AI-Based Predictive Models for Cardiogenic Shock in STEMI: Real-World Data for Early Risk Assessment and Prognostic Insights
Abstract
1. Introduction
2. Materials and Methods
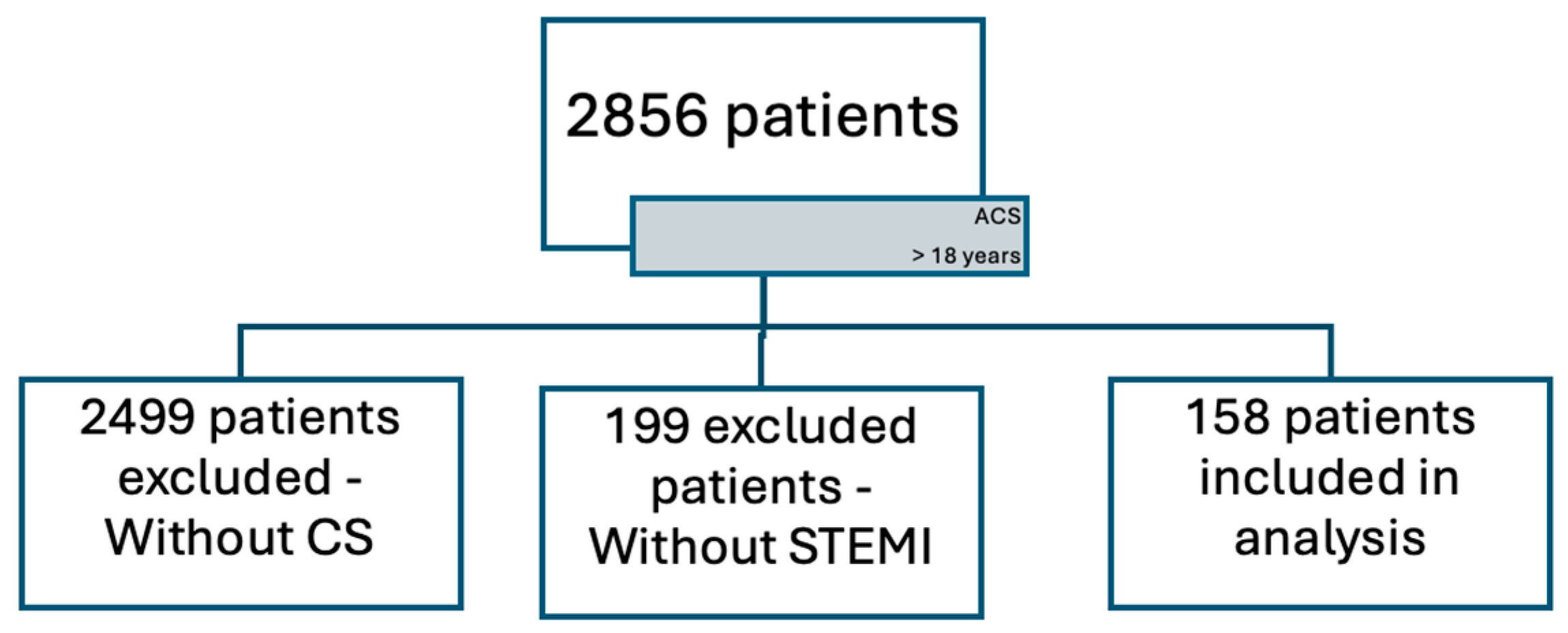
2.1. Dataset
- Inclusion Criteria:
- Acute coronary syndrome complicated by cardiogenic shock in patients who received care in the Cardiology Unit of the University Emergency Hospital of Bucharest.
- Age > 18 years.
- Exclusion Criteria:
- Medical records with missing hospitalization data.
- Patients who requested to be discharged against medical advice.
- Patients with end-stage liver disease.
- Patients with a diagnosis of sepsis [35].
- Patients with other severe infections without a diagnosis of sepsis.
- Patients with severe malnutrition.
- Patients receiving large-volume blood transfusions.
- Patients with a diagnosis of active malignancy.
- Patients with coagulation disorders, such as patients with a diagnosis of thrombophilia and patients with coagulopathy.
2.2. Analyzed Variables
- o
- Demographic and Clinical Data: Age, sex, time from symptom onset, heart rate, and Killip class (according to the definition [36];
- o
- o
- ECG Features: ECG rhythm (sinus rhythm, atrial fibrillation, ventricular tachycardia, ventricular fibrillation, and junctional rhythm), conduction abnormalities, localization of ST elevation, ST elevation in aVR, QRS duration, Q wave presence, and reciprocal ST depression;
- o
- Laboratory Findings (in ED): Hemoglobin, leukocyte count, troponin, creatine kinase–MB isoenzyme (CKMB), creatine kinase isoenzyme (CKI), glucose, creatinine, potassium (K), sodium (Na), aspartate aminotransferase (AST), ALT (alanine aminotransferase), urea nitrogen (BUN), and fibrinogen;
- o
- Echocardiographic Data (ED cardiology consultation phase): left ventricular ejection fraction (LVEF) (EF > 50, EF 40–50, EF < 40), mitral regurgitation, right ventricular (RV) dysfunction, left ventricular (LV) thrombosis, LV aneurysm, pericardial effusion, and mechanical complications.
- Metabolic and renal function (e.g., creatinine, potassium) [41];
2.3. Data Preprocessing
2.4. Statistical Analysis
2.5. Machine Learning Models
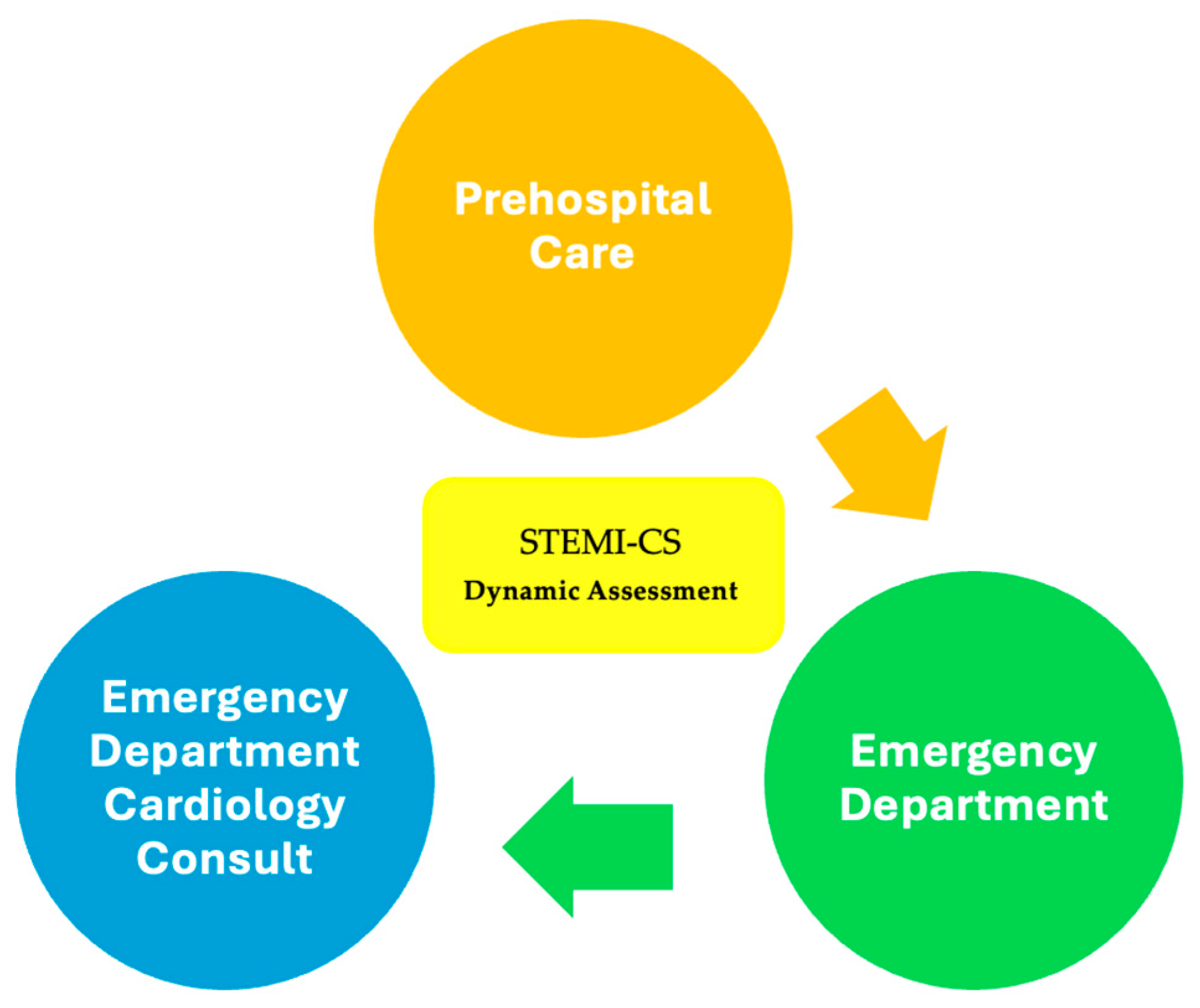
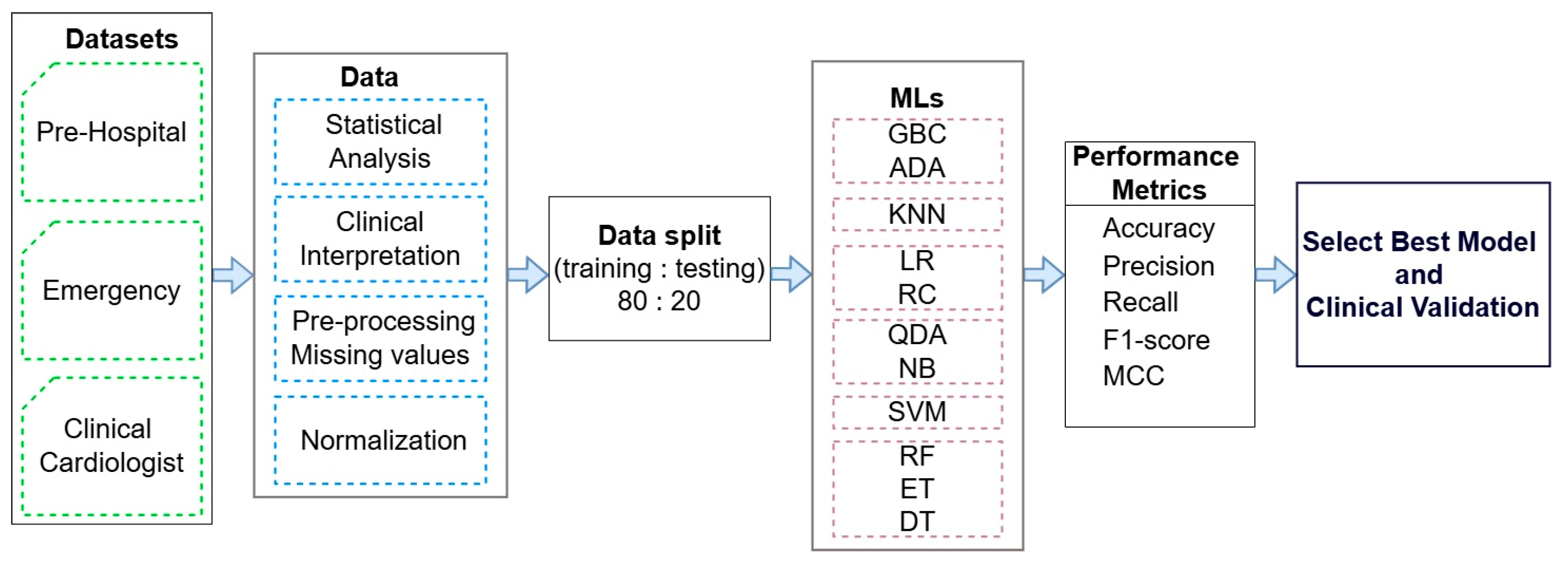
2.6. Framework
- Three datasets (marked with a green color) from the prehospital phase, emergency department phase, and ED cardiology consultation phase with real clinical data were collected by providers from the Cardiology Department of the University Emergency Hospital of Bucharest, Romania, in accordance with the inclusion and exclusion criteria outlined in the Section 2.1.
