Management of Systemic Cardiotoxicity Associated with Antidepressant Use in Patients with Depressive Disorders: A Systematic Review
Abstract
1. Introduction
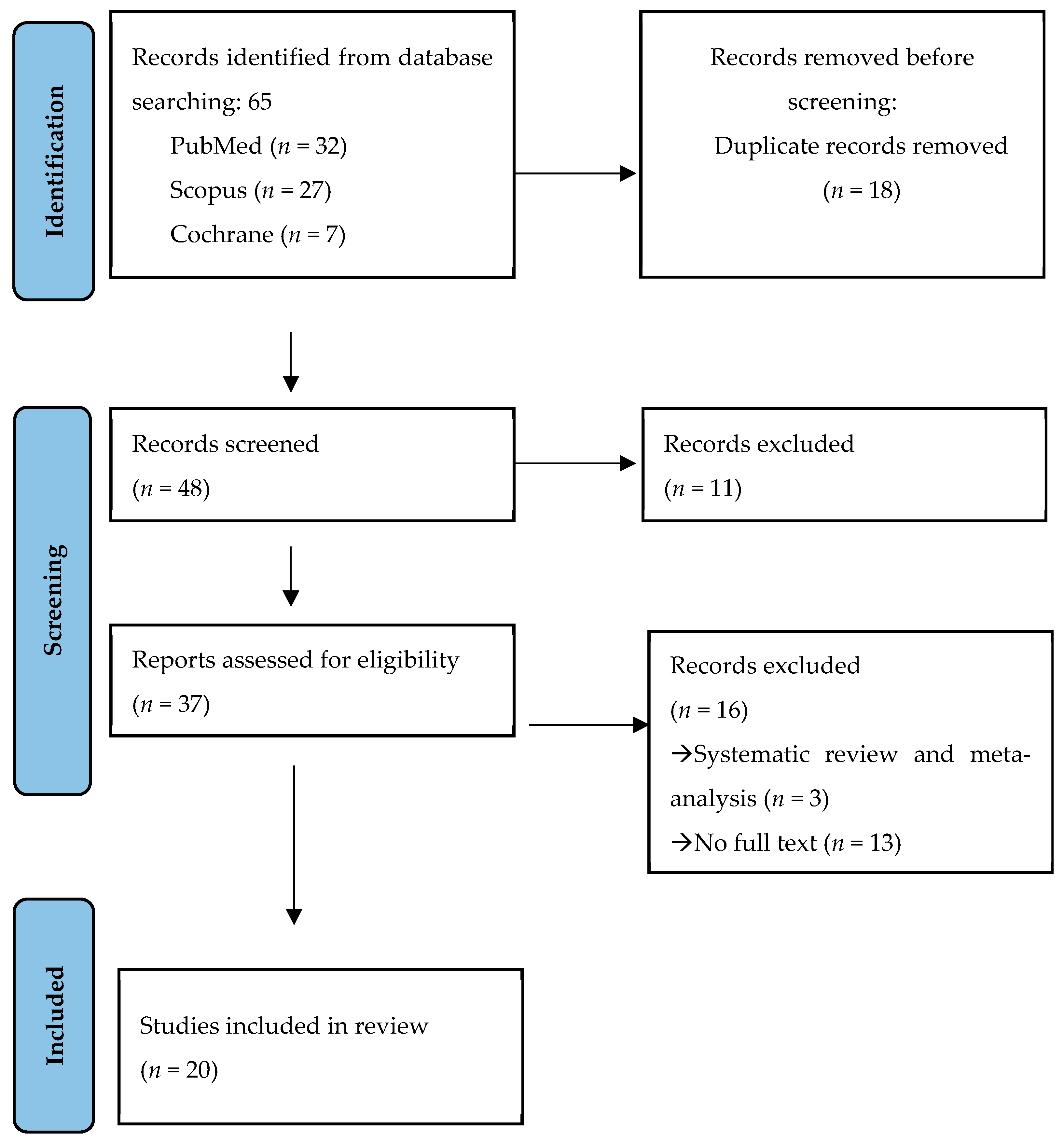
2. Materials and Methods
3. Results
3.1. Selection of the Studies
3.2. Results of the Studies
3.2.1. Age and Gender Distribution of Patients
3.2.2. Antidepressant
3.2.3. Signs and Symptoms
3.2.4. Treatment and Post-Operative Conditions
3.2.5. Follow-Up
3.2.6. Risk of Bias
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Carney, R.M.; E Freedland, K.; E Miller, G.; Jaffe, A.S. Depression as a risk factor for cardiac mortality and morbidity: A review of potential mechanisms. J. Psychosom. Res. 2002, 53, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Coupland, C.; Hill, T.; Morriss, R.; Moore, M.; Arthur, A.; Hippisley-Cox, J. Antidepressant use and risk of cardiovascular outcomes in people aged 20 to 64: Cohort study using primary care database. BMJ 2016, 352, i1350. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Viswanathan, T.; Lang, C.C.; Petty, R.D.; Baxter, M.A. Cardiotoxicity and Chemotherapy-The Role of Precision Medicine. Diseases 2021, 9, 90. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yekehtaz, H.; Farokhnia, M.; Akhondzadeh, S. Cardiovascular considerations in antidepressant therapy: An evidence-based review. J. Tehran Heart Cent. 2013, 8, 169–176. [Google Scholar] [PubMed] [PubMed Central]
- Kerr, G.W.; McGuffie, A.C.; Wilkie, S. Tricyclic antidepressant overdose: A review. Emerg. Med. J. 2001, 18, 236–241. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- MA, M.S.M.; McFarland, B.H. Trends in antidepressant overdoses. Pharmacoepidemiol. Drug Saf. 2007, 16, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Ventetuolo, C.E.; Muratore, C.S. Extracorporeal life support in critically ill adults. Am. J. Respir. Crit. Care Med. 2014, 190, 497–508. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mooney, M.R.; Unger, B.T.; Boland, L.L.; Burke, M.N.; Kebed, K.Y.; Graham, K.J.; Henry, T.D.; Katsiyiannis, W.T.; Satterlee, P.A.; Sendelbach, S.; et al. Therapeutic hypothermia after out-of-hospital cardiac arrest: Evaluation of a regional system to increase access to cooling. Circulation 2011, 124, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Holliday, S.M.; Benfield, P. Venlafaxine: A review of its pharmacology and therapeutic potential in depression. Drugs 1995, 49, 280–294. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, G.L.; VadeBoncouer, T.; Ramaraju, G.A.; Garcia-Amaro, M.F.; Cwik, M.J. Pretreatment or resuscitation with a lipid infusion shifts the dose-response to bupivacaine-induced asystole in rats. Anesthesiology 1998, 88, 1071–1075. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moola, S.; Munn, Z.; Tufanaru, C.; Aromataris, E.; Sears, K.; Sfetcu, R.; Currie, M.; Qureshi, R.; Mattis, P.; Lisy, K.; et al. Systematic reviews of etiology and risk; Chapter 7. In JBI Manual for Evidence Synthesis; Aromataris, E., Munn, Z., Eds.; JBI: London, UK, 2020; Available online: https://synthesismanual.jbi.global (accessed on 10 April 2025).
