Long-Term Ocular Outcomes of Prematurity: Morphological Alterations, Visual Aspects and Implications for Age-Related Ocular Diseases
Abstract
1. Introduction
2. Visual Acuity
3. Strabismus and Amblyopia
4. Refractive Error
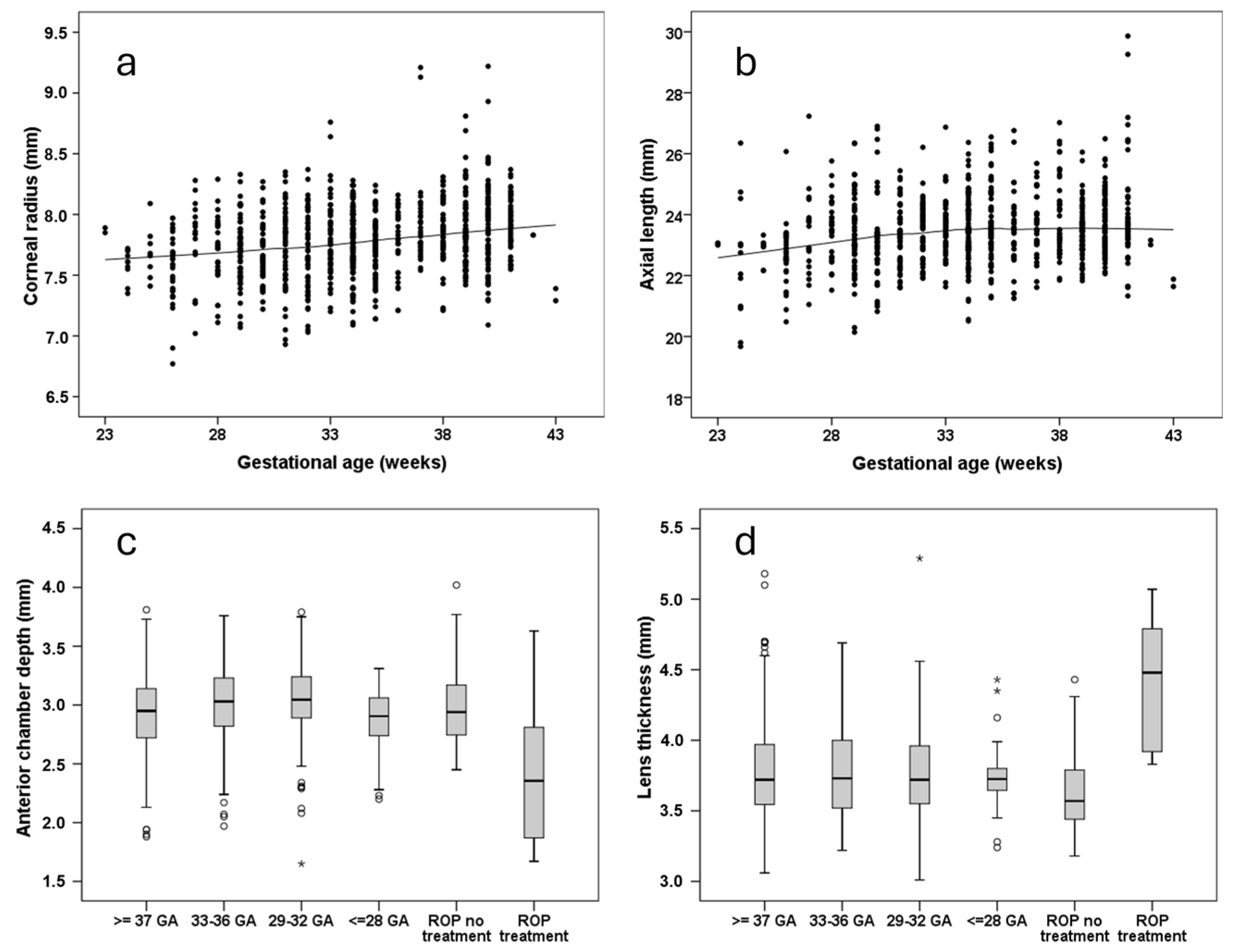
5. Anterior Segment of the Eye: Ocular Geometry
6. Posterior Segment of the Eye
6.1. Macula and Fovea
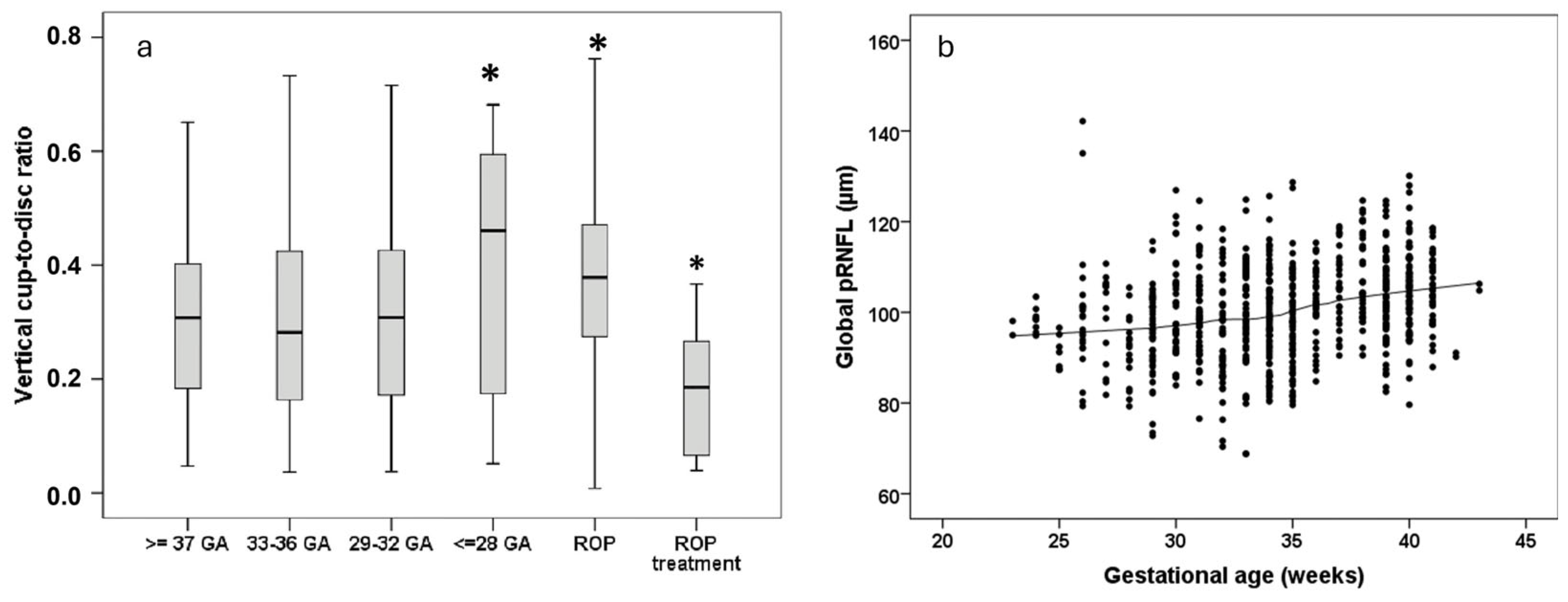
6.2. Retinal Nerve Fiber Layer and Optic Disc
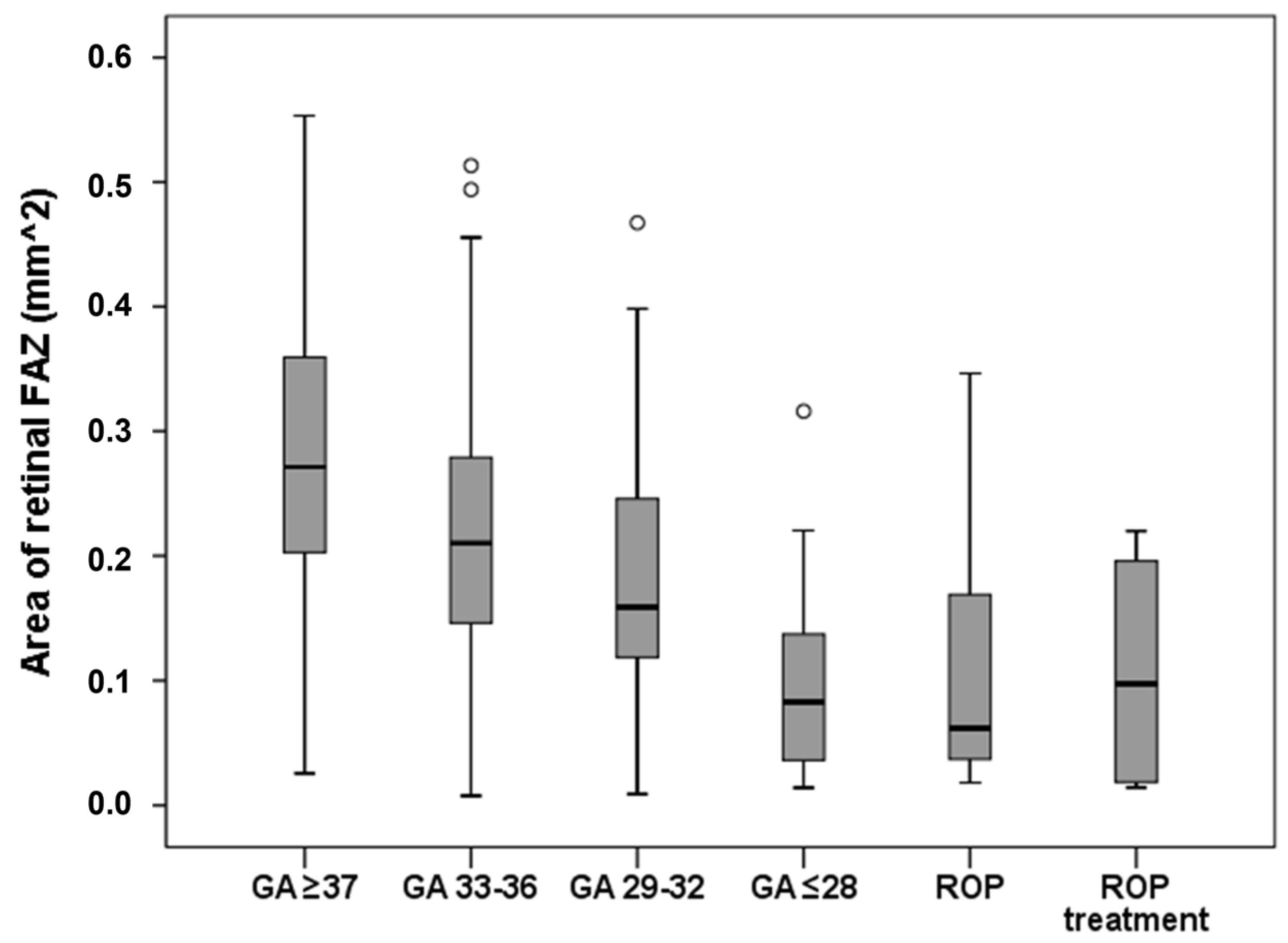
6.3. Retinal Vasculature and Choroid
7. Ocular Diseases with Fetal Origins in Adulthood
8. Critical Appraisal of the Literature
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| GA | Gestational age |
| BW | Birth weight |
| ROP | Retinopathy of prematurity |
| GHS | Gutenberg Health Study |
| GPES | Gutenberg Prematurity Eye Study |
| VLBW | Very low birth weight |
| BCVA | Best-corrected visual acuity |
| DCVA | Distant-corrected visual acuity |
| VRQoL | Vision-related quality of life |
| D | Diopter |
| VEGF | Vascular endothelial growth factor |
| ACA | Anterior chamber angle |
| pRNFL | Peripapillary Retinal Nerve Fiber Layer |
| VCDR | Vertical cup-to-disc-ratio |
| FAZ | Foveal avascular zone |
| AMD | Age-related macular degeneration |
References
- Ohuma, E.O.; Moller, A.-B.; Bradley, E.; Chakwera, S.; Hussain-Alkhateeb, L.; Lewin, A.; Okwaraji, Y.B.; Mahanani, W.R.; Johansson, E.W.; Lavin, T.; et al. National, regional, and global estimates of preterm birth in 2020, with trends from 2010: A systematic analysis. Lancet 2023, 402, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Crump, C. An overview of adult health outcomes after preterm birth. Early Hum. Dev. 2020, 150, 105187. [Google Scholar] [CrossRef] [PubMed]
- Crump, C. Preterm birth and mortality in adulthood: A systematic review. J. Perinatol. 2020, 40, 833–843. [Google Scholar] [CrossRef] [PubMed]
- Darlow, B.A.; Clemett, R.S.; Horwood, L.J.; Mogridge, N. Prospective study of New Zealand infants with birth weight less than 1500 g and screened for retinopathy of prematurity: Visual outcome at age 7–8 years. Br. J. Ophthalmol. 1997, 81, 935–940. [Google Scholar] [CrossRef]