- Advanced statistical analysis and clinical interpretation were performed to select the most relevant clinical parameters for predicting cardiogenic shock, reducing dimensionality while preserving predictive performance. Multicollinearity was evaluated using the variance inflation factor, and McNemar’s test assessed interaction effects and model consistency. Model performance was measured using accuracy for overall correctness and the F1-score to balance precision and sensitivity, minimizing both false positives and false negatives. Then, 95% confidence intervals (CIs) for accuracy were computed to assess robustness in clinical settings. Following parameter selection, logistic regression was used to evaluate the explanatory power of individual predictors, while Random Forest captured non-linear interactions to enhance performance in complex datasets.
- Data preprocessing steps were applied, including the handling of missing values and feature normalization to ensure data quality and consistency.
- The pre-processed data were split into training and testing sets in an 80:20 ratio for the training and testing of eleven ML models (marked with a purple color). These models were evaluated using standard performance metrics (accuracy—ACC; precision; recall; F1-score; and Matthews correlation coefficient—MCC) to identify the most accurate and clinically relevant model.
- Clinical validation was conducted for the best-performing ML model to assess its applicability and reliability in real-world medical settings.
3. Results
3.1. Study Population and Cardiovascular Risk Factors
3.2. Model Performance
3.2.1. Predictive Parameters for Prehospital Phase
3.2.2. Emergency Department Evaluation Phase
3.2.3. Cardiology Consultation Phase in Emergency Department
4. Discussion
4.1. Key Prognostic Variables for Risk of Progression to Cardiogenic Shock in STEMI Patients
- Main Objective and Comparison with the Literature
- Key Predictors of Progression to CS
- Comparison with Existing Studies
- Importance of Predictive Models in Prehospital Setting
- Phase-Specific Model Performance Insights
4.2. Necessity of Study on Predictive Models for Progression to Cardiogenic Shock in STEMI Patients
4.3. Comparative Analysis of Predictive Models for Cardiogenic Shock Progression in STEMI Patients and Our Study
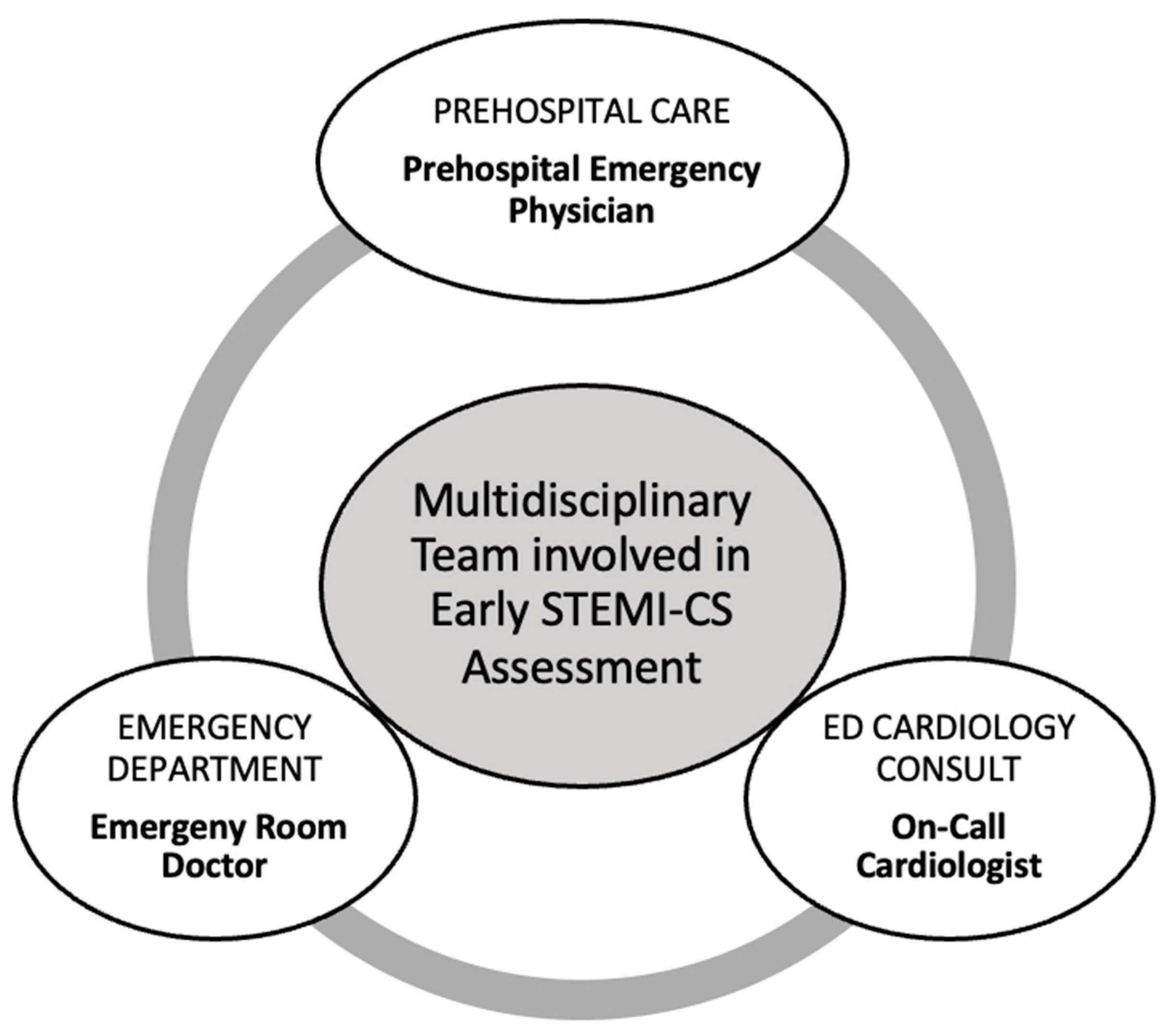
4.3.1. Prehospital Care
4.3.2. Emergency Department
4.3.3. Emergency Department Cardiology Consult
4.4. Clinical Implications and Directions for Digital Implementation
4.5. Lessons Learned
- Phase-specific predictive modeling enables a more granular understanding of cardiogenic shock risk in STEMI patients.