- Nguyen, H.; Kidron, A.; Ghildyal, C.; Veluri, S.; Nguyen, N.; Nguyen, Q.; Nguyen, H. Novel Presentation of Cardiotoxicity and Other Complications in Tricyclic Antidepressant Poisoning. Cureus 2021, 13, e17181. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ikejiri, K.; Akama, Y.; Ieki, Y.; Kawamoto, E.; Suzuki, K.; Yokoyama, K.; Ishikura, K.; Imai, H. Veno-arterial extracorporeal membrane oxygenation and targeted temperature management in tricyclic antidepressant-induced cardiac arrest: A case report and literature review. Medicine 2021, 100, e24980. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zitoune, Z.; Kugener, L.; Jonckheer, J.; Lanckmans, K.; Hantson, P.; Devriendt, J.; Honore, P.M. Effective Treatment of Acute Tricyclic Antidepressant Poisoning with Cardiogenic Shock and Severe Rhabdomyolysis Using ECMO and CytoSorb® Adsorber. Am. J. Case Rep. 2023, 24, e939884. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Amiri, H.; Zamani, N.; Hassanian-Moghaddam, H.; Shadnia, S. Cardiotoxicity of tricyclic antidepressant treated by 2650 mEq sodium bicarbonate: A case report. JRSM Cardiovasc. Dis. 2016, 5, 2048004016682178. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kassim, T.; Haddad, T.M.; Rakhra, A.; Kabach, A.; Qurie, A.; Selim, M.; Nayfeh, A.S.; Aly, A.; Holmberg, M.J. A Case of Amitriptyline-induced Myocarditis. Cureus 2018, 10, e2840. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Robinson, S. Treatment of status epilepticus and prolonged QT after massive intentional bupropion overdose with lidocaine. Am. J. Emerg. Med. 2022, 55, 232.e3–232.e4. [Google Scholar] [CrossRef] [PubMed]
- Koshy, P.; Chavan, G.; Nagdev, G. T-Wave Inversion After Escitalopram Overdose: A Case Report. Cureus 2023, 15, e34523. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cobilinschi, C.; Mirea, L.; Andrei, C.-A.; Ungureanu, R.; Cotae, A.-M.; Avram, O.; Isac, S.; Grințescu, I.M.; Țincu, R. Biodetoxification Using Intravenous Lipid Emulsion, a Rescue Therapy in Life-Threatening Quetiapine and Venlafaxine Poisoning: A Case Report. Toxics 2023, 11, 917. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ando, M.; Tamura, R.; Nakasako, S.; Takimoto, S.; Ariyoshi, K.; Yamaguchi, M.; Sakizono, K.; Eto, M.; Fukushima, S.; Sugioka, N.; et al. Plasma concentration of amitriptyline and metabolites after resuscitation from cardiopulmonary arrest following an overdose: A case report. Clin. Case Rep. 2020, 9, 805–811. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Reinsch, N.; Haq, E.; Ramakrishna, K.; Craft, L. Cardiovascular Collapse Requiring Veno-Arterial Extracorporeal Membrane Oxygenation and Lidocaine: A Case of Massive Bupropion Overdose. Cureus 2024, 16, e62873. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Isaza, A.M.A.; Bustamante-Cristancho, L.A.; Uribe-B, F.L. Successful Outcome Following Intravenous Lipid Emulsion Rescue Therapy in a Patient with Cardiac Arrest Due to Amitriptyline Overdose. Am. J. Case Rep. 2020, 21, e922206. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ungureanu, R.; Dumitriu, A.-M.; Cobilinschi, C.; Ene, R.; Buiuc, M.; Grințescu, I.M.; Mirea, L. An Atypical Case of Rhabdomyolysis Following an Atypical Antidepressant Overdose. J. Clin. Med. 2025, 14, 276. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Franco, V. Wide complex tachycardia after bupropion overdose. Am. J. Emerg. Med. 2015, 33, e3–e5. [Google Scholar] [CrossRef] [PubMed]