- Fieß, A.; Kölb-Keerl, R.; Elflein, H.M.; Schuster, A.K.; Knuf, M.; Kirchhof, B.; Oberacher-Velten, I.; Muether, P.S.; Bauer, J. Evaluation of Ophthalmic Follow-up Care of Former Pre-term and Full-term Infants Aged from 4 to 10 Years in Germany—Results of the Wiesbaden Prematurity Study (WPS). Klin. Monatsblatter Augenheilkd. 2019, 236, 1174–1181. [Google Scholar] [CrossRef]
- Wu, W.C.; Lin, R.I.; Shih, C.P.; Wang, N.K.; Chen, Y.P.; Chao, A.N.; Chen, K.J.; Chen, T.L.; Hwang, Y.S.; Lai, C.C.; et al. Visual acuity, optical components, and macular abnormalities in patients with a history of retinopathy of prematurity. Ophthalmology 2012, 119, 1907–1916. [Google Scholar] [CrossRef]
- Fieß, A.; Kolb-Keerl, R.; Knuf, M.; Kirchhof, B.; Blecha, C.; Oberacher-Velten, I.; Muether, P.S.; Bauer, J. Axial Length and Anterior Segment Alterations in Former Preterm Infants and Full-Term Neonates Analyzed with Scheimpflug Imaging. Cornea 2017, 36, 821–827. [Google Scholar] [CrossRef]
- Fieß, A.; Schuster, A.K.; Pfeiffer, N.; Nickels, S. Association of birth weight with corneal power in early adolescence: Results from the National Health and Nutrition Examination Survey (NHANES) 1999–2008. PLoS ONE 2017, 12, e0186723. [Google Scholar] [CrossRef]
- Akula, J.D.; Arellano, I.A.; Swanson, E.A.; Favazza, T.L.; Bowe, T.S.; Munro, R.J.; Ferguson, R.D.; Hansen, R.M.; Moskowitz, A.; Fulton, A.B. The Fovea in Retinopathy of Prematurity. Investig. Ophthalmol. Vis. Sci. 2020, 61, 28. [Google Scholar] [CrossRef]
- Rothman, A.L.; Sevilla, M.B.; Mangalesh, S.; Gustafson, K.E.; Edwards, L.; Cotten, C.M.; Shimony, J.S.; Pizoli, C.E.; El-Dairi, M.A.; Freedman, S.F.; et al. Thinner Retinal Nerve Fiber Layer in Very Preterm Versus Term Infants and Relationship to Brain Anatomy and Neurodevelopment. Am. J. Ophthalmol. 2015, 160, 1296–1308.e1292. [Google Scholar] [CrossRef]
- Mangalesh, S.; McGeehan, B.; Tai, V.; Chen, X.; Tran-Viet, D.; Vajzovic, L.; Viehland, C.; Izatt, J.A.; Cotten, C.M.; Freedman, S.F.; et al. Macular Optical Coherence Tomography Characteristics at 36 weeks Postmenstrual Age in Infants Examined for Retinopathy of Prematurity. Ophthalmol. Retin. 