- Early identification of key clinical parameters—such as Killip class, creatinine, potassium, ECG rhythm, and symptom onset—can significantly improve triage decisions.
- Prehospital and emergency department models are critical for timely reperfusion and resource allocation, especially in settings with limited catheterization lab access.
- Random Forest and Extra Trees algorithms performed robustly in early phases, highlighting their potential for clinical integration.
- Predictive tools relying on simple, routine data can be feasibly implemented into standard workflows, including mobile applications and clinical alert systems.
5. Limitations
- Sample Size and External Validation: As a retrospective, single-center study, these findings are also subject to potential selection and information biases. To enhance generalizability, future research should include larger, multicenter cohorts or validate the model on external datasets. Statistical findings should be interpreted accordingly and confirmed by more extensive prospective research. We acknowledge that the exclusion of NSTEMI and unstable angina patients may limit the generalizability of our findings. However, this methodological decision was made to enhance model robustness by focusing on STEMI patients, who follow a more standardized and time-sensitive emergency care pathway. Future studies will aim to expand this approach to other ACS subtypes with appropriate modeling strategies.Additionally, the current sample size and low number of cardiogenic shock events within subgroups limited the feasibility of conducting statistically robust subgroup analyses (e.g., by age, sex, infarct location, or comorbidity profile). Performing such analyses under these constraints could result in unstable estimates or misleading interpretations. This limitation will be addressed in future multicenter studies, where stratified performance evaluation across clinically relevant subgroups will be feasible.
- Lack of Ethnic Diversity: The study population consists exclusively of Caucasian patients, which may restrict the applicability of the model to other ethnic groups. Further validation in more diverse populations is necessary to broaden its clinical utility.
- Geographical Context: This study was conducted in an Eastern European country, where access to advanced mechanical circulatory support is more limited compared to Western Europe. To strengthen the predictive model’s robustness, validation on international datasets is required. Future research may also include subgroup analyses by geographic region or healthcare system characteristics to better understand potential disparities in model performance across different clinical environments.
- Socioeconomic Factors: The study was performed in a Romanian center of excellence, where patients have access to more resources than those treated in smaller regional hospitals. This may affect the model’s applicability in different healthcare settings. A subgroup analysis comparing STEMI-CS patients from high-resource centers with those from smaller hospitals could provide further insight into these disparities.
- Missing Data for Key Clinical Variables: Several clinically relevant parameters—such as NT-proBNP, lactate, blood pressure, oxygen saturation, and signs of hypoperfusion—were excluded from the final analysis due to incomplete data, particularly during early phases of care. Although these variables are known to contribute to cardiogenic shock risk prediction, including them would have introduced bias. Future prospective studies should incorporate systematic data collection to evaluate their predictive value more accurately.
6. Future Research Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AI | Artificial intelligence |
| ACS | Acute coronary syndrome |
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| AMI | Acute myocardial infarction |
| CI | Confidence intervals |
| CICU | Cardiac intensive care unit |
| CS | Cardiogenic shock |
| CS-Team | Team-based cardiogenic shock |
| CKD | Chronic kidney disease |
| DES | Drug eluting stent |
| DM | Diabetes mellitus |
| DT | Decision tree |
| ECG | Electrocardiographic |
| EHR | Electronic health record |
| ET | Extra Trees |
| GBC | Gradient boosting |
| HTN | Hypertension |
| KNN | K-nearest neighbors |
| LDH | Lactic acid dehydrogenase |
| LR | Logistic regression |
| LR | Logistic regression |
| MCC | Matthews correlation coefficient |
| MCS | Mechanical circulatory support |
| ML | Machine learning |
| NSTEMI | Non ST-elevation myocardial infarction |
| NB | Naïve Bayes |
| P-E-I-CI | Prioritization and evolving ischemia in cardiogenic instability |
| PREMs | Patient-reported experience measures |
| PROMs | Patient-reported outcome measures |
| QDA | Quadratic discriminant analysis |
| RF | Random Forest |
| SCAI SHOCK | Society for Cardiovascular Angiography and Interventions Shock |
| STEMI | ST-elevation myocardial infarction |
| SVM | Support vector machine |
| VIF | Variance inflation factor |
| WBC | White blood cell |
References
- So, D.Y.; Boudreau, R.; Chih, S. The Role of a Cardiogenic Shock Team in Decision Making Surrounding Mechanical Circulatory Support. Can. J. Cardiol. 2025, 41, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Yuen, T.; Senaratne, J.M. Definition, Classification, and Management of Primary Noncardiac Causes of Cardiogenic Shock. Can. J. Cardiol. 2024, 41, 587–604. [Google Scholar] [CrossRef] [PubMed]
- Lüsebrink, E.; Binzenhöfer, L.; Adamo, M.; Lorusso, R.; Mebazaa, A.; Morrow, D.A.; Price, S.; Jentzer, J.C.; Brodie, D.; Combes, A.; et al. Cardiogenic shock. Lancet 2024, 404, 2006–2020. [Google Scholar] [CrossRef] [PubMed]
- Kanhouche, G.; Nicolau, J.C.; Furtado, R.H.d.M.; Carvalho, L.S.; Dalçoquio, T.F.; Pileggi, B.; Marchi, M.F.d.S.; Abi-Kair, P.; Lopes, N.; Giraldez, R.R.; et al. Long-term outcomes of cardiogenic shock and cardiac arrest complicating ST-elevation myocardial infarction according to timing of occurrence. Eur. Heart J. Open 2024, 4, oeae075. [Google Scholar] [CrossRef]
- Lee, K.H.; Harrison, W.; Chow, K.L.; Lee, M.; Kerr, A.J. Cardiogenic Shock Prior to Percutaneous Coronary Intervention in ST-Elevation Myocardial Infarction: Outcomes and Predictors of Mortality (ANZACS-QI 73). Heart Lung Circ. 2024, 33, 450–459. [Google Scholar] [CrossRef]
- Pöss, J.; Desch, S.; Thiele, H. Shock management in acute myocardial infarction. EuroIntervention 2014, 10, T74–T82. [Google Scholar] [CrossRef]
- Tamis-Holland, J.E.; Abbott, J.D.; Al-Azizi, K.; Barman, N.; Bortnick, A.E.; Cohen, M.G.; Dehghani, P.; Henry, T.D.; Latif, F.; Madjid, M.; et al. SCAI Expert Consensus Statement on the Management of Patients with STEMI Referred for Primary PCI. J. Soc. Cardiovasc. Angiogr. Interv. 2024, 3, 102294. [Google Scholar] [CrossRef]
- Parlow, S.; Fernando, S.M.; Pugliese, M.; Qureshi, D.; Talarico, R.; Sterling, L.H.; van Diepen, S.; Herridge, M.S.; Price, S.; Brodie, D.; et al. Resource Utilization and Costs Associated with Cardiogenic Shock Complicating Myocardial Infarction: A Population-Based Cohort Study. JACC Adv. 2024, 3, 101047. [Google Scholar] [CrossRef]
- Jamil, Y.; Park, D.Y.; Rao, S.V.; Ahmad, Y.; Sikand, N.V.; Bosworth, H.B.; Coles, T.; Damluji, A.A.; Nanna, M.G.; Samsky, M.D. Association Between Frailty and Management and Outcomes of Acute Myocardial Infarction Complicated by Cardiogenic Shock. JACC Adv. 2024, 3, 100949. [Google Scholar] [CrossRef]
- Warren, A.F.; Rosner, C.; Gattani, R.; Truesdell, A.G.; Proudfoot, A.G. Cardiogenic Shock: Protocols, Teams, Centers, and Networks. US Cardiol. Rev. 2021, 15, e18. [Google Scholar] [CrossRef]
- Shenoy, N.; Devasia, T. Comparison of Outcomes between Early and Late Presentation of ST-elevation Myocardial Infarction in Patients with Cardiogenic Shock. Int. J. Cardiovasc. Acad. 2024, 10, 53–59. [Google Scholar] [CrossRef]
- Sachdeva, P.; Kaur, K.; Fatima, S.; Mahak, F.; Noman, M.; Siddenthi, S.M.; Surksha, M.A.; Munir, M.; Fatima, F.; Sultana, S.S.; et al. Advancements in Myocardial Infarction Management: Exploring Novel Approaches and Strategies. Cureus 2023, 15, e45578. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.V.; O’Donoghue, M.L.; Ruel, M.; Rab, T.; Tamis-Holland, J.E.; Alexander, J.H.; Baber, U.; Baker, H.; Cohen, M.G.; Cruz-Ruiz, M.; et al. 2025 ACC/AHA/ACEP/NAEMSP/SCAI Guideline for the Management of Patients with Acute Coronary Syndromes: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2025, 151, e771–e862. [Google Scholar] [PubMed]
- Naidu, S.S.; Baran, D.A.; Jentzer, J.C.; Hollenberg, S.M.; van Diepen, S.; Basir, M.B.; Grines, C.L.; Diercks, D.B.; Hall, S.; Kapur, N.K.; et al. SCAI SHOCK Stage Classification Expert Consensus Update: A Review and Incorporation of Validation Studies. J. Soc. Cardiovasc. Angiogr. Interv. 2022, 1, 100008. [Google Scholar] [CrossRef]
- Leung, C.; Fong, Y.H.; Chiang, M.C.S.; Wong, I.M.H.; Ho, C.B.; Yeung, Y.K.; Leung, C.Y.; Lee, P.H.; So, T.C.; Cheng, Y.W.; et al. Protocol-Driven Best Practices and Cardiogenic Shock Survival in Asian Patients. J. Am. Hear. Assoc. 2025, 14, e037742. [Google Scholar] [CrossRef]
- Elhaddad, M.; Hamam, S. AI-Driven Clinical Decision Support Systems: An Ongoing Pursuit of Potential. Cureus 2024, 16, e57728. [Google Scholar] [CrossRef]
- Artificial Intelligence in Healthcare—European Commission. Available online: https://health.ec.europa.eu/ehealth-digital-health-and-care/artificial-intelligence-healthcare_en (accessed on 15 March 2025).
