Diagnosing Ulcerative Colitis: Should We Go Beyond the Surface?
Abstract
1. Introduction
- Those who go beneath the surface do so at their peril (Oscar Wilde, 1891)
2. Methods
- Clarifying the definitions
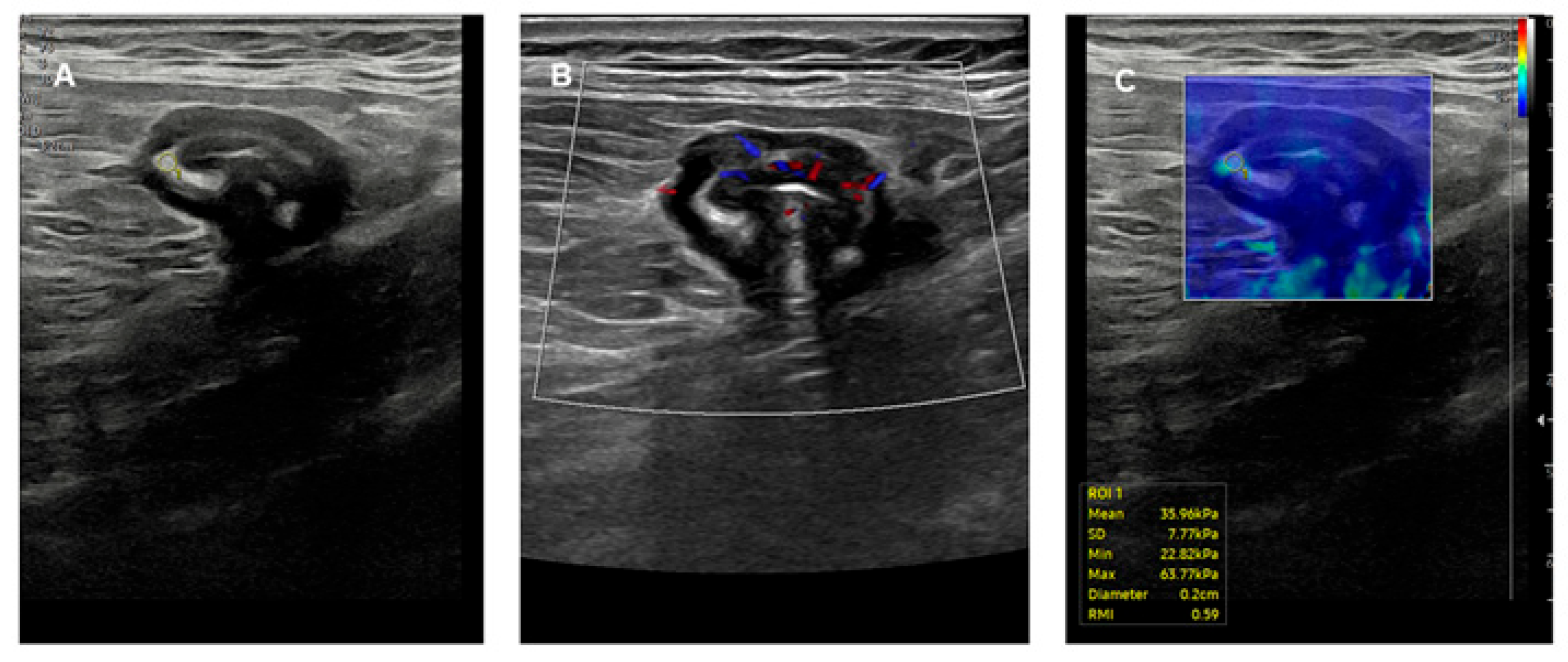
- Beyond the surface…
- …and resurfacing
3. Future Perspectives
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADC | apparent diffusion coefficient |
| AUC | area under the curve |
| CD | Crohn’s disease |
| DWI | diffusion-weighted imaging |
| ECCO | European Crohn’s and Colitis Organisation |
| GI | gastrointestinal |
| IBD | inflammatory bowel diseases |
| IUS | intestinal ultrasound |
| MRE | magnetic resonance enterography |
| MUS | Milan ultrasound criteria |
| SWD | shear wave dispersion |
| SWE | shear wave elastography |
| UC | ulcerative colitis |
| VSOPs | very small superparamagnetic iron oxide nanoparticles |
References
- Magro, F.; Gionchetti, P.; Eliakim, R.; Ardizzone, S.; Armuzzi, A.; Barreiro-de Acosta, M.; Burisch, J.; Gecse, K.B.; Hart, A.L.; Hindryckx, P.; et al. Third European Evidence-based Consensus on diagnosis and management of ulcerative colitis. Part 1: Definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J. Crohn’s Colitis 2017, 11, 649–670. [Google Scholar] [CrossRef] [PubMed]
- Centanni, L.; Bencardino, S.; D’Amico, F.; Zilli, A.; Parigi, T.L.; Allocca, M.; Danese, S.; Furfaro, F. Targeting mucosal healing in Crohn’s disease: Efficacy of novel pathways and therapeutic targets. Expert. Opin. Ther. Targets 2024, 28, 963–978. [Google Scholar] [CrossRef] [PubMed]
- Krugliak Cleveland, N.; Torres, J.; Rubin, D.T. What does disease progression look like in ulcerative colitis, and how might it be prevented? Gastroenterology 2022, 162, 1396–1408. [Google Scholar] [CrossRef]
- Ippolito, C.; Colucci, R.; Segnani, C.; Errede, M.; Girolamo, F.; Virgintino, D.; Dolfi, A.; Tirotta, E.; Buccianti, P.; Di Candio, G.; et al. Fibrotic and vascular remodelling of colonic wall in patients with active ulcerative colitis. J. Crohn’s Colitis 2016, 10, 1194–1204. [Google Scholar] [CrossRef]
- Antonelli, E.; Giuliano, V.; Casella, G.; Villanacci, V.; Baldini, V.; Baldoni, M.; Morelli, O.; Bassotti, G. Ultrasonographic assessment of colonic wall in moderate-severe ulcerative colitis: Comparison with endoscopic findings. Dig. Liver Dis. 2011, 43, 703–706. [Google Scholar] [CrossRef]
- Palmela, C.; Maaser, C. The use of intestinal ultrasound in ulcerative colitis-more than a mucosal disease? Gastroenterology 2022, 163, 1485–1487. [Google Scholar] [CrossRef]
- Rubio, C.A.; Lang-Schwarz, C.; Vieth, M. Architectural crypt distortions in ulcerative colitis: Time for reappraisal. J. Gastroenterol. Hepatol. 2024, 39, 2479–2486. [Google Scholar] [CrossRef]
- Magro, F.; Langner, C.; Driessen, A.; Ensari, A.; Geboes, K.; Mantzaris, G.J.; Villanacci, V.; Becheanu, G.; Borralho Nunes, P.; Cathomas, G.; et al. European consensus on the histopathology of inflammatory bowel disease. J. Crohn’s Colitis 2013, 7, 827–851. [Google Scholar] [CrossRef]