2020, 5, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Bowl, W.; Bowl, M.; Schweinfurth, S.; Holve, K.; Andrassi-Darida, M.; Stieger, K.; Lorenz, B. Choroidal Thickness with Swept-Source Optical Coherence Tomography versus Foveal Morphology in Young Children with a History of Prematurity. Ophthalmic Res. 2018, 60, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.F.; Ramasamy, B.; Lythgoe, D.T.; Clark, D. Choroidal thickness in regressed retinopathy of prematurity. Eye 2014, 28, 1461–1468. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Recchia, F.M.; Recchia, C.C. Foveal dysplasia evident by optical coherence tomography in patients with a history of retinopathy of prematurity. Retina 2007, 27, 1221–1226. [Google Scholar] [CrossRef] [PubMed]
- Fieß, A.; Christian, L.; Janz, J.; Kolb-Keerl, R.; Knuf, M.; Kirchhof, B.; Muether, P.S.; Bauer, J. Functional analysis and associated factors of the peripapillary retinal nerve fibre layer in former preterm and full-term infants. Br. J. Ophthalmol. 2017, 101, 1405–1411. [Google Scholar] [CrossRef]
- Fieß, A.; Janz, J.; Schuster, A.K.; Kölb-Keerl, R.; Knuf, M.; Kirchhof, B.; Muether, P.S.; Bauer, J. Macular morphology in former preterm and full-term infants aged 4 to 10 years. Graefe’s Arch. Clin. Exp. Ophthalmol. 2017, 255, 1433–1442. [Google Scholar] [CrossRef]
- Schalij-Delfos, N.E.; de Graaf, M.E.; Treffers, W.F.; Engel, J.; Cats, B.P. Long term follow up of premature infants: Detection of strabismus, amblyopia, and refractive errors. Br. J. Ophthalmol. 2000, 84, 963–967. [Google Scholar] [CrossRef][Green Version]
- Snir, M.; Friling, R.; Weinberger, D.; Sherf, I.; Axer-Siegel, R. Refraction and keratometry in 40 week old premature (corrected age) and term infants. Br. J. Ophthalmol. 2004, 88, 900–904. [Google Scholar] [CrossRef]
- Fieß, A.; Kolb-Keerl, R.; Schuster, A.K.; Knuf, M.; Kirchhof, B.; Muether, P.S.; Bauer, J. Prevalence and associated factors of strabismus in former preterm and full-term infants between 4 and 10 Years of age. BMC Ophthalmol. 2017, 17, 228. [Google Scholar] [CrossRef]
- Kelly, C.E.; Cheong, J.L.; Molloy, C.; Anderson, P.J.; Lee, K.J.; Burnett, A.C.; Connelly, A.; Doyle, L.W.; Thompson, D.K. Neural correlates of impaired vision in adolescents born extremely preterm and/or extremely low birthweight. PLoS ONE 2014, 9, e93188. [Google Scholar] [CrossRef]