- Niculet, E.; Bobeica, C.; Stefanopol, I.A.; Pelin, A.M.; Nechifor, A.; Onisor, C.; Tatu, A.L. Once-Daily Abrocitinib for the Treatment of Moderate-to-Severe Atopic Dermatitis in Adults and Adolescents Aged 12 Years and Over: A Short Review of Current Clinical Perspectives. Ther. Clin. Risk Manag. 2022, 18, 399–407. [Google Scholar] [CrossRef]
- Böhm, A.; Segev, A.; Jajcay, N.; Krychtiuk, K.A.; Tavazzi, G.; Spartalis, M.; Kollarova, M.; Berta, I.; Jankova, J.; Guerra, F.; et al. Machine learning-based scoring system to predict cardiogenic shock in acute coronary syndrome. Eur. Heart J.-Digit. Heal. 2025, 6, 240–251. [Google Scholar] [CrossRef]
- Stamate, E.; Piraianu, A.-I.; Ciobotaru, O.R.; Crassas, R.; Duca, O.; Fulga, A.; Grigore, I.; Vintila, V.; Fulga, I.; Ciobotaru, O.C. Revolutionizing Cardiology through Artificial Intelligence—Big Data from Proactive Prevention to Precise Diagnostics and Cutting-Edge Treatment—A Comprehensive Review of the Past 5 Years. Diagnostics 2024, 14, 1103. [Google Scholar] [CrossRef]
- Patrascanu, O.S.; Tutunaru, D.; Musat, C.L.; Dragostin, O.M.; Fulga, A.; Nechita, L.; Ciubara, A.B.; Piraianu, A.I.; Stamate, E.; Poalelungi, D.G.; et al. Future Horizons: The Potential Role of Artificial Intelligence in Cardiology. J. Pers. Med. 2024, 14, 656. [Google Scholar] [CrossRef]
- Piraianu, A.-I.; Fulga, A.; Musat, C.L.; Ciobotaru, O.-R.; Poalelungi, D.G.; Stamate, E.; Ciobotaru, O.; Fulga, I. Enhancing the Evidence with Algorithms: How Artificial Intelligence Is Transforming Forensic Medicine. Diagnostics 2023, 13, 2992. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, A.; Nakamura, Y.; Sato, E.; Kagiyama, N. Artificial Intelligence in Clinics: Enhancing Cardiology Practice. JMA J. 2025, 8, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Alowais, S.A.; Alghamdi, S.S.; Alsuhebany, N.; Alqahtani, T.; Alshaya, A.I.; Almohareb, S.N.; Aldairem, A.; Alrashed, M.; Bin Saleh, K.; Badreldin, H.A.; et al. Revolutionizing healthcare: The role of artificial intelligence in clinical practice. BMC Med. Educ. 2023, 23, 689. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.; Vavilin, I.; Nguyen, A.H.; Batchelor, W.B.; Blumer, V.; Cilia, L.; Dewanjee, A.; Desai, M.; Desai, S.S.; Flanagan, M.C.; et al. Contemporary approach to cardiogenic shock care: A state-of-the-art review. Front. Cardiovasc. Med. 2024, 11, 1354158. [Google Scholar] [CrossRef]
- Doolub, G.; Khurshid, S.; Theriault-Lauzier, P.; Lapalme, A.N.; Tastet, O.; So, D.; Langlais, E.L.; Cobin, D.; Avram, R. Revolutionising Acute Cardiac Care with Artificial Intelligence: Opportunities and Challenges. Can. J. Cardiol. 2024, 40, 1813–1827. [Google Scholar] [CrossRef]
- Karalis, V.D. The Integration of Artificial Intelligence into Clinical Practice. Appl. Biosci. 2024, 3, 14–44. [Google Scholar] [CrossRef]
- Stamate, E.; Ciobotaru, O.R.; Arbune, M.; Piraianu, A.I.; Duca, O.M.; Fulga, A.; Fulga, I.; Balta, A.A.S.; Dumitrascu, A.G.; Ciobotaru, O.C. Multidisciplinary Perspectives of Challenges in Infective Endocarditis Complicated by Septic Embolic-Induced Acute Myocardial Infarction. Antibiotics 2024, 13, 513. [Google Scholar] [CrossRef]
- Totolici, G.; Miron, M.; Culea-Florescu, A.-L. Automatic Segmentation and Statistical Analysis of the Foveal Avascular Zone. Technologies 2024, 12, 235. [Google Scholar] [CrossRef]
- Recommendations on Digital Interventions for Health System Strengthening. Available online: https://iris.who.int/bitstream/handle/10665/311941/9789241550505-eng.pdf (accessed on 5 April 2025).