- Garcia, S.; Schuh, M.; Cheema, A.; Atwal, H.; Atwal, P.S. Palpitations and Asthenia Associated with Venlafaxine in a CYP2D6 Poor Metabolizer and CYP2C19 Intermediate Metabolizer. Case Rep. Genet. 2017, 2017, 6236714. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Avcil, M.; Kapçı, M.; Yavaşoğlu, I.; Kantekin, B.; Akpek, M. Simultaneous Use of Intravenous Lipid Emulsion and Plasma Exchange Therapies in Multiple Drug Toxicity. Med. Princ. Pract. 2016, 25, 577–579. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Castanares-Zapatero, D.; Gillard, N.; Capron, A.; Haufroid, V.; Hantson, P. Reversible cardiac dysfunction after venlafaxine overdose and possible influence of genotype and metabolism. Forensic Sci. Int. 2016, 266, e48–e51. [Google Scholar] [CrossRef] [PubMed]
- Kontio, T.; Salo, A.; Kantola, T.; Toivonen, L.; Skrifvars, M.B. Successful use of therapeutic hypothermia after cardiac arrest due to amitriptyline and venlafaxine intoxication. Ther. Hypothermia Temp. Manag. 2015, 5, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Azdaki, N.; Nakhaee, S.; Zamani, N.; Mehrpour, O. Refractory Arrhythmias in a Young Patient Poisoned by Imipramine. Cardiovasc. Toxicol. 2019, 19, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Ohnishi, M.; Takegawa, R.; Hirose, T.; Hattori, Y.; Shimazu, T. Effect of lipid emulsion during resuscitation of a patient with cardiac arrest after overdose of chlorpromazine and mirtazapine. Am. J. Emerg. Med. 2015, 33, 1541.e1-2. [Google Scholar] [CrossRef] [PubMed]
- Galust, H.; Seltzer, J.A.; Hardin, J.R.; Friedman, N.A.; Minns, A. Tianeptine abuse via novel, extended-release, star-shaped, drug delivery device. Toxicol. Rep. 2023, 11, 162–164. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Subramanian, S.; Oughli, H.A.; Gebara, M.A.; Palanca, B.J.A.; Lenze, E.J. Treatment-Resistant Late-Life Depression: A Review of Clinical Features, Neuropsychology, Neurobiology, and Treatment. Psychiatr. Clin. N. Am. 2023, 46, 371–389. [Google Scholar] [CrossRef] [PubMed]
- Nezafati, M.H.; Vojdanparast, M.; Nezafati, P. Antidepressants and cardiovascular adverse events: A narrative review. ARYA Atheroscler 2015, 11, 295–304. [Google Scholar] [PubMed] [PubMed Central]
- Khawam, E.A.; Laurencic, G.; Malone, D.A., Jr. Side effects of antidepressants: An overview. Cleve Clin. J. Med. 2006, 73, 351. [Google Scholar] [CrossRef] [PubMed]
- Simon, L.V.; Torrico, T.J.; Keenaghan, M. Serotonin Syndrome; Updated 2 March 2024. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025; Available online: https://www.ncbi.nlm.nih.gov/books/NBK482377/ (accessed on 10 April 2025).
