Karabinowska-Małocha, A.; Sachajko, Z.; et al. Transthyretin amyloid cardiomyopathy in patients with unexplained increased left ventricular wall thickness. Int. J. Cardiovasc. Imaging 2024, 40, 1693–1703. [Google Scholar] [CrossRef]
- Sperry, B.W.; Vranian, M.N.; Hachamovitch, R.; Joshi, H.; McCarthy, M.; Ikram, A.; Hanna, M. Are classic predictors of voltage valid in cardiac amyloidosis? A contemporary analysis of electrocardiographic findings. Int. J. Cardiol. 2016, 214, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Pavia, P.; Rapezzi, C.; Adler, Y.; Arad, M.; Basso, C.; Brucato, A.; Burazor, I.; Caforio, A.L.P.; Damy, T.; Eriksson, U.; et al. Diagnosis and treatment of cardiac amyloidosis: A position statement of the ESC Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2021, 42, 1554–1568. [Google Scholar] [CrossRef] [PubMed]
| Cases | Gender, Age | Cardiovascular Risk Factors/Disease | Perugini | Severity of CTS |
|---|---|---|---|---|
| 1 | M, 79 | HBP; dyslipidemia | 2 | Right: Severe; Left: Severe |
| 2 | M, 69 | Dyslipidemia | 0 | Right: Moderate; Left: Severe |
| 3 | M, 64 | Dyslipidemia; previous smoker; coronary disease | 0 | Right: Moderate; Left: Moderate |
| 4 | F, 63 | - | 0 | Right: NA; Left: Moderate |
| 5 | F, 63 | HBP | 0 | Right: Moderate; Left: Moderate |
| 6 | F, 75 | Dyslipidemia; obesity | 0 | Right: Mild; Left: Mild |
| 7 | M, 74 | HBP; dyslipidemia; obesity; coronary disease | 3 | Right: Severe; Left: Mild |
| 8 | F, 83 | HBP | 0 | Right: Severe; Left: Severe |
| 9 | M, 67 | HBP | 0 | Right: Severe; Left: Severe |
| 10 | F, 62 | - | 0 | Right: Mild; Left: Moderate |
| 11 | F, 60 | HBP | 0 | Right: Severe; Left: Moderate |
| 12 | F, 84 | HBP | 0 | Right: Severe; Left: Mild |
| 13 | M, 86 | HBP; dyslipidemia | 3 | Right: Moderate; Left: Mild |
| 14 | F, 81 | - | 0 | Right: Severe; Left: Severe |
| 15 | F, 70 | HBP | 0 | Right: Moderate; Left: Moderate |
| 16 | F, 72 | HBP; obesity | 0 | Right: Severe; Left: Moderate |
| 17 | M, 81 | HBP; dyslipidemia; obesity | 3 | Right: Severe; Left: Severe |
| 18 | F, 61 | - | 0 | Right: Mild; Left: Mild |
| 19 | F, 85 | HBP; stroke | 0 | Right: Moderate; Left: Severe |
| 20 | M, 70 | Diabetes mellitus type 2 | 0 | Right: Moderate; Left: Moderate |
| 21 | F, 60 | - | 0 | Right: Severe; Left: Severe |
| 22 | F, 66 | HBP | 0 | Right: Moderate; Left: Severe |
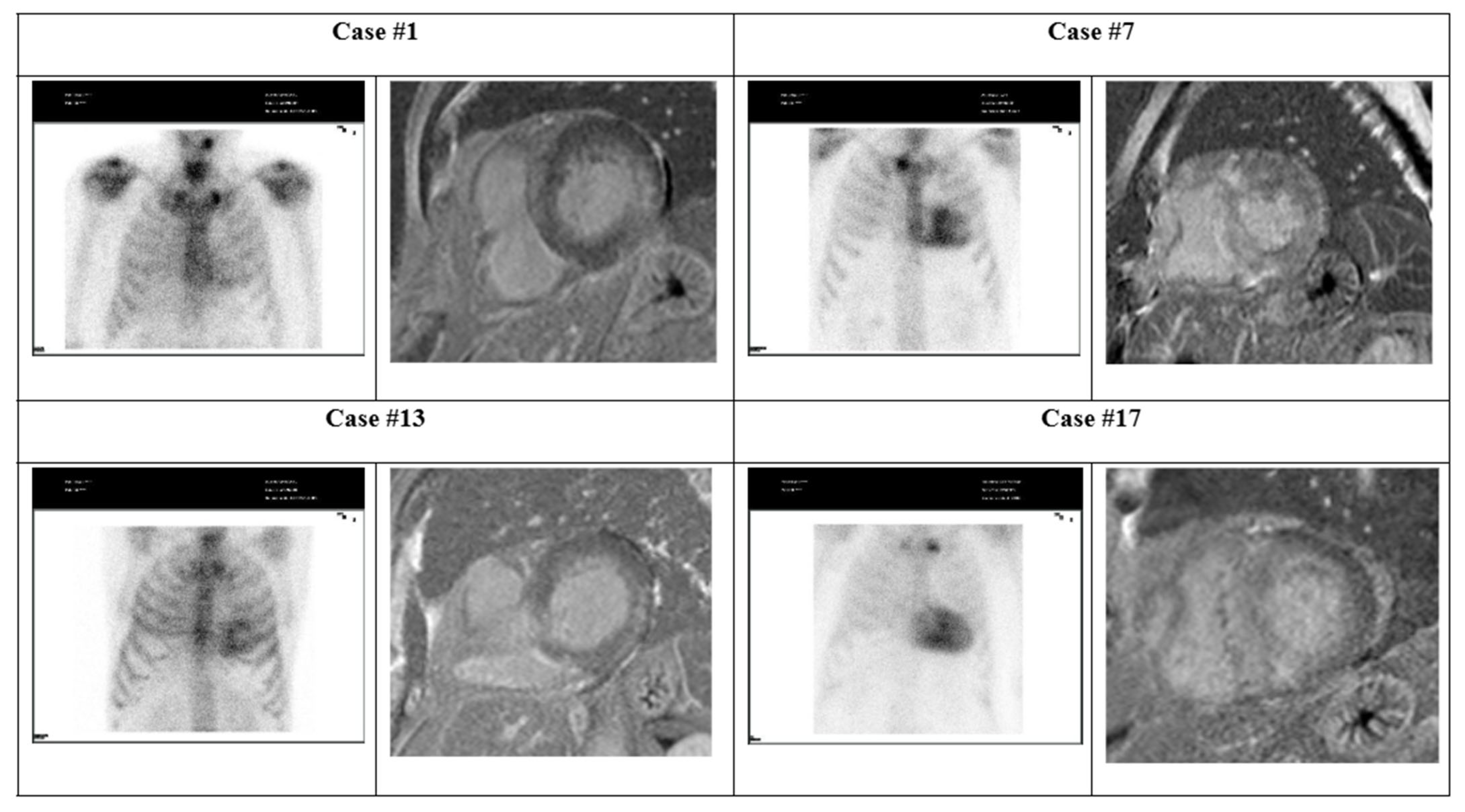
| Exam | Parameter | Case #1 | Case #7 | Case #13 | Case #17 |