- Molloy, C.S.; Di Battista, A.M.; Anderson, V.A.; Burnett, A.; Lee, K.J.; Roberts, G.; Cheong, J.L.; Anderson, P.J.; Doyle, L.W. The contribution of visual processing to academic achievement in adolescents born extremely preterm or extremely low birth weight. Child Neuropsychol. 2017, 23, 361–379. [Google Scholar] [CrossRef] [PubMed]
- Darlow, B.A.; Elder, M.J.; Kimber, B.; Martin, J.; Horwood, L.J. Vision in former very low birthweight young adults with and without retinopathy of prematurity compared with term born controls: The NZ 1986 VLBW follow-up study. Br. J. Ophthalmol. 2018, 102, 1041–1046. [Google Scholar] [CrossRef] [PubMed]
- Darlow, B.A.; Lui, K.; Kusuda, S.; Reichman, B.; Håkansson, S.; Bassler, D.; Modi, N.; Lee, S.K.; Lehtonen, L.; Vento, M.; et al. International variations and trends in the treatment for retinopathy of prematurity. Br. J. Ophthalmol. 2017, 101, 1399–1404. [Google Scholar] [CrossRef] [PubMed]
- Pétursdóttir, D.; Holmström, G.; Larsson, E.; Böhm, B. Visual-motor functions are affected in young adults who were born premature and screened for retinopathy of prematurity. Acta Paediatr. 2021, 110, 127–133. [Google Scholar] [CrossRef]
- Pétursdóttir, D.; Holmström, G.; Larsson, E. Visual function is reduced in young adults formerly born prematurely: A population-based study. Br. J. Ophthalmol. 2020, 104, 541–546. [Google Scholar] [CrossRef]
- Pétursdóttir, D.; Holmström, G.; Larsson, E. Strabismus, stereoacuity, accommodation and convergence in young adults born premature and screened for retinopathy of prematurity. Acta Ophthalmol. 2022, 100, e791–e797. [Google Scholar] [CrossRef]
- Pétursdóttir, D.; Holmström, G.; Larsson, E. Refraction and its development in young adults born prematurely and screened for retinopathy of prematurity. Acta Ophthalmol. 2021, 100, 189–195. [Google Scholar] [CrossRef]
- Pétursdóttir, D.; Åkerblom, H.; Holmström, G.; Larsson, E. Central macular morphology and optic nerve fibre layer thickness in young adults born premature and screened for retinopathy of prematurity. Acta Ophthalmol. 2024, 102, 391–400. [Google Scholar] [CrossRef]