- Hetrick, S.E.; McKenzie, J.E.; Bailey, A.P.; Sharma, V.; Moller, C.I.; Badcock, P.B.; Cox, G.R.; Merry, S.N.; Meader, N. New generation antidepressants for depression in children and adolescents: A network meta-analysis. Cochrane Database Syst. Rev. 2021, 5, CD013674. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012. Available online: https://www.ncbi.nlm.nih.gov/books/NBK547852/ (accessed on 10 April 2025).
- Kharofa, J.; Sekar, P.; Haverbusch, M.; Moomaw, C.; Flaherty, M.; Kissela, B.; Broderick, J.; Woo, D. Selective serotonin reuptake inhibitors and risk of hemorrhagic stroke. Stroke 2007, 38, 3049–3051. [Google Scholar] [CrossRef] [PubMed]
- De Picker, L.; Eede, F.V.D.; Dumont, G.; Moorkens, G.; Sabbe, B.G. Antidepressants and the risk of hyponatremia: A class-by-class review of literature. Psychosomatics 2014, 55, 536–547. [Google Scholar] [CrossRef] [PubMed]
- De Luca, B.; Canozzi, A.; Mosconi, C.; Gastaldon, C.; Papola, D.; Metelli, A.; Tedeschi, F.; Amaddeo, F.; Purgato, M.; Solmi, M.; et al. Efficacy and tolerability of antidepressants in individuals suffering from physical conditions and depressive disorders: Network meta-analysis. Br. J. Psychiatry 2025, 1–14. [Google Scholar] [CrossRef]
- Kelly, K.; Posternak, M.; Jonathan, E.A.; Je, A. Toward achieving optimal response: Understanding and managing antidepressant side effects. Dialogues Clin. Neurosci. 2008, 10, 409–418. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Khalid, M.M.; Waseem, M. Tricyclic Antidepressant Toxicity; Updated 17 July 2023. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025; Available online: https://www.ncbi.nlm.nih.gov/books/NBK430931/ (accessed on 10 April 2025).
- Chan, C.Y.Y.; Waring, W.S. Tricyclic Cardiotoxicity Treated With Sodium Bicarbonate. Circulation 2007, 115, e63–e64. [Google Scholar] [CrossRef] [PubMed]
- Eken, C. Hypertonic saline: An alternative therapy in TCA overdoses failed to respond sodium bicarbonate. Clin. Toxicol. 2008, 46, 488. [Google Scholar] [CrossRef] [PubMed]
- Bruccoleri, R.E.; Burns, M.M. A Literature Review of the Use of Sodium Bicarbonate for the Treatment of QRS Widening. J. Med. Toxicol. 2016, 12, 121–129. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sabri, M.A.; Saber-Ayad, M.M. MAO Inhibitors; Updated 5 Jun 2023. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025; Available online: https://www.ncbi.nlm.nih.gov/books/NBK557395/ (accessed on 10 April 2025).
- Nassef, M.; Abdulhalim, N. VA ECMO and drug intoxication. In Evolving Therapies and Technologies in Extracorporeal Membrane Oxygenation; IntechOpen: London, UK, 2024. [Google Scholar] [CrossRef]
- Weiner, L.; Mazzeffi, M.A.; Hines, E.Q.; Gordon, D.; Herr, D.L.; Kim, H.K. Clinical utility of venoarterial-extracorporeal membrane oxygenation (VA-ECMO) in patients with drug-induced cardiogenic shock: A retrospective study of the Extracorporeal Life Support Organizations’ ECMO case registry. Clin. Toxicol. 2019, 58, 705–710. [Google Scholar] [CrossRef] [PubMed]
- Maina, G.; Adami, M.; Ascione, G.; Bondi, E.; De Berardis, D.; Delmonte, D.; Maffezzoli, S.; Martinotti, G.; Nivoli, A.; Ottavianelli, E.; et al. Nationwide consensus on the clinical management of treatment-resistant depression in Italy: A Delphi panel. Ann. Gen. Psychiatry 2023, 22, 48. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Checklist |
|---|
| 1. Were the Patient’s Demographic Characteristics Clearly Described? Yes/No/Unclear/Not Applicable |
| 2. Was the Patient’s History Clearly Described and Presented as a Timeline? Yes/No/Unclear/Not Applicable |
| 3. Was the Current Clinical Condition of the Patient on Presentation Clearly Described? Yes/No/Unclear/Not Applicable |
| 4. Were Diagnostic Tests or Assessment Methods and the Results Clearly Described? Yes/No/Unclear/Not Applicable |
| 5. Was the Intervention(s) or Treatment Procedure(s) Clearly Described? Yes/No/Unclear/Not Applicable |
| 6. Was the Postintervention Clinical Condition Clearly Described? Yes/No/Unclear/Not Applicable |
| 7. Were Adverse Events (Harms) or Unanticipated Events Identified and Described? Yes/No/Unclear/Not Applicable |
| 8. Does the Case Report Provide Takeaway Lessons? Yes/No/Unclear/Not Applicable |
| S. No | First Author’s Name/Year of Publication [Reference Number] | Age and Sex of Patient | Antidepressant | Signs and Symptoms | Treatment and Post-Operative Conditions | Follow Up |
|---|---|---|---|---|---|---|
| 1 | Nguyen H/2021 [13] | 35-year-old female | Tricyclic antidepressant (amitriptyline) | Severe cardiovascular toxicities with arrhythmia characterized by QT elongation. | The patient was admitted to the intensive care unit, where hemodynamic parameters and ECG were monitored; she regained consciousness after 2 days. | After 7 days the patient did not reveal any recurrence. |