- Villanacci, V.; Antonelli, E.; Reboldi, G.; Salemme, M.; Casella, G.; Bassotti, G. Endoscopic biopsy samples of naïve “colitides” patients: Role of basal plasmacytosis. J. Crohn’s Colitis 2014, 8, 1438–1443. [Google Scholar] [CrossRef]
- Canavese, G.; Villanacci, V.; Antonelli, E.; Cadei, M.; Sapino, A.; Rocca, R.; Daperno, M.; Suriani, R.; Di Santo, M.G.; Cassoni, P.; et al. Eosinophilia-associated basal plasmacytosis: An early and sensitive histologic feature of inflammatory bowel disease. APMIS 2017, 125, 179–183. [Google Scholar] [CrossRef]
- Villanacci, V.; Reggiani-Bonetti, L.; Caprioli, F.; Saragoni, L.; Salviato, T.; Mescoli, C.; Canavese, G.; Manenti, S.; Spada, E.; Baron, L.; et al. Histopathology of inflammatory bowel disease—Position statement of the Pathologists of the Italian Group for the Study of Inflammatory Bowel Disease (IG-IBD) and Italian Group of Gastrointestinal Pathologists (GIPAD-SIAPEC). Dig. Liver Dis. 2020, 52, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Cheon, J.H. Pathogenesis of inflammatory bowel disease and recent advances in biologic therapies. Immune Netw. 2017, 17, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Liu, C.; Jiang, S.; Qian, D.; Duan, J. Cross talk between gut microbiota and intestinal mucosal immunity in the development of ulcerative colitis. Infect. Immun. 2021, 89, e0001421. [Google Scholar] [CrossRef]
- Subramanian, A.; Kumarasamy, V.; Begum, M.Y.; Sekar, M.; Subramaniyan, V.; Wong, L.S.; Al Fatease, A. Exploring the connections: Autophagy, gut microbiota, and inflammatory bowel disease pathogenesis. J. Inflamm. Res. 2024, 17, 10453–10470. [Google Scholar] [CrossRef]
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.F. Ulcerative colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef]
- Harbord, M.; Eliakim, R.; Bettenworth, D.; Karmiris, K.; Katsanos, K.; Kopylov, U.; Kucharzik, T.; Molnár, T.; Raine, T.; Sebastian, S.; et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 2: Current management. J. Crohn’s Colitis 2017, 11, 769–784. [Google Scholar] [CrossRef]
- Pai, R.K.; D’Haens, G.; Kobayashi, T.; Sands, B.E.; Travis, S.; Jairath, V.; De Hertogh, G.; Park, B.; McGinnis, K.; Redondo, I.; et al. Histologic assessments in ulcerative colitis: The evidence behind a new endpoint in clinical trials. Expert. Rev. Gastroenterol. Hepatol. 2024, 18, 73–87. [Google Scholar] [CrossRef]
- Villanacci, V.; Del Sordo, R.; Mino, S.; Locci, G.; Bassotti, G. Histological healing in IBD: Ready for prime time? Dig. Liver Dis. 2025, 57, 504–510. [Google Scholar] [CrossRef]
- Villanacci, V.; Del Sordo, R.; Parigi, T.L.; Leoncini, G.; Bassotti, G. Inflammatory bowel diseases: Does one histological score fit all? Diagnostics 2023, 13, 2112. [Google Scholar] [CrossRef]
- Parigi, T.L.; Solitano, V.; Armuzzi, A.; Barreiro de Acosta, M.; Begun, J.; Ben-Horin, S.; Biedermann, L.; Colombel, J.F.; Dignass, A.; Fumery, M.; et al. Defining mucosal healing in randomized controlled trials of inflammatory bowel disease: A systematic review and future perspective. United Eur. Gastroenterol. J. 2024, 12, 1266–1279. [Google Scholar] [CrossRef]