- Fieß, A.; Gißler, S.; Mildenberger, E.; Urschitz, M.S.; Laspas, P.; Stoffelns, B.; Pfeiffer, N.; Schuster, A.K. A short report on: Postnatal environmental factors associated with corneal shaping: Results from the Gutenberg Prematurity Eye Study. Acta Ophthalmol. 2024, 102, e405–e407. [Google Scholar] [CrossRef]
- Ernst, M.; Reiner, I.; Fieß, A.; Tibubos, A.N.; Schulz, A.; Burghardt, J.; Klein, E.M.; Brähler, E.; Wild, P.S.; Münzel, T.; et al. Sex-dependent associations of low birth weight and suicidal ideation in adulthood: A community-based cohort study. Sci. Rep. 2020, 10, 12969. [Google Scholar] [CrossRef]
- Fieß, A.; Nickels, S.; Schulz, A.; Münzel, T.; Wild, P.S.; Beutel, M.E.; Urschitz, M.S.; Lackner, K.J.; Pfeiffer, N.; Schuster, A.K. The relationship of ocular geometry with refractive error in normal and low birth weight adults. J. Optom. 2021, 14, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Fieß, A.; Dautzenberg, K.; Gißler, S.; Mildenberger, E.; Urschitz, M.S.; Elflein, H.M.; Laspas, P.; Stoffelns, B.M.; Pfeiffer, N.; Schuster, A.K. Prevalence of strabismus and risk factors in adults born preterm with and without retinopathy of prematurity: Results from the Gutenberg Prematurity Eye study. Br. J. Ophthalmol. 2024, 108, 1590–1597. [Google Scholar] [CrossRef] [PubMed]
- Wagner, F.M.; Hoffmann, E.M.; Nickels, S.; Fiess, A.; Münzel, T.; Wild, P.S.; Beutel, M.E.; Schmidtmann, I.; Lackner, K.J.; Pfeiffer, N.; et al. Peripapillary Retinal Nerve Fiber Layer Profile in Relation to Refractive Error and Axial Length: Results From the Gutenberg Health Study. Transl. Vis. Sci. Technol. 2020, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Fieß, A.; Elbaz, H.; Korb, C.A.; Nickels, S.; Schulz, A.; Münzel, T.; Wild, P.S.; Beutel, M.E.; Schmidtmann, I.; Lackner, K.J.; et al. Low Birth Weight Is Linked to Age-Related Macular Degeneration: Results from the Population-Based Gutenberg Health Study (GHS). Investig. Ophthalmol. Vis. Sci. 2019, 60, 4943–4950. [Google Scholar] [CrossRef]
- Fieß, A.; Gißler, S.; Wild, P.S.; Lackner, K.J.; Münzel, T.; Michal, M.; Urschitz, M.S.; Pfeiffer, N.; Schuster, A.K. Hypertensive Retinopathy is Not Associated with Low or High Birth Weight—Results from the Population-Based German Gutenberg Health Study. Clin. Ophthalmol. 2024, 18, 1797–1800. [Google Scholar] [CrossRef]