| 2 | Ikejiri K/2021 [14] | 19-year-old male | Tricyclic antidepressant (amitriptyline) | The patient presented with altered mental status and a seizure. The following values were assessed: initial body temperature (36.1 °C), heart rate (86 bpm), blood pressure (92/39 mm Hg), and SpO2 (98%). Upon admission, the patient exhibited a comatose state with a Glasgow Coma Scale score of 3 (E1V1M1) with a generalized tonic-clonic seizure. His urine test result was positive for Amitriptyline. | The patient was admitted and intubated. The ECG showed a wide QRS complex tachycardia. Following ingestion, the patient experienced a TCA-induced cardiac arrest. The patient was initiated on VA-ECMO 240 min later, which stabilized the hemodynamic status, and the ECG gradually improved. The patient was weaned off ECMO after 27 h. After completing targeted temperature management, their mental status improved, and they were extubated on day 5. | He presented with no neurological deficits and was discharged on day 15. |
| 3 | Zitoune Z/2023 [15] | 55-year-old female | Tricyclic antidepressant (imipramine) | The patient was found unconscious on site and presented with arrhythmia, cardiac arrest, cardiogenic shock, coma, and respiratory distress. | The patient presented with severe rhabdomyolysis and rapidly developed cardiogenic shock and malignant arrhythmias requiring VA-ECMO. Continuous renal replacement therapy was initiated after admission. Serial blood measurements of imipramine and its active metabolite desipramine were performed. Cardiac function improved, and ECMO was explanted after 4 days. The severe acute respiratory distress syndrome resolved spontaneously, and the neurological outcome was favorable despite early myoclonus. The patient regained consciousness on the fifth day. | The patient was discharged after 4 weeks. |
| 4 | Amiri H/2016 [16] | 27-year-old male | Clonazepam and tricyclic antidepressant (amitriptyline) | The patient presented with status epilepticus, hypotension, and refractory QRS complex widening. | The patient was admitted to intensive care unit, where he resolved after intravenous administration of 2650 mEq sodium bicarbonate. | He was discharged after 1 week with no symptoms. |
| 5 | Kassim T/2018 [17] | 21-year-old male | Tricyclic antidepressant (amitriptyline) | The patient presented unconscious after an amitriptyline overdose as a suicide attempt. | The patient was admitted to the intensive care unit. The ECG showed sinus tachycardia. Intravenous fluids and sodium bicarbonate were administered. The patient was taken off mechanical ventilation after 2 days with signs of improvement. | The patient was discharged after 7 days of hospitalization. At the 1-month follow-up, the troponin level was repeated and was within normal limits. |
| 6 | Robinson S/2022 [18] | 14-year-old female | Bupropion | The patient ingested 15 g of bupropion, resulting in the onset of status epilepticus, with QT prolongation evolving into ventricular tachycardia and ventricular fibrillation, requiring five cardioversions and one defibrillation. | The QT interval eventually narrowed after supportive care and lidocaine drip. The patient was able to be extubated two days later, with cognitive function and good echocardiogram. | - |
| 7 | Koshy P/2023 [19] | 22-year-old female | Selective serotonin reuptake inhibitor (escitalopram) | The patient presented with the following values: heart rate 102 bpm, blood pressure 130/80 mmHg, respiratory rate 20 rpm, oxygen saturation 97% on room air, and temperature 36.4 °C. She was fully conscious and oriented. | Her ECG showed T-wave inversions that resolved the next day with supportive management. After 24 h, she developed dystonia, which resolved with low doses of benzodiazepines. | She became stable and was discharged on the fifth day after admission. |
| 8 | Cobilinschi C/2023 [20] | 33-year-old male | Selective serotonin and norepinephrine reuptake inhibitor (venlafaxine) | The patient was cardiorespiratory stable but unresponsive with a GCS of 4, was intubated, and mechanical ventilation was initiated. | After admission to the intensive care unit, he developed cardiovascular collapse refractory to vasopressors with junctional bradycardia, followed by spontaneous conversion to sinus rhythm. This was followed by ventricular extrasystoles, trigeminy, and even episodes of non-sustained ventricular tachycardia. Generalized tonic-clonic seizures were observed. Antiarrhythmic and anticonvulsant therapy, an intravenous lipid emulsion bolus, and continuous infusion were then administered. His condition progressively improved over the following hours, and 24 h later the vasopressor was gradually discontinued. On day 2, the patient had a recurrence of cardiovascular collapse, and a second dose of intravenous lipid emulsion was administered. | The patient was discharged in good condition on day 15 and referred to a psychiatrist. |