- Estevinho, M.M.; Roseira, J.; Teixeira, P.V.; Dignass, A.; Magro, F. Clinical Significance of histologic healing in IBD: Evidence from randomized controlled trials (RCT) and real world (RW) data. Dig. Liver Dis. 2025, 57, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.; Jangi, S.; Dulai, P.S.; Boland, B.S.; Prokop, L.J.; Jairath, V.; Feagan, B.G.; Sandborn, W.J.; Singh, S. Incremental benefit of achieving endoscopic and histologic remission in patients with ulcerative colitis: A systematic review and meta-analysis. Gastroenterology 2020, 159, 1262–1275. [Google Scholar] [CrossRef] [PubMed]
- Villanacci, V.; Del Sordo, R.; Lanzarotto, F.; Ricci, C.; Sidoni, A.; Manenti, S.; Mino, S.; Bugatti, M.; Bassotti, G. Claudin-2: A marker for a better evaluation of histological mucosal healing in inflammatory bowel diseases. Dig. Liver Dis. 2025, 57, 827–832. [Google Scholar] [CrossRef]
- Warren, B.F.; Sheperd, N.A. What are the controversies in histopathological diagnosis? In Challenges in Inflammatory Bowel Disease; Jewell, D.P., Warren, B.F., Mortensen, N.J., Eds.; Blackwell Science: Oxford, UK, 2001. [Google Scholar]
- Latella, G.; Viscido, A. Controversial contribution of Th17/IL-17 toward the immune response in intestinal fibrosis. Dig. Dis. Sci. 2020, 65, 1299–1306. [Google Scholar] [CrossRef]
- de Bruyn, J.R.; Meijer, S.L.; Wildenberg, M.E.; Bemelman, W.A.; van den Brink, G.R.; D’Haens, G.R. Development of fibrosis in acute and longstanding ulcerative colitis. J. Crohn’s Colitis 2015, 9, 966–972. [Google Scholar] [CrossRef]
- Gordon, I.O.; Agrawal, N.; Willis, E.; Goldblum, J.R.; Lopez, R.; Allende, D.; Liu, X.; Patil, D.Y.; Yerian, L.; El-Khider, F.; et al. Fibrosis in ulcerative colitis is directly linked to severity and chronicity of mucosal inflammation. Aliment. Pharmacol. Ther. 2018, 47, 922–939. [Google Scholar] [CrossRef]
- Bassotti, G.; Antonelli, E.; Villanacci, V.; Baldoni, M.; Dore, M.P. Colonic motility in ulcerative colitis. United Eur. Gastroenterol. J. 2014, 2, 457–462. [Google Scholar] [CrossRef]
- Villanacci, V.; Bassotti, G.; Nascimbeni, R.; Antonelli, E.; Cadei, M.; Fisogni, S.; Salerni, B.; Geboes, K. Enteric nervous system abnormalities in inflammatory bowel diseases. Neurogastroenterol. Motil. 2008, 20, 1009–1016. [Google Scholar] [CrossRef]
- Yamagata, M.; Mikami, T.; Tsuruta, T.; Yokoyama, K.; Sada, M.; Kobayashi, K.; Katsumata, T.; Koizumi, W.; Saigenji, K.; Okayasu, I. Submucosal fibrosis and basic-fibroblast growth factor-positive neutrophils correlate with colonic stenosis in cases of ulcerative colitis. Digestion 2011, 84, 12–21. [Google Scholar] [CrossRef]
- Gordon, I.O.; Agrawal, N.; Goldblum, J.R.; Fiocchi, C.; Rieder, F. Fibrosis in ulcerative colitis: Mechanisms, features, and consequences of a neglected problem. Inflamm. Bowel Dis. 2014, 20, 2198–2206. [Google Scholar] [CrossRef]