|---|---|---|---|---|---|
| - | CV symptoms | Asymptomatic | Asymptomatic | Asymptomatic | Tiredness |
| - | Troponin I (ng/mL) | 35.3 | 37.9 | 10.5 | 71.7 |
| Scintigraphy | Perugini grade (0–3) | 2 | 3 | 3 | 3 |
| Echocardiogram | LVEF (%) | 60.2 | 60.0 | 77.4 | 61.4 |
| GLS (%) | −19.9 | −11.7 | −18.7 | −10.4 | |
| PW (mm) | 12.0 | 14.1 | 12.0 | 13.0 | |
| Changes | Concentric LVH | LVH | LVH | Concentric LVH | |
| E-wave (cm/s) | 10.8 | 9.5 | 7.3 | - | |
| ECG | Changes | None | None | Left anterior fascicular block | 1st-degree AV block |
| Heart rate (bpm) | 76 | 72 | 50 | 88 | |
| QRS (ms) | 104 | 104 | 102 | 98 | |
| PR (ms) | 167 | 172 | 173 | 270 | |
| QT (ms) | 346 | 387 | 420 | 355 | |
| Cardiac Magnetic Resonance | LVEF (%) | 67 | 50 | 58 | 61 |
| LVH | Moderate | Severe | Moderate | Severe | |
| Max. thickness (mm) | 13 | 19 | 14 | 21 | |
| LGE | 1 | 1 | 1 | 1 | |
| LGE ≥ 3 segments | 0 | 0 | 0 | 1 | |
| Conclusions | Fibrosis (probable amyloid infiltration) | Findings suggestive of amyloidosis | Fibrosis (probable amyloid infiltration) | Findings suggestive of amyloidosis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pimenta, S.; Santos, L.; Martins, A.; Santos, J.; Fortuna, I.; Pereira, B.; Vasconcelos, M.; Carvalho, M.; Carvalho, A.; Gonçalves, M.; et al. Screening for Cardiac Amyloidosis When Conducting Carpal Tunnel Surgery. J. Clin. Med. 2025, 14, 3710. https://doi.org/10.3390/jcm14113710
Pimenta S, Santos L, Martins A, Santos J, Fortuna I, Pereira B, Vasconcelos M, Carvalho M, Carvalho A, Gonçalves M, et al. Screening for Cardiac Amyloidosis When Conducting Carpal Tunnel Surgery. Journal of Clinical Medicine. 2025; 14(11):3710. https://doi.org/10.3390/jcm14113710
Chicago/Turabian StylePimenta, Sofia, Luís Santos, Ana Martins, Janete Santos, Inês Fortuna, Barbara Pereira, Mariana Vasconcelos, Miguel Carvalho, André Carvalho, Micaela Gonçalves, and et al. 2025. "Screening for Cardiac Amyloidosis When Conducting Carpal Tunnel Surgery" Journal of Clinical Medicine 14, no. 11: 3710. https://doi.org/10.3390/jcm14113710
APA StylePimenta, S., Santos, L., Martins, A., Santos, J., Fortuna, I., Pereira, B., Vasconcelos, M., Carvalho, M., Carvalho, A., Gonçalves, M., Pinto, I., Fidalgo, I., Pereira, J., Faria, T., Costa, L., & Martins, E. (2025). Screening for Cardiac Amyloidosis When Conducting Carpal Tunnel Surgery. Journal of Clinical Medicine, 14(11), 3710. https://doi.org/10.3390/jcm14113710