| 9 | Ando M/2020 [21] | 68-year-old male | Tricyclic antidepressant (amitriptyline) | The patient was found with impaired awareness. Upon admission, the GCS score was 3 (E1; V1; M1); systolic/diastolic blood pressure, 101/62 mm Hg; oxygen saturation, 94%; body temperature, 38.7 °C; heart rate, 120/min; respiration rate, 20/min; blood pH, 7.022; QTc interval, 610 ms; and QRS interval, 270 ms. | Atrial fibrillation was evident on the electrocardiogram. The patient was subsequently intubated and treated for a shock-like hemodynamic status. The patient’s level of consciousness improved 60 h after admission. On the 10th day, he was transferred to the medical psychiatry unit. | In the unit, the patient reported transient suicidal feelings that gradually dissipated. On the 23rd day, he was discharged. |
| 10 | Reinsch N/2021 [22] | 18-year-old female | Bupropion | Bupropion ingestion triggered status epilepticus, prolonged QTc, widened QRS, pulseless ventricular tachycardia, and cardiovascular collapse necessitating ECMO and Impella support. | This patient exhibited a widening QRS complex despite aggressive bicarbonate therapy and recurrent episodes of pulseless ventricular tachycardia, which were ultimately resolved with lidocaine. Neurological prognosis was complicated by the absence of brainstem reflexes. Following therapy, the patient was weaned off Impella, ECMO, and the ventilator after the seventh day of hospitalization. | She was discharged on hospital day 17 with a plan for intensive outpatient psychiatric therapy. |
| 11 | Angel-Isaza AM/2020 [23] | 28-year-old female | Tricyclic antidepressant (amitriptyline) | The patient developed cardiac arrest and received advanced cardiopulmonary resuscitation. | Hypotension and pulselessness did not respond to sodium treatment. The patient stabilized following treatment with lipid emulsion therapy and was weaned off vasopressors and mechanical ventilation over the next 24 h without residual neurological deficits. | - |
| 12 | Ungureanu R/2025 [24] | 25-year-old male | Bupropion | The patient was admitted to a psychiatric clinic for a suicide attempt by self-poisoning with bupropion; shortly thereafter, she was transferred to the hospital for rhabdomyolysis and hepatic cytolysis syndrome. No abnormalities were observed during the physical examination. | The patient presented with a bupropion overdose. The absence of typical neurotoxic or muscular symptoms and the subsequent involvement of multiple factors led to a decision to initiate early renal replacement therapy, despite the absence of overt acute kidney injury. | On day 4, the patient was discharged in stable condition and referred to a mental health center. |
| 13 | Franco V/2015 [25] | 30-year-old female | Bupropion | The patient presented to the emergency department with four seizure episodes following the ingestion of extended-release bupropion. The patient’s vital signs upon arrival were blood pressure 97/45 mm Hg, heart rate 102 beats/minute, respiratory rate 19 breaths/minute, and O2 saturation 97%. | The patient was intubated and sedated. Subsequently, she was treated with sodium bicarbonate for tachycardia with QT interval prolongation. | - |
| 14 | Garcia S/2017 [26] | 58-year-old male | Selective serotonin and norepinephrine reuptake inhibitor (venlafaxine) | The patient reported experiencing intermittent palpitations associated with exertional dyspnea and added that he had suffered from asthenia and a lack of endurance since early childhood; however, his fatigue symptoms worsened when he started taking venlafaxine approximately twelve years ago. | Electrocardiogram (ECG) examination revealed symptoms of skipped beats and a racing heart, associated with sinus rhythm and occasional premature atrial beats. The patient underwent a dobutamine stress test. His ejection fraction response increased from 60% at rest to 80% at peak stress. The patient’s genetic test results established the phenotype of a CYP2D6 poor metabolizer and a CYP2C19 intermediate metabolizer. | The recommendations included switching the patient to alternative agents. Desvenlafaxine was recommended. |