- Grip, O.; Malm, J.; Veress, B.; Bjartell, A.; Lindgren, S.; Egesten, A. Increased presence of cells containing transforming growth factor alpha (TGF-alpha) in ulcerative colitis, both during active inflammation and in remission. Eur. J. Gastroenterol. Hepatol. 2000, 12, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Gundersen, M.D.; Goll, R.; Fenton, C.G.; Anderssen, E.; Sørbye, S.W.; Florholmen, J.R.; Paulssen, R.H. Fibrosis mediators in the colonic mucosa of acute and healed ulcerative colitis. Clin. Transl. Gastroenterol. 2019, 10, e00082. [Google Scholar] [CrossRef] [PubMed]
- Franzè, E.; Monteleone, I.; Laudisi, F.; Rizzo, A.; Dinallo, V.; Di Fusco, D.; Colantoni, A.; Ortenzi, A.; Giuffrida, P.; Di Carlo, S.; et al. Cadherin-11 is a regulator of intestinal fibrosis. J. Crohn’s Colitis 2020, 14, 406–417. [Google Scholar] [CrossRef]
- Jharap, B.; Sandborn, W.J.; Reinisch, W.; D’Haens, G.; Robinson, A.M.; Wang, W.; Huang, B.; Lazar, A.; Thakkar, R.B.; Colombel, J.F. Randomised clinical study: Discrepancies between patient-reported outcomes and endoscopic appearance in moderate to severe ulcerative colitis. Aliment. Pharmacol. Ther. 2015, 42, 1082–1092. [Google Scholar] [CrossRef]
- Gupta, A.; Yu, A.; Peyrin-Biroulet, L.; Ananthakrishnan, A.N. Treat to target: The role of histologic healing in inflammatory bowel diseases: A systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 2021, 19, 1800–1813. [Google Scholar] [CrossRef]
- Magro, F.; Alves, C.; Lopes, J.; Lopes, S.; Tavares de Sousa, H.; Cotter, J.; Macedo da Silva, V.; Lago, P.; Vieira, A.; Brito, M.; et al. Histologic features of colon biopsies (Geboes Score) associated with progression of ulcerative colitis for the first 36 months after biopsy. Clin. Gastroenterol. Hepatol. 2021, 19, 2567–2576.e9. [Google Scholar] [CrossRef]
- Loy, L.; Roda, G.; Fiorino, G.; Allocca, M.; Furfaro, F.; Argollo, M.; Peyrin-Biroulet, L.; Danese, S. Detection and management of early stage inflammatory bowel disease: An update for clinicians. Expert. Rev. Gastroenterol. Hepatol. 2019, 13, 547–555. [Google Scholar] [CrossRef]
- Yanofsky, R.; Rubin, D.T. A practical approach to positioning therapies in ulcerative colitis. J. Can. Assoc. Gastroenterol. 2025, 8 (Suppl. S2), S6–S14. [Google Scholar] [CrossRef]
- Chaemsupaphan, T.; Arzivian, A.; Leong, R.W. Comprehensive care of ulcerative colitis: New treatment strategies. Expert Rev. Gastroenterol. Hepatol. 2025. ahead of print. [Google Scholar] [CrossRef]
- Krugliak Cleveland, N.; Bressler, B.; Siegel, C.A.; BRIDGe UC Progression Summit Collaborators. A summary of the BRIDGe summit on damage-related progression of ulcerative colitis: Establishing research priorities. Gastroenterology 2022, 163, 1505–1509. [Google Scholar] [CrossRef]
- Coulie, B.; Camilleri, M.; Bharucha, A.E.; Sandborn, W.J.; Burton, D. Colonic motility in chronic ulcerative proctosigmoiditis and the effects of nicotine on colonic motility in patients and healthy subjects. Aliment. Pharmacol. Ther. 2001, 15, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Drewes, A.M.; Frøkjaer, J.B.; Larsen, E.; Reddy, H.; Arendt-Nielsen, L.; Gregersen, H. Pain and mechanical properties of the rectum in patients with active ulcerative