- Johnson, K.B.; Horn, I.B.; Horvitz, E. Pursuing Equity with Artificial Intelligence in Health Care. JAMA Healt Forum 2025, 6, e245031. [Google Scholar] [CrossRef]
- Bergmark, B.A.; Mathenge, N.; Merlini, P.A.; Lawrence-Wright, M.B.; Giugliano, R.P. Acute coronary syndromes. Lancet 2022, 399, 1347–1358. [Google Scholar] [CrossRef]
- Ammirati, E.; Frigerio, M.; Adler, E.D.; Basso, C.; Birnie, D.H.; Brambatti, M.; Friedrich, M.G.; Klingel, K.; Lehtonen, J.; Moslehi, J.J.; et al. Management of Acute Myocarditis and Chronic Inflammatory Cardiomyopathy. Circ. Heart Fail. 2020, 13, e007405. [Google Scholar] [CrossRef] [PubMed]
- Borger, M.; de Waha, S.; Marsan, N.A.; Zühlke, L.; Mestres, C.A.; Fosbol, E.; Bonaros, N.; Pizzi, M.N.; Brida, M.; Sionis, A.; et al. Key priorities for the implementation of the 2023 ESC Guidelines for the management of endocarditis in low-resource settings. Eur. Heart J.-Qual. Care Clin. Outcomes 2025. online ahead of print. [Google Scholar] [CrossRef]
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef] [PubMed]
- de Mello, B.H.G.; Oliveira, G.B.F.; Ramos, R.F.; Lopes, B.B.C.; Barros, C.B.S.; de Oliveira Carvalho, E.; Teixeira, F.B.P.; Arruda, A.S.; Revelo, M.S.C.; Piegas, L.S. Validation of the Killip-Kimball Classification and Late Mortality after Acute Myocardial Infarction. Arq. Bras. Cardiol. 2014, 103, 107. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee; ElSayed, N.A.; Aleppo, G.; Bannuru, R.R.; Bruemmer, D.; Collins, B.S.; Ekhlaspour, L.; Gaglia, J.L.; Hilliard, M.E.; Johnson, E.L.; et al. 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes—2024. Diabetes Care 2023, 47, S20–S42. [Google Scholar] [CrossRef]
- McEvoy, J.W.; McCarthy, C.P.; Bruno, R.M.; Brouwers, S.; Canavan, M.D.; Ceconi, C.; Christodorescu, R.M.; Daskalopoulou, S.S.; Ferro, C.J.; Gerdts, E.; et al. 2024 ESC Guidelines for the management of elevated blood pressure and hypertension. Eur. Heart J. 2024, 45, 3912–4018. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk: The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Fox, K.A.A.; Dabbous, O.H.; Goldberg, R.J.; Pieper, K.S.; Eagle, K.A.; Van de Werf, F.; Avezum, Á.; Goodman, S.G.; Flather, M.D.; Anderson, F.A.; et al. Prediction of risk of death and myocardial infarction in the six months after presentation with acute coronary syndrome: Prospective multinational observational study (GRACE). BMJ 2006, 333, 1091. [Google Scholar] [CrossRef]
- Thiele, H.; Zeymer, U.; Neumann, F.-J.; Ferenc, M.; Olbrich, H.-G.; Hausleiter, J.; Richardt, G.; Hennersdorf, M.; Empen, K.; Fuernau, G.; et al. Intraaortic Balloon Support for Myocardial Infarction with Cardiogenic Shock. N. Engl. J. Med. 2012, 367, 1287–1296. [Google Scholar] [CrossRef]
- Yamaji, H.; Iwasaki, K.; Kusachi, S.; Murakami, T.; Hirami, R.; Hamamoto, H.; Hina, K.; Kita, T.; Sakakibara, N.; Tsuji, T. Prediction of acute left main coronary artery obstruction by 12-lead electrocardiography: ST segment elevation in lead aVR with less ST segment elevation in lead V1. J. Am. Coll. Cardiol. 2001, 38, 1348–1354. [Google Scholar] [CrossRef]
- Byrne, R.; Coughlan, J.J.; Rossello, X.; Ibanez, B.; Members of the Task Force for the 2023 ESC Guidelines for the Management of Acute Coronary Syndromes; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.-A.; et al. Key priorities for the implementation of the 2023 ESC Guidelines for the management of acute coronary syndromes in low-resource settings. Eur. Heart J.-Qual. Care Clin. Outcomes 2025. online ahead of print. [Google Scholar] [CrossRef]
- Byrne, R.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.-A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes: Developed by the task force on the management of acute coronary syndromes of the European Society of Cardiology (ESC). Eur. Heart J. Acute Cardiovasc. Care 2023, 13, 55–161. [Google Scholar] [CrossRef] [PubMed]
- Hochman, J.S.; Sleeper, L.A.; Webb, J.G.; Dzavik, V.; Buller, C.E.; Aylward, P.; Col, J.; White, H.D.; for the SHOCK Investigators. Early Revascularization and Long-term Survival in Cardiogenic Shock Complicating Acute Myocardial Infarction. JAMA 2006, 295, 2511–2515. [Google Scholar] [CrossRef] [PubMed]
- Emilia Babe, E.; Florin Pop, C.; Alexandra Coadă, C.; Lupu, M.; Florin Ferent, I.; Ioana Hodas, R.; Ursu, M.-Ş. Factors Associated with Mortality Risk in Patients with Cardiogenic Shock Post-ST-Elevation Myocardial Infarction: Insights from a Regional Centre in Northwest Romania. Medicina 2025, 61, 725. [Google Scholar]
- Syntila, S.; Chatzis, G.; Markus, B.; Ahrens, H.; Waechter, C.; Luesebrink, U.; Divchev, D.; Schuett, H.; Tsalouchidou, P.-E.; Jerrentrup, A.; et al. Comparison of Mechanical Support with Impella or Extracorporeal Life Support in Post-Cardiac Arrest Cardiogenic Shock: A Propensity Scoring Matching Analysis. J. Clin. Med. 2021, 10, 3583. [Google Scholar] [CrossRef]
- Taha, H.S.; Gohar, A.; Ammar, W.; Alhossary, H.; Adel, A.; Diab, R.; Mahfouz, H.; Shaker, M.M.; Samy, M. Predictors of short-term mortality in cardiogenic shock: Insights from an Egyptian multicenter registry. Egypt. Heart J. 2024, 76, 94. [Google Scholar] [CrossRef]
- García-García, C.; López-Sobrino, T.; Sanz-Girgas, E.; Cueto, M.R.; Aboal, J.; Pastor, P.; Buera, I.; Sionis, A.; Andrea, R.; Rodríguez-López, J.; et al. Cardiogenic shock mortality according to Aetiology in a Mediterranean cohort: Results from the Shock-CAT study. ESC Heart Fail. 2024, 12, 1336–1345. [Google Scholar] [CrossRef]
- Muzafarova, T.; Motovska, Z.; Hlinomaz, O.; Kala, P.; Hromadka, M.; Precek, J.; Mrozek, J.; Matejka, J.; Kettner, J.; Bis, J.; et al. The Prognosis of Cardiogenic Shock Following Acute Myocardial Infarction—An Analysis of 2693 Cases from a Prospective Multicenter Registry. Dtsch. Aerzteblatt Online 2023, 120, 538–539. [Google Scholar] [CrossRef]
- Zhang, Q.; Xu, L.; Xie, Z.; He, W.; Huang, X. Machine learning-based prediction of mortality in acute myocardial infarction with cardiogenic shock. Front. Cardiovasc. Med. 2024, 11, 1402503. [Google Scholar] [CrossRef]
- Senman, B.; Jentzer, J.C.; Barnett, C.F.; Bartos, J.A.; Berg, D.D.; Chih, S.; Drakos, S.G.; Dudzinski, D.M.; Elliott, A.; Gage, A.; et al. Need for a Cardiogenic Shock Team Collaborative—Promoting a Team-Based Model of Care to Improve Outcomes and Identify Best Practices. J. Am. Heart Assoc. 2024, 13, e031979. [Google Scholar] [CrossRef]
- Hérion, F.-X.; Beurton, A.; Oddos, C.; Nubret, K.; Aguerreche, C.; Quessard, A.; Faure, M.; Gerbaud, E.; Pernot, M.; Imbault, J.; et al. Multidisciplinary cardiogenic shock team approach improves the long-term outcomes of patients suffering from refractory cardiogenic shock treated with short-term mechanical circulatory support. Eur. Heart J. Acute Cardiovasc. Care 2023, 12, 821–830. [Google Scholar] [CrossRef] [PubMed]