| 15 | Avcil M/2016 [27] | 30-year-old female | Aripiprazole | She arrived at the emergency department in critical condition approximately 2 h after drug ingestion. Upon arrival, she exhibited signs of cardiotoxicity, including QT interval prolongation and atrial fibrillation, in addition to profound hypotension and severe depression of the central nervous and respiratory systems. | The patient was admitted to the emergency critical care unit, where she was treated with a sodium bicarbonate infusion (20 mEq/h) for acidosis found in the blood gas analysis, and potassium replacement was also started. Because the patient exhibited persistent hypotension, and her metabolic condition did not improve, intravenous lipid emulsion (ILE) therapy was administered. Following this procedure, the ECG returned to sinus rhythm, and the QT interval normalized. | Her overall condition improved after extubation, no further issues were encountered, and she was discharged from the hospital 4 days after admission. Her QTc at discharge was 425 ms. During a follow-up appointment 20 days later, the patient’s only complaint was mild hoarseness. |
| 16 | Castanares-Zapatero D/2016 [28] | 45-year-old female | Selective serotonin and norepinephrine reuptake inhibitor (venlafaxine) | Upon arrival at the emergency facilities, the patient was found to be drowsy, with a Glasgow Coma Scale (GCS) score of 13/15, and presented with the following vital signs: arterial blood pressure 145/84 mmHg, heart rate 100 min−1. The neurological examination showed no particularities, with the exception of horizontal nystagmus. The electrocardiogram revealed a sinus rhythm at 100 min−1, with narrow QRS complexes and a QTc duration of 430 ms. | Due to the progression of altered consciousness, the patient was transferred to the intensive care unit. The venlafaxine overdose resulted in marked left ventricular dysfunction, in the absence of significant conduction disorders on the electrocardiogram. | The patient left the ICU on day 3, without having manifested any symptoms associated with serotonin syndrome. Complete left ventricular function recovery was observed on echocardiogaphy at the 2-week follow-up. |
| 17 | Kontio/2015 [29] | 19-year-old female | Tricyclic antidepressant (amitriptyline) | The cardiac arrest was witnessed, but no bystander cardiopulmonary resuscitation (CPR) was performed. Three defibrillations, magnesium sulfate, and sodium bicarbonate were given and her trachea was intubated, after which return of spontaneous circulation (ROSC) was achieved in 26 min. After ROSC, she had seizures and was sedated. | The patient was unconscious and had dilated pupils. She was tachycardic with a body temperature of 33.5 °C. She was transferred to the intensive care unit was maintained with invasive cooling. During the treatment, she did not experience any serious cardiac arrhythmia, transthoracic echocardiogram was normal, and the electrocardiogram (ECG) returned to normal. The patient was extubated 45 h after the cardiac arrest. After the extubation, she was alert and cooperative, but slightly delusional. | She was transferred to a ward on the third day and discharged from hospital on the sixth day of admission. Ambulatory psychiatric follow-up was organized. |
| 18 | Azdaki N/2019 [30] | 19-year-old female | Tricyclic antidepressant (imipramine) | The patient presented to our emergency department with primary complaints of heart palpitations, weakness, and lethargy. She reportedly had experienced two to three episodes of palpitations within the 2 weeks prior to presentation. Upon admission to the emergency department, the patient became unconscious and experienced a drop attack. She immediately underwent cardiopulmonary monitoring, and an electrocardiogram (ECG) was performed, which showed regular wide complex tachycardia. | The patient was diagnosed with PSVT. A drug screening urine test was performed. She had ingested imipramine. Sodium bicarbonate (15 cc twice) and calcium gluconate were administered. Despite the administration of amiodarone, the patient still had arrhythmia and, given the patient’s better reaction to lidocaine, amiodarone was substituted with lidocaine. Infusion of lidocaine and sodium bicarbonate and gastric decontamination were continued in the ICU, and the QRS complex narrowed on the ECG. | - |
| 19 | Matsumoto H/2015 [31] | 24-year-old female | Mirtazapine | The patient with depression was found in a comatose state, and an emergency life-saving technology service was called. After transport to the hospital, she stopped breathing, became pulseless, and immediate cardiopulmonary life support was initiated. Electrocardiographic monitoring showed asystole during resuscitation even after arrival at the hospital. | In addition to the conventional administration of adrenaline (total dose, 5 mg), 20% intralipid 100 mL was administered intravenously 8 min after arrival at the hospital and administered again 27 min after re-arrival at the hospital due to persistent asystole. Return of spontaneous circulation occurred 29 min after arrival (70 min after cardiac arrest). | The patient recovered without major complications and was transferred to another hospital for psychiatric care 70 days after admission. |