colitis. Inflamm. Bowel Dis. 2006, 12, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Bassotti, G.; de Roberto, G.; Chistolini, F.; Sietchiping-Nzepa, F.; Morelli, O.; Morelli, A. Twenty-four-hour manometric study of colonic propulsive activity in patients with diarrhea due to inflammatory (ulcerative colitis) and non-inflammatory (irritable bowel syndrome) conditions. Int. J. Color. Dis. 2004, 19, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Dong, T.; Tang, C.; Wei, J.; Guo, W.; Ding, C.; Gui, L.; Gong, J. A mesenteric fat-derived radiomic model to identify colonic fibrosis and predict treatment response to biologics in chronic ulcerative colitis. Dis. Colon. Rectum 2024, 67, 1544–1554. [Google Scholar] [CrossRef]
- Lu, B.; Lin, J.; Du, J.; He, S.; Cao, Q.; Huang, L.; Mao, R.; Sun, C.; Li, Z.; Feng, S.; et al. Native T1 mapping and magnetization transfer imaging in grading bowel fibrosis in Crohn’s disease: A comparative animal study. Biosensors 2021, 11, 302. [Google Scholar] [CrossRef]
- Caruso, A.; Angriman, I.; Scarpa, M.; D’Incà, R.; Mescoli, C.; Rudatis, M.; Sturniolo, G.C.; Schifano, G.; Lacognata, C. Diffusion-weighted magnetic resonance for assessing fibrosis in Crohn’s disease. Abdom. Radiol. 2020, 45, 2327–2335. [Google Scholar] [CrossRef]
- Jensen, J.H.; Helpern, J.A.; Ramani, A.; Lu, H.; Kaczynski, K. Diffusional kurtosis imaging: The quantification of non-gaussian water diffusion by means of magnetic resonance imaging. Magn. Reson. Med. 2005, 53, 1432–1440. [Google Scholar] [CrossRef]
- Golusda, L.; Kühl, A.A.; Lehmann, M.; Dahlke, K.; Mueller, S.; Boehm-Sturm, P.; Saatz, J.; Traub, H.; Schnorr, J.; Freise, C.; et al. Visualization of inflammation in experimental colitis by magnetic resonance imaging using very small superparamagnetic iron oxide particles. Front. Physiol. 2022, 13, 862212. [Google Scholar] [CrossRef]
- Zhu, F.; Chen, X.; Qiu, X.; Guo, W.; Wang, X.; Cao, J.; Gong, J. Seeing beyond the surface: Superior performance of ultrasound elastography over Milan ultrasound criteria in distinguishing fibrosis of ulcerative colitis. J. Crohn’s Colitis 2024, 18, 1795–1803. [Google Scholar] [CrossRef]
- Yamada, K.; Ishikawa, T.; Kawashima, H.; Ohno, E.; Iida, T.; Ishikawa, E.; Mizutani, Y.; Sawada, T.; Maeda, K.; Yamamura, T.; et al. Evaluation of ulcerative colitis activity using transabdominal ultrasound shear wave elastography. Quant. Imaging Med. Surg. 2022, 12, 618–626. [Google Scholar] [CrossRef]
- Rustemovic, N.; Cukovic-Cavka, S.; Brinar, M.; Radić, D.; Opacic, M.; Ostojic, R.; Vucelic, B. A pilot study of transrectal endoscopic ultrasound elastography in inflammatory bowel disease. BMC Gastroenterol. 2011, 11, 113. [Google Scholar] [CrossRef] [PubMed]
- Odze, R.D. A contemporary and critical appraisal of ‘indeterminate colitis’. Mod. Pathol. 2015, 28 (Suppl. S1), S30–S46. [Google Scholar] [CrossRef] [PubMed]
- Geboes, K.; Van Eyken, P. Inflammatory bowel disease unclassified and indeterminate colitis: The role of the pathologist. J. Clin. Pathol. 2009, 62, 201–205. [Google Scholar] [CrossRef]