- El-Mughayyar, D.; Marshall, T.; D’souza, K.; MacLeod, J.B.; McCoy, A.; Morris, S.; Smith, M.; White, C.W.; Sarkar, S.; Brunt, K.R.; et al. Implementation of a Multidisciplinary Cardiogenic Shock Team in a Nonacademic Canadian Heart Centre: An Implementation Study. CJC Open 2025, 7, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Yuriditsky, E.; Horowitz, J.M. Prioritizing Rapid Reperfusion in ST-Segment–Elevation Myocardial Infarction Complicated by Cardiogenic Shock: Leveraging Regionalized Systems of Care. Circ. Cardiovasc. Interv. 2024, 17, e013848. [Google Scholar] [CrossRef] [PubMed]
- Scholz, K.H.; Friede, T.; Meyer, T.; Jacobshagen, C.; Lengenfelder, B.; Jung, J.; Fleischmann, C.; Moehlis, H.; Olbrich, H.G.; Ott, R.; et al. Prognostic significance of emergency department bypass in stable and unstable patients with ST-segment elevation myocardial infarction. Eur. Heart J. Acute Cardiovasc. Care 2018, 9, 34–44. [Google Scholar] [CrossRef]
- Rahman, F.; Finkelstein, N.; Alyakin, A.; Gilotra, N.A.; Trost, J.; Schulman, S.P.; Saria, S. Using Machine Learning for Early Prediction of Cardiogenic Shock in Patients with Acute Heart Failure. J. Soc. Cardiovasc. Angiogr. Interv. 2022, 1, 100308. [Google Scholar] [CrossRef]
- Couronné, R.; Probst, P.; Boulesteix, A.L. Random forest versus logistic regression: A large-scale benchmark experiment. BMC Bioinf. 2018. [Google Scholar] [CrossRef]
- Eliakundu, A.L.; Bloom, J.E.; Ball, J.; Nehme, E.; Okyere, D.; Heritier, S.; Voskoboinik, A.; Dawson, L.; Cox, S.; Anderson, D.; et al. Prehospital factors predicting mortality in patients with shock: State-wide linkage study. Open Heart 2024, 11, e002799. [Google Scholar] [CrossRef]
- Meertens, M.M.; Tichelbäcker, T.; Macherey-Meyer, S.; Heyne, S.; Braumann, S.; Nießen, S.F.; Baldus, S.; Adler, C.; Lee, S. Meta-analysis of extracorporeal membrane oxygenation in combination with intra-aortic balloon pump vs. extracorporeal membrane oxygenation only in patients with cardiogenic shock due to acute myocardial infarction. Front. Cardiovasc. Med. 2023, 9, 1104357. [Google Scholar] [CrossRef]
- Savage, M.L.; Hay, K.; Vollbon, W.; Doan, T.; Murdoch, D.J.; Hammett, C.; Poulter, R.; Walters, D.L.; Denman, R.; Ranasinghe, I.; et al. Prehospital Activation of the Cardiac Catheterization Laboratory in ST-Segment–Elevation Myocardial Infarction for Primary Percutaneous Coronary Intervention. J. Am. Heart Assoc. 2023, 12, e029346. [Google Scholar] [CrossRef]
- Shen, C.; Wang, S.; Huo, R.; Huang, Y.; Yang, S. Comparison of machine learning and nomogram to predict 30-day in-hospital mortality in patients with acute myocardial infarction combined with cardiogenic shock: A retrospective study based on the eICU-CRD and MIMIC-IV databases. BMC Cardiovasc. Disord. 2025, 25, 197. [Google Scholar] [CrossRef]
- Jaskiewicz, F.; Zielinska, M. Prehospital clinical presentation in patients with acute coronary syndrome complicated by cardiogenic shock: A single center study. Aust. Crit. Care 2019, 32, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Shang, Z.; Liu, Y.; Yuan, Y.-Y.; Wang, X.-Y.; Yu, H.-Y.; Gao, W. Early identification of STEMI patients with emergency chest pain using lipidomics combined with machine learning. J. Geriatr. Cardiol. 2022, 19, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Lee, T.; Kochan, A.; Barker, M.; Roston, T.; Singer, J.; Grunau, B.; Helmer, J.; Wong, G.C.; Fordyce, C. Abstract 14054: Pre-Hospital Predictors of Cardiogenic Shock Among ST-Segment Elevation Myocardial Infarction Patients with and without Cardiac Arrest: Implications for Shock Teams. Circulation 2023, 148 (Suppl. S1), 4. [Google Scholar] [CrossRef]
- Krychtiuk, K.; Vrints, C.; Wojta, J.; Huber, K.; Speidl, W.S. Basic mechanisms in cardiogenic shock: Part 2—Biomarkers and treatment options. Eur. Heart J. Acute Cardiovasc. Care 2022, 11, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Galusko, V.; Wenzl, F.A.; Vandenbriele, C.; Panoulas, V.; Lüscher, T.F.; Gorog, D.A. Current and novel biomarkers in cardiogenic shock. Eur. J. Heart Fail. 2025. online ahead of print. [Google Scholar] [CrossRef]
- Tatu, A.L.; Nadasdy, T.; Arbune, A.; Chioncel, V.; Bobeica, C.; Niculet, E.; Iancu, A.V.; Dumitru, C.; Popa, V.T.; Kluger, N.; et al. Interrelationship and Sequencing of Interleukins 4, 13, 31, and 33—An Integrated Systematic Review: Dermatological and Multidisciplinary Perspectives. J. Inflamm. Res. 2022, 15, 5163–5184. [Google Scholar] [CrossRef]
- Jäntti, T.; Tarvasmäki, T.; Harjola, V.-P.; Parissis, J.; Pulkki, K.; Sionis, A.; Silva-Cardoso, J.; Køber, L.; Banaszewski, M.; Spinar, J.; et al. Frequency and Prognostic Significance of Abnormal Liver Function Tests in Patients with Cardiogenic Shock. Am. J. Cardiol. 2017, 120, 1090–1097. [Google Scholar] [CrossRef]
- Li, J.; Zhao, Z.; Jiang, H.; Jiang, M.; Yu, G.; Li, X. Predictive value of elevated alanine aminotransferase for in-hospital mortality in patients with acute myocardial infarction. BMC Cardiovasc. Disord. 2021, 21, 82. [Google Scholar] [CrossRef]
- Shariefuddin, W.W.A.; Pramudyo, M.; Martha, J.W. Shock index creatinine: A new predictor of mortality in acute coronary syndrome patients. BMC Cardiovasc. Disord. 2024, 24, 87. [Google Scholar] [CrossRef]
- Ran, P.; Wei, X.-B.; Lin, Y.-W.; Li, G.; Huang, J.-L.; He, X.-Y.; Yang, J.-Q.; Yu, D.-Q.; Chen, J.-Y. Shock Index-C: An Updated and Simple Risk-Stratifying Tool in ST-Segment Elevation Myocardial Infarction. Front. Cardiovasc. Med. 2021, 8, 657817. [Google Scholar] [CrossRef]
- Kanabar, K.; Sharma, Y.P.; Krishnappa, D.; Santosh, K.; Dhudasia, M. A study of the predictive role of multiple variables for the incidence of acute kidney injury and its outcomes in Indian patients with ST-elevation myocardial infarction and cardiogenic shock. Egypt. Heart J. 2024, 76, 123. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Liu, X.; Sun, D.; Liu, X.; Wang, Y.; Zhang, Z.; Han, Y.; Wang, X. CCC-ACS investigators Machine-learning models to predict serious adverse hospitalization events after ACS. Postgrad. Med. J. 2025. online ahead of print. [Google Scholar] [CrossRef]
- Zweck, E.; Thayer, K.L.; Helgestad, O.K.L.; Kanwar, M.; Ayouty, M.; Garan, A.R.; Hernandez-Montfort, J.; Mahr, C.; Wencker, D.; Sinha, S.S.; et al. Phenotyping cardiogenic shock. J. Am. Heart Assoc. 2021, 10, e020085. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, J.M.; Gonzalez-Ascaso, A.; Matas, J.F.R. The mechanisms of potassium loss in acute myocardial ischemia: New insights from computational simulations. Front. Physiol. 2023, 14, 1074160. [Google Scholar] [CrossRef] [PubMed]
- Kraft, P.L.; Newman, S.; Hanson, D.; Anderson, W.; Bastani, A. Emergency physician discretion to activate the cardiac catheterization team decreases door-to-balloon time for acute ST-elevation myocardial infarction. Ann. Emerg. Med. 2007, 50, 520–526. [Google Scholar] [CrossRef]