- Fieß, A.; Ponto, K.A.; Urschitz, M.S.; Nickels, S.; Schulz, A.; Münzel, T.; Wild, P.S.; Beutel, M.E.; Lackner, K.J.; Pfeiffer, N.; et al. Birthweight and its association with retinal vessel equivalents—Results from the population-based German Gutenberg Health Study. Acta Ophthalmol. 2021, 99, e773–e774. [Google Scholar] [CrossRef]
- Fieß, A.; Stingl, J.; Urschitz, M.S.; Hoffmann, E.M.; Münzel, T.; Wild, P.S.; Beutel, M.E.; Lackner, K.J.; Pfeiffer, N.; Schuster, A.K. Birth weight and its association with optic nerve head morphology—Results from the population-based German Gutenberg Health Study. Acta Ophthalmol. 2022, 100, e1349–e1350. [Google Scholar] [CrossRef]
- Fieß, A.; Lamparter, J.; Raum, P.; Peto, T.; Ponto, K.A.; Nickels, S.; Münzel, T.; Wild, P.S.; Beutel, M.E.; Urschitz, M.S.; et al. Birth Weight and Diabetic Retinopathy: Results From the Population-Based Gutenberg Health Study (GHS). Ophthalmic Epidemiol. 2021, 28, 122–130. [Google Scholar] [CrossRef]
- Fieß, A.; Schuster, A.K.; Nickels, S.; Elflein, H.M.; Schulz, A.; Beutel, M.E.; Blettner, M.; Pfeiffer, N. Association of low birth weight with myopic refractive error and lower visual acuity in adulthood: Results from the population-based Gutenberg Health Study (GHS). Br. J. Ophthalmol. 2019, 103, 99–105. [Google Scholar] [CrossRef]
- Fieß, A.; Schuster, A.K.; Nickels, S.; Urschitz, M.S.; Elflein, H.M.; Schulz, A.; Munzel, T.; Wild, P.S.; Beutel, M.E.; Schmidtmann, I.; et al. Association of Low Birth Weight With Altered Corneal Geometry and Axial Length in Adulthood in the German Gutenberg Health Study. JAMA Ophthalmol. 2019, 137, 507–514. [Google Scholar] [CrossRef]
- Fieß, A.; Wagner, F.M.; Urschitz, M.S.; Nagler, M.; Stoffelns, B.; Wild, P.S.; Münzel, T.; Beutel, M.E.; Lackner, K.J.; Pfeiffer, N.; et al. Association of Birth Weight With Foveolar Thickness in Adulthood: Results From a Population-Based Study. Investig. Ophthalmol. Vis. Sci. 2021, 62, 9. [Google Scholar] [CrossRef] [PubMed]