| 20 | Galust H/2023 [32] | 53-year-old female | Selective serotonin and norepinephrine reuptake inhibitor (venlafaxine) | The electrocardiogram showed sinus tachycardia with an enlarged QRS complex but normal corrected QT interval. The patient was rapidly intubated and mechanically ventilated. She received fluids and 8.4% sodium bicarbonate. Norepinephrine infusion was started. | Initial management of cardiovascular failure included dobutamine, norepinephrine, and epinephrine infusions to maintain blood pressure. Due to refractoriness of the presumed drug-induced cardiac failure with rapidly worsening multiorgan failure, veno-arterial ECMO was implemented via femoral vessel cannulation at the bedside. | The ICU stay was complicated by the onset of ventilator-associated pneumonia related to Enterobacter cloacae, treated with cefepime, and by several hemorrhage episodes at the cannulation site requiring red blood cell, platelet, and fresh plasma transfusions. The patient was discharged to the medical ward after 25 days of hospitalization. |
| Sl. No | First Author’s Name/Year of Publication | 1. Were the Patient’s Demographic Characteristics Clearly Described? | 2. Was the Patient’s History Clearly Described and Presented as a Timeline? | 3. Was the Current Clinical Condition of the Patient on Presentation Clearly Described? | 4. Were the Diagnostic Tests or Assessment Methods and the Results Clearly Described? | 5. Was the Intervention(s) or Treatment Procedure(s) Clearly Described? | 6. Was the Postintervention Clinical Condition Clearly Described? | 7. Were Adverse Events (Harms) or Unanticipated Events Identified and Described? | 8. Does the Case Report Provide Takeaway Lessons? | Overall Risk of Bias |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Nguyen H/2021 [13] | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | L |
| 2 | Ikejiri K/2021 [14] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | L |
| 3 | Zitoune Z/2023 [15] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | L |
| 4 | Amiri H/2016 [16] | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | L |
| 5 | Kassim T/2018 [17] | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | L |
| 6 | Robinson S/2022 [18] | Yes | Yes | Yes | Yes | Yes | No | No | Yes | M |
| 7 | Koshy P/2023 [19] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | L |
| 8 | Cobilinschi C/2023 [20] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | L |
| 9 | Ando M/2020 [21] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | L |
| 10 | Reinsch N/2021 [22] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | L |
| 11 | Angel-Isaza AM/2020 [23] | Yes | Yes | Yes | Yes | Yes | No | No | Yes | M |
| 12 | Ungureanu R/2025 [24] | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | L |
| 13 | Franco V/2015 [25] | Yes | Yes | Yes | No | Yes | No | No | Yes | M |
| 14 | Garcia S/2017 [26] | Yes | Yes | Yes | Yes | Yes | No | No | Yes | L |
| 15 | Mucahit A/2016 [27] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | L |
| 16 | Castanares-Zapatero D/2016 [28] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | L |
| 17 | Kontio/2015 [29] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | L |
| 18 | Azdaki N/2019 [30] | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | L |
| 19 | Matsumoto H/2015 [31] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | L |
| 20 | Galust H/2023 [32] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | L |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Nakhebi, O.A.S.; Albu-Kalinovic, R.; Bosun, A.; Neda-Stepan, O.; Gliga, M.; Crișan, C.-A.; Marinescu, I.; Enătescu, V.-R. Management of Systemic Cardiotoxicity Associated with Antidepressant Use in Patients with Depressive Disorders: A Systematic Review. J. Clin. Med. 2025, 14, 3696. https://doi.org/10.3390/jcm14113696
Al Nakhebi OAS, Albu-Kalinovic R, Bosun A, Neda-Stepan O, Gliga M, Crișan C-A, Marinescu I, Enătescu V-R. Management of Systemic Cardiotoxicity Associated with Antidepressant Use in Patients with Depressive Disorders: A Systematic Review. Journal of Clinical Medicine. 2025; 14(11):3696. https://doi.org/10.3390/jcm14113696
Chicago/Turabian StyleAl Nakhebi, Omar Anwar Saleh, Raluka Albu-Kalinovic, Adela Bosun, Oana Neda-Stepan, Marius Gliga, Cătălina-Angela Crișan, Ileana Marinescu, and Virgil-Radu Enătescu. 2025. "Management of Systemic Cardiotoxicity Associated with Antidepressant Use in Patients with Depressive Disorders: A Systematic Review" Journal of Clinical Medicine 14, no. 11: 3696. https://doi.org/10.3390/jcm14113696
APA StyleAl Nakhebi, O. A. S., Albu-Kalinovic, R., Bosun, A., Neda-Stepan, O., Gliga, M., Crișan, C.-A., Marinescu, I., & Enătescu, V.-R. (2025). Management of Systemic Cardiotoxicity Associated with Antidepressant Use in Patients with Depressive Disorders: A Systematic Review. Journal of Clinical Medicine, 14(11), 3696. https://doi.org/10.3390/jcm14113696