- Tehrani, B.N.; Truesdell, A.G.; Sherwood, M.; Desai, S.; Tran, H.A.; Epps, K.C.; Singh, R.; Psotka, M.; Shah, P.; Cooper, L.B.; et al. Standardized Team-Based Care for Cardiogenic Shock. J. Am. Coll. Cardiol. 2019, 73, 1659–1669. [Google Scholar] [CrossRef]
- Sinha, S.S.; Morrow, D.A.; Kapur, N.K.; Kataria, R.; Roswell, R.O. 2025 Concise Clinical Guidance: An ACC Expert Consensus Statement on the Evaluation and Management of Cardiogenic Shock. J. Am. Coll. Cardiol. 2025, 85, 1618–1641. [Google Scholar] [CrossRef]
- Naidu, S.S.; Baran, D.A.; Jentzer, J.C.; Hollenberg, S.M.; van Diepen, S.; Basir, M.B.; Grines, C.L.; Diercks, D.B.; Hall, S.; Kapur, N.K.; et al. SCAI SHOCK Stage Classification Expert Consensus Update: A Review and Incorporation of Validation Studies: This statement was endorsed by the American College of Cardiology (ACC), American College of Emergency Physicians (ACEP), American Heart Association (AHA), European Society of Cardiology (ESC) Association for Acute Cardiovascular Care (ACVC), International Society for Heart and Lung Transplantation (ISHLT), Society of Critical Care Medicine (SCCM), and Society of Thoracic Surgeons (STS) in December 2021. J. Am. Coll. Cardiol. 2022, 79, 933–946. [Google Scholar]
- Lim, H.S.; González-Costello, J.; Belohlavek, J.; Zweck, E.; Blumer, V.; Schrage, B.; Hanff, T.C. Hemodynamic management of cardiogenic shock in the intensive care unit. J. Heart Lung Transplant. 2024, 43, 1059–1073. [Google Scholar] [CrossRef]
- Kwak, M.J.; Kim, K.; Joong, E.R.; Jung, H.S.; Gil, J.S.; Jo, Y.S.; Youn, T.-J.; Chung, W.-Y.; Chae, I.-H.; Choi, D.-J.; et al. The Effect of Direct Communication Between Emergency Physicians and Interventional Cardiologists on Door to Balloon Times in STEMI. J. Korean Med. Sci. 2008, 23, 706. [Google Scholar] [CrossRef][Green Version]
- Bai, Z.; Hu, S.; Wang, Y.; Deng, W.; Gu, N.; Zhao, R.; Zhang, W.; Ma, Y.; Wang, Z.; Liu, Z.; et al. Development of a machine learning model to predict the risk of late cardiogenic shock in patients with ST-segment elevation myocardial infarction. Ann. Transl. Med. 2021, 9, 1162. [Google Scholar] [CrossRef] [PubMed]
- Laghlam, D.; Benghanem, S.; Ortuno, S.; Bouabdallaoui, N.; Manzo-Silberman, S.; Hamzaoui, O.; Aissaoui, N. Management of cardiogenic shock: A narrative review. Ann. Intensiv. Care 2024, 14, 45. [Google Scholar] [CrossRef] [PubMed]
- Djakpo, D.K.; Wang, Z.Q.; Shrestha, M. The significance of transaminase ratio (AST/ALT) in acute myocardial infarction. Arch. Med. Sci.-Atheroscler. Dis. 2020, 5, 279–283. [Google Scholar] [CrossRef]
- Sundermeyer, J.; Kellner, C.; Beer, B.N.; Besch, L.; Dettling, A.; Bertoldi, L.F.; Blankenberg, S.; Dauw, J.; Dindane, Z.; Eckner, D.; et al. Association between left ventricular ejection fraction, mortality and use of mechanical circulatory support in patients with non-ischaemic cardiogenic shock. Clin. Res. Cardiol. 2023, 113, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Sundermeyer, J.; Beer, B.N.; Blankenberg, S.; Kirchhof, P.; Luedike, P.; Mangner, N.; Nordbeck, P.; Orban, M.; Pazdernik, M.; Proudfoot, A.; et al. Impact of left ventricular ejection fraction on mortality and use of mechanical circulatory support in non-ischaemic cardiogenic shock. Eur. Heart J. 2022, 43 (Suppl. S2), ehac544.1013. [Google Scholar] [CrossRef]
- Jeong, J.H.; Kook, H.; Lee, S.H.; Joo, H.J.; Park, J.H.; Hong, S.J.; Kim, M.-N.; Park, S.-M.; Jung, J.S.; Yang, J.H.; et al. Prediction of In-Hospital Mortality for Ischemic Cardiogenic Shock Requiring Venoarterial Extracorporeal Membrane Oxygenation. J. Am. Heart Assoc. Cardiovasc. Cerebrovasc. Dis. 2024, 13, e032701. [Google Scholar] [CrossRef]
- Yogyakarta, I.; Bagaswoto, H.P.; Dinarti, L.K. Left Ventricle Ejection Fraction as a Predictor of Cardiogenic Shock in Patients with ST Elevation Myocardial Infarct at Dr. Sardjito General Hospital Yogyakarta. In Proceedings of the International Conference on Medical Science and Health (ICOMESH 2024), Bandar Lampung, Indonesia, 7–8 August 2024; pp. 104–118. [Google Scholar]
- Moreira, A.; Crispim, J. The importance of the health information systems in value-based healthcare initiatives: A scoping review. Procedia Comput. Sci. 2024, 239, 1476–1482. [Google Scholar] [CrossRef]
- Brahmbhatt, D.H.; Kalra, S.; Luk, A.; Billia, F. From Escalate to Elevate: A New Paradigm for Comprehensive Cardiogenic Shock Management. Can. J. Cardiol. 2025, 41, 630–644. [Google Scholar] [CrossRef]
- Goh, E.; Gallo, R.; Hom, J.; Strong, E.; Weng, Y.; Kerman, H.; Cool, J.A.; Kanjee, Z.; Parsons, A.S.; Ahuja, N.; et al. Large Language Model Influence on Diagnostic Reasoning: A Randomized Clinical Trial. JAMA Netw. Open 2024, 7, e2440969. [Google Scholar] [CrossRef]
- Mebazaa, A.; Soussi, S. Precision Medicine in Cardiogenic Shock: We Are Almost There! JACC Heart Fail. 2023, 11, 1316–1319. [Google Scholar] [CrossRef]
- Soussi, S.; Tarvasmäki, T.; Kimmoun, A.; Ahmadiankalati, M.; Azibani, F.; dos Santos, C.C.; Duarte, K.; Gayat, E.; Jentzer, J.C.; Harjola, V.-P.; et al. Identifying biomarker-driven subphenotypes of cardiogenic shock: Analysis of prospective cohorts and randomized controlled trials. eClinicalMedicine 2024, 79, 103013. [Google Scholar] [CrossRef] [PubMed]
| STEMI—CS (158 Patients) | |||
|---|---|---|---|
| STEMI patients with CS present on admission 87 patients (55.1%) | STEMI patients with CS post-admission 71 patients (44.9%) | ||
| Females | Males | Females | Males |
| 43 patients (49.43%) | 44 patients (50.57%) | 17 patients (24%) | 51 patients (76%) |
| Cardiovascular risk factors | Cardiovascular risk factors | ||
| Diabetes mellitus 23 (26.43%) | Diabetes mellitus 27 (38.02%) | ||
| Hypertension 31 (36.78%) | Hypertension 55 (77.46%) | ||
| Smoking 27 (31.03%) | Smoking 30 (49.29%) | ||
| Dyslipidemia 65 (74.71%) | Dyslipidemia 50 (71.83%) | ||
| Model | Accuracy | Sensitivity | Specificity | F1-Score | AUC | Brier Score |
|---|---|---|---|---|---|---|
| Random Forest | 0.7742 | 0.7778 | 0.7692 | 0.7735 | 0.7735 | 0.1927 |
| Logistic Regression | 0.7419 | 0.7222 | 0.7692 | 0.7450 | 0.8248 | 0.1738 |
| Parameter | Coefficient | p-Value | 95% Confidence Interval |
|---|---|---|---|
| Killip at Presentation | 1.1394 | 0.0000 | [0.6708, 1.6080] |
| Age | 0.0188 | 0.3489 | [−0.0210, 0.0587] |
| ECG Rhythm at Presentation | 1.0267 | 0.0188 | [0.1614, 1.8920] |
| Pain Onset | 0.3753 | 0.1185 | [−0.1007, 0.8512] |
| Sex | 0.3764 | 0.4649 | [−0.6435, 1.3963] |
| HR at Presentation | −0.0009 | 0.9133 | [−0.0165, 0.0148] |
| ST Elevation in aVR | −0.1368 | 0.8899 | [−2.0937, 1.8201] |
| Parameter | VIF |
|---|---|
| Killip at Presentation | 1.1415 |
| Age | 1.2938 |
| ECG Rhythm at Presentation | 1.1443 |
| Pain Onset | 1.1968 |
| Sex | 1.2141 |
| HR at Presentation | 1.0706 |
| ST Elevation in aVR | 1.0768 |
| Model | Accuracy | Sensitivity | Specificity | F1-Score | AUC | Brier Score |
|---|---|---|---|---|---|---|
| Random Forest | 0.7419 | 0.9444 | 0.4615 | 0.6201 | 0.8291 | 0.1737 |