- Fieß, A.; Urschitz, M.S.; Nagler, M.; Nickels, S.; Marx-Groß, S.; Münzel, T.; Wild, P.S.; Beutel, M.E.; Lackner, K.J.; Pfeiffer, N.; et al. Association of birth weight with corneal aberrations in adulthood—Results from a population-based study. J. Optom. 2023, 16, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Fieß, A.; Urschitz, M.S.; Marx-Groß, S.; Nagler, M.; Wild, P.S.; Münzel, T.; Beutel, M.E.; Lackner, K.J.; Pfeiffer, N.; Schuster, A.K. Association of Birth Weight with Central and Peripheral Corneal Thickness in Adulthood-Results from the Population-Based German Gutenberg Health Study. Children 2021, 8, 1006. [Google Scholar] [CrossRef] [PubMed]
- Fieß, A.; Greven, K.; Mildenberger, E.; Urschitz, M.S.; Elflein, H.M.; Zepp, F.; Stoffelns, B.; Pfeiffer, N.; Schuster, A.K. Visual acuity, amblyopia, and vision-related quality of life in preterm adults with and without ROP: Results from the Gutenberg prematurity eye study. Eye 2023, 37, 1794–1801. [Google Scholar] [CrossRef]
- Fieß, A.; Gißler, S.; Fauer, A.; Riedl, J.C.; Mildenberger, E.; Urschitz, M.S.; Zepp, F.; Stoffelns, B.; Pfeiffer, N.; Schuster, A.K. Short report on retinal vessel metrics and arterial blood pressure in adult individuals born preterm with and without retinopathy of prematurity: Results from the Gutenberg Prematurity Eye Study. Acta Ophthalmol. 2022, 100, e1769–e1770. [Google Scholar] [CrossRef]
- Fieß, A.; Hartmann, A.; Mildenberger, E.; Urschitz, M.S.; Laspas, P.; Schultheis, A.; Stoffelns, B.; Pfeiffer, N.; Gißler, S.; Schuster, A.K. Sex-Specific Differences in the Relationship Between Prematurity and Ocular Geometry. Investig. Ophthalmol. Vis. Sci. 2024, 65, 23. [Google Scholar] [CrossRef]
- Fieß, A.; Berger, L.A.; Riedl, J.C.; Mildenberger, E.; Urschitz, M.S.; Hampel, U.; Wasielica-Poslednik, J.; Zepp, F.; Stoffelns, B.; Pfeiffer, N.; et al. The role of preterm birth, retinopathy of prematurity and perinatal factors on corneal aberrations in adulthood: Results from the Gutenberg prematurity eye study. Ophthalmic Physiol. Opt. 2022, 42, 1379–1389. [Google Scholar] [CrossRef]
- Fieß, A.; Pfisterer, A.; Gißler, S.; Korb, C.; Mildenberger, E.; Urschitz, M.S.; Zepp, F.; Stoffelns, B.; Pfeiffer, N.; Schuster, A.K. Retinal Thickness and Foveal Hypoplasia in Adults Born Preterm with and Without Retinopathy of Prematurity: The Gutenberg