- Kim, S.H.; Buhle, A.; Roberts, A.; Singh, N.; Mir, A.; Kesar, V.; Lozano, A.; Ji, W.; Hanlon, A.; Grider, D. Multidisciplinary inflammatory bowel disease conference: The impact of the expert pathologist on patient care. Inflamm. Bowel Dis. 2024, 30, 1482–1491. [Google Scholar] [CrossRef]
- Radmard, A.R.; Amouei, M.; Torabi, A.; Sima, A.R.; Saffar, H.; Geahchan, A.; Davarpanah, A.H.; Taouli, B. MR Enterography in ulcerative colitis: Beyond endoscopy. Radiographics 2024, 44, e230131. [Google Scholar] [CrossRef]
- Yuan, B.; Huang, P.; Yang, M.; Tang, G.; Wang, F. Intestinal ultrasound scan predicts corticosteroid failure and colectomy risk in patients with ulcerative colitis. Eur. J. Gastroenterol. Hepatol. 2024, 36, 884–889. [Google Scholar] [CrossRef]
- Su, H.Y.; Taylor, K.M.; Friedman, A.B.; Cataletti, G.; Maconi, G. Ultrasound assessment of gastrointestinal luminal contents: A narrative review. J. Ultrasound 2024, 27, 781–792. [Google Scholar] [CrossRef]
- Huynh, D.; Rubtsov, D.; Basu, D.; Khaing, M.M. The diagnostic utility of biochemical markers and intestinal ultrasound compared with endoscopy in patients with Crohn’s disease and ulcerative colitis: A systemic review and meta-analysis. J. Clin. Med. 2024, 13, 3030. [Google Scholar] [CrossRef]
- Madsen, G.R.; Wilkens, R.; Attauabi, M.; Ilvemark, J.F.K.F.; Theede, K.; Bjerrum, J.T.; Bendtsen, F.; Seidelin, J.B.; Boysen, T.; Burisch, J. Intestinal ultrasound as a prognostic tool in new-onset ulcerative colitis-a Copenhagen IBD Cohort Study. J. Crohn’s Colitis 2025, 19, jjaf033. [Google Scholar] [CrossRef]
- Abid, H.; Cherkaoui, H.; Benahsine, F.; Lamine, A.; Lahlali, M.; Chaouche, I.; Bartal, F.; Lahmidani, N.; Elmekkaoui, A.; Benajah, D.A.; et al. Non-invasive monitoring of inflammatory bowel disease using intestinal ultrasound. World J. Gastrointest. Endosc. 2025, 17, 97016. [Google Scholar] [CrossRef]
- Hoerning, A.; Jüngert, J.; Siebenlist, G.; Knieling, F.; Regensburger, A.P. Ultrasound in pediatric inflammatory bowel disease-a review of the state of the art and future perspectives. Children 2024, 11, 156. [Google Scholar] [CrossRef] [PubMed]
- Pillet, J.; Voirol-Perrin, J.; Martel, M.; Kherad, O.; Restellini, S. Intestinal ultrasonography diagnostic performance and feasibility in IBD during pregnancy: A systematic review and narrative synthesis. Inflamm. Intest. Dis. 2024, 9, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Latella, G. Redox Imbalance in intestinal fibrosis: Beware of the TGFβ-1, ROS, and Nrf2 connection. Dig. Dis. Sci. 2018, 63, 312–320. [Google Scholar] [CrossRef]
- Rogler, G. New therapeutic avenues for treatment of fibrosis: Can we learn from other diseases? Dig. Dis. 2014, 32 (Suppl. S1), 39–49. [Google Scholar] [CrossRef]
- Mannon, P.; Reinisch, W. Interleukin 13 and its role in gut defence and inflammation. Gut 2012, 61, 1765–1773. [Google Scholar] [CrossRef]
- Rieder, F.; Mukherjee, P.K.; Massey, W.J.; Wang, Y.; Fiocchi, C. Fibrosis in IBD: From pathogenesis to therapeutic targets. Gut 2024, 73, 854–866. [Google Scholar] [CrossRef]