| Logistic Regression | 0.6452 | 0.7778 | 0.4615 | 0.5793 | 0.6752 | 0.2164 |
| Parameter | Coefficient | p-Value | 95% Confidence Interval |
|---|---|---|---|
| Killip Presentation | 1.1891 | 0.0000 | [0.6680, 1.7102] |
| K (Potassium) | 0.3240 | 0.3066 | [−0.3037, 0.9518] |
| Creatinine | −0.0429 | 0.9123 | [−0.8136, 0.7279] |
| Age | 0.0209 | 0.3201 | [−0.0207, 0.0626] |
| CKI | −0.0006 | 0.0051 | [−0.0010, −0.0002] |
| Electrocardiogram Rhythm at Presentation | 1.6462 | 0.0173 | [0.2770, 3.0155] |
| ALT (Alanine Aminotransferase) | −0.0001 | 0.9747 | [−0.0042, 0.0041] |
| Parameter | VIF |
|---|---|
| Killip Presentation | 1.1229 |
| K (Potassium) | 1.4220 |
| Creatinine | 1.6557 |
| Age | 1.1044 |
| CKI | 1.0257 |
| EKG Rhythm at Presentation | 1.1188 |
| ALT | 1.2160 |
| Feature | Importance Score | Rank (of 33 Variables) | Interpretation |
|---|---|---|---|
| Killip Presentation | 0.9922 | 1 | Most predictive; reflects clinical severity |
| Age | 0.5915 | 2 | Important demographic risk factor |
| Creatinine | 0.5891 | 3 | Indicator of renal function and systemic status |
| CKI | 0.5784 | 4 | Reflects severe myocardial damage and dysfunction |
| Potassium | 0.5758 | 5 | Electrolyte balance linked to arrhythmia risk |
| AST | 0.4723 | 6 | Marker of tissue injury |
| EKG Rhythm at Presentation | 0.4409 | 7 | Reflects electrical instability |
| LVEF at Presentation | 0.2524 | 19 | Moderate predictor; less informative alone |
| Model | Accuracy | Sensitivity | Specificity | F1-Score | AUC | Brier Score |
|---|---|---|---|---|---|---|
| Random Forest | 0.7742 | 0.7500 | 0.8182 | 0.7826 | 0.9091 | 0.1498 |
| Logistic Regression | 0.7419 | 0.7000 | 0.8182 | 0.7545 | 0.8273 | 0.1771 |
| Parameter | Coefficient | p-Value | 95% Confidence Interval |
|---|---|---|---|
| Killip Presentation | 1.1263 | 0.0000 | [0.6150, 1.6376] |
| Age | 0.0277 | 0.1448 | [−0.0099, 0.0652] |
| Creatinine | −0.0575 | 0.8723 | [−0.7658, 0.6508] |
| CKI | −0.0004 | 0.0596 | [−0.0008, 0.0000] |
| Potassium | 0.8889 | 0.0208 | [0.1274, 1.6505] |
| AST | −0.0009 | 0.4306 | [−0.0033, 0.0014] |
| EKG Rhythm at Presentation | 1.2979 | 0.0096 | [0.3052, 2.2906] |
| Parameter | VIF |
|---|---|
| Killip Presentation | 1.1268 |
| Age | 1.0959 |
| Creatinine | 1.5216 |
| CKI | 1.1398 |
| Potassium (K) | 1.4051 |
| AST | 1.2527 |
| EKG Rhythm at Presentation | 1.1134 |
| MLs | ACC | Precision | Recall | F1-Score | MCC | TN | FP | FN | TP |
|---|---|---|---|---|---|---|---|---|---|
| ET | 0.90625 | 0.907843 | 0.90625 | 0.906158 | 0.814092 | 14 | 2 | 1 | 15 |
| RF | 0.78125 | 0.791498 | 0.78125 | 0.779310 | 0.572656 | 11 | 5 | 2 | 14 |
| DT | 0.62500 | 0.633333 | 0.62500 | 0.619048 | 0.258199 | 8 | 8 | 4 | 12 |
| QDA | 0.75000 | 0.766667 | 0.75000 | 0.746032 | 0.516398 | 10 | 6 | 2 | 14 |
| NB | 0.84375 | 0.845098 | 0.84375 | 0.843597 | 0.688847 | 14 | 2 | 3 | 13 |
| SVM | 0.75000 | 0.750000 | 0.75000 | 0.750000 | 0.500000 | 12 | 4 | 4 | 12 |
| LR | 0.71875 | 0.719608 | 0.71875 | 0.718475 | 0.438357 | 11 | 5 | 4 | 12 |
| RC | 0.68750 | 0.690476 | 0.68750 | 0.686275 | 0.377964 | 10 | 6 | 4 | 12 |
| GBC | 0.75000 | 0.753968 | 0.75000 | 0.749020 | 0.503953 | 11 | 5 | 3 | 13 |
| ADA | 0.68750 | 0.690476 | 0.68750 | 0.686275 | 0.377964 | 10 | 6 | 4 | 12 |
| KNN | 0.68750 | 0.687500 | 0.68750 | 0.687500 | 0.375000 | 11 | 5 | 5 | 11 |
| MLs | ACC | Precision | Recall | F1-Score | MCC | TN | FP | FN | TP |
|---|---|---|---|---|---|---|---|---|---|
| ET | 0.62500 | 0.626984 | 0.62500 | 0.623529 | 0.251976 | 11 | 5 | 7 | 9 |
| RF | 0.75000 | 0.753968 | 0.75000 | 0.749020 | 0.503953 | 11 | 5 | 3 | 13 |
| DT | 0.62500 | 0.626984 | 0.62500 | 0.623529 | 0.251976 | 9 | 7 | 5 | 11 |
| QDA | 0.75000 | 0.790909 | 0.75000 | 0.740891 | 0.539360 | 9 | 7 | 1 | 15 |
| NB | 0.75000 | 0.790909 | 0.75000 | 0.740891 | 0.539360 | 15 | 1 | 7 | 9 |
| SVM | 0.78125 | 0.811688 | 0.78125 | 0.775776 | 0.592157 | 10 | 6 | 1 | 15 |
| LR | 0.71875 | 0.726721 | 0.71875 | 0.716256 | 0.445399 | 10 | 6 | 3 | 13 |
| RC | 0.75000 | 0.766667 | 0.75000 | 0.746032 | 0.516398 | 10 | 6 | 2 | 14 |
| GBC | 0.68750 | 0.690476 | 0.68750 | 0.686275 | 0.377964 | 10 | 6 | 4 | 12 |
| ADA | 0.75000 | 0.750000 | 0.75000 | 0.750000 | 0.500000 | 12 | 4 | 4 | 12 |
| KNN | 0.62500 | 0.625000 | 0.62500 | 0.625000 | 0.250000 | 10 | 6 | 6 | 10 |
| MLs | ACC | Precision | Recall | F1-Score | MCC | TN | FP | FN | TP |
|---|---|---|---|---|---|---|---|---|---|
| ET | 0.62500 | 0.633333 | 0.62500 | 0.619048 | 0.258199 | 8 | 8 | 4 | 12 |
| RF | 0.81250 | 0.833333 | 0.81250 | 0.809524 | 0.645497 | 11 | 5 | 1 | 15 |
| DT | 0.71875 | 0.726721 | 0.71875 | 0.716256 | 0.445399 | 10 | 6 | 3 | 13 |
| QDA | 0.75000 | 0.790909 | 0.75000 | 0.740891 | 0.539360 | 9 | 7 | 1 | 15 |
| NB | 0.75000 | 0.790909 | 0.75000 | 0.740891 | 0.539360 | 15 | 1 | 7 | 9 |
| SVM | 0.78125 | 0.811688 | 0.78125 | 0.775776 | 0.592157 | 10 | 6 | 1 | 15 |
| LR | 0.71875 | 0.726721 | 0.71875 | 0.716256 | 0.445399 | 10 | 6 | 3 | 13 |
| RC | 0.75000 | 0.766667 | 0.75000 | 0.746032 | 0.516398 | 10 | 6 | 2 | 14 |
| GBC | 0.68750 | 0.690476 | 0.68750 | 0.686275 | 0.377964 | 10 | 6 | 4 | 12 |
| ADA | 0.75000 | 0.750000 | 0.75000 | 0.750000 | 0.500000 | 12 | 4 | 4 | 12 |
| KNN | 0.62500 | 0.625000 | 0.62500 | 0.625000 | 0.250000 | 10 | 6 | 6 | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stamate, E.; Culea-Florescu, A.-L.; Miron, M.; Piraianu, A.-I.; Dumitrascu, A.G.; Fulga, I.; Fulga, A.; Patrascanu, O.S.; Iancu, D.; Ciobotaru, O.C.; et al. AI-Based Predictive Models for Cardiogenic Shock in STEMI: Real-World Data for Early Risk Assessment and Prognostic Insights. J. Clin. Med. 2025, 14, 3698. https://doi.org/10.3390/jcm14113698
Stamate E, Culea-Florescu A-L, Miron M, Piraianu A-I, Dumitrascu AG, Fulga I, Fulga A, Patrascanu OS, Iancu D, Ciobotaru OC, et al. AI-Based Predictive Models for Cardiogenic Shock in STEMI: Real-World Data for Early Risk Assessment and Prognostic Insights. Journal of Clinical Medicine. 2025; 14(11):3698. https://doi.org/10.3390/jcm14113698
Chicago/Turabian StyleStamate, Elena, Anisia-Luiza Culea-Florescu, Mihaela Miron, Alin-Ionut Piraianu, Adrian George Dumitrascu, Iuliu Fulga, Ana Fulga, Octavian Stefan Patrascanu, Doriana Iancu, Octavian Catalin Ciobotaru, and et al. 2025. "AI-Based Predictive Models for Cardiogenic Shock in STEMI: Real-World Data for Early Risk Assessment and Prognostic Insights" Journal of Clinical Medicine 14, no. 11: 3698. https://doi.org/10.3390/jcm14113698
APA StyleStamate, E., Culea-Florescu, A.-L., Miron, M., Piraianu, A.-I., Dumitrascu, A. G., Fulga, I., Fulga, A., Patrascanu, O. S., Iancu, D., Ciobotaru, O. C., & Ciobotaru, O. R. (2025). AI-Based Predictive Models for Cardiogenic Shock in STEMI: Real-World Data for Early Risk Assessment and Prognostic Insights. Journal of Clinical Medicine, 14(11), 3698. https://doi.org/10.3390/jcm14113698