- Raine, T.; Bonovas, S.; Burisch, J.; Kucharzik, T.; Adamina, M.; Annese, V.; Bachmann, O.; Bettenworth, D.; Chaparro, M.; Czuber-Dochan, W.; et al. ECCO guidelines on herapeutics in ulcerative colitis: Medical treatment. J. Crohn’s Colitis 2022, 16, 2–17. [Google Scholar] [CrossRef]
- Solitano, V.; Jairath, V.; Ungaro, F.; Peyrin-Biroulet, L.; Danese, S. TL1A inhibition for inflammatory bowel disease treatment: From inflammation to fibrosis. Med 2024, 5, 386–400. [Google Scholar] [CrossRef]
- Di Gregorio, J.; Sferra, R.; Speca, S.; Vetuschi, A.; Dubuquoy, C.; Desreumaux, P.; Pompili, S.; Cristiano, L.; Gaudio, E.; Flati, V.; et al. Role of glycogen synthase kinase-3β and PPAR-γ on epithelial-to-mesenchymal transition in DSS-induced colorectal fibrosis. PLoS ONE 2017, 12, e0171093. [Google Scholar] [CrossRef]
- Gordon, I.O.; Abushamma, S.; Kurowski, J.A.; Holubar, S.D.; Kou, L.; Lyu, R.; Rieder, F. Paediatric ulcerative colitis is a fibrotic disease and is linked with chronicity of inflammation. J. Crohn’s Colitis 2022, 16, 804–821. [Google Scholar] [CrossRef]
- Ohama, T.; Hori, M.; Fujisawa, M.; Kiyosue, M.; Hashimoto, M.; Ikenoue, Y.; Jinno, Y.; Miwa, H.; Matsumoto, T.; Murata, T.; et al. Downregulation of CPI-17 contributes to dysfunctional motility in chronic intestinal inflammation model mice and ulcerative colitis patients. J. Gastroenterol. 2008, 43, 858–865. [Google Scholar] [CrossRef] [PubMed]
- da Silva Watanabe, P.; Cavichioli, A.M.; D’Arc de Lima Mendes, J.; Aktar, R.; Peiris, M.; Blackshaw, L.A.; de Almeida Araújo, E.J. Colonic motility adjustments in acute and chronic DSS-induced colitis. Life Sci. 2023, 321, 121642. [Google Scholar] [CrossRef] [PubMed]
- Villanacci, V.; Bugatti, M.; Zini, S.; Del Sordo, R.; Bassotti, G. Letter: The importance of histological assessment-a further stride in STRIDE. Aliment. Pharmacol. Ther. 2023, 58, 1244–1245. [Google Scholar] [CrossRef]
- Bassotti, G.; Del Sordo, R.; Lanzarotto, F.; Mino, S.; Ricci, C.; Villanacci, V. Claudin-2 simplifies histological assessment of activity/remission of ulcerative colitis in real-life daily practice. Eur. J. Gastroenterol. Hepatol. 2025, 37, 409–413. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villanacci, V.; Maconi, G.; Laschi, L.; Bassotti, G. Diagnosing Ulcerative Colitis: Should We Go Beyond the Surface? J. Clin. Med. 2025, 14, 3690. https://doi.org/10.3390/jcm14113690
Villanacci V, Maconi G, Laschi L, Bassotti G. Diagnosing Ulcerative Colitis: Should We Go Beyond the Surface? Journal of Clinical Medicine. 2025; 14(11):3690. https://doi.org/10.3390/jcm14113690
Chicago/Turabian StyleVillanacci, Vincenzo, Giovanni Maconi, Lucrezia Laschi, and Gabrio Bassotti. 2025. "Diagnosing Ulcerative Colitis: Should We Go Beyond the Surface?" Journal of Clinical Medicine 14, no. 11: 3690. https://doi.org/10.3390/jcm14113690
APA StyleVillanacci, V., Maconi, G., Laschi, L., & Bassotti, G. (2025). Diagnosing Ulcerative Colitis: Should We Go Beyond the Surface? Journal of Clinical Medicine, 14(11), 3690. https://doi.org/10.3390/jcm14113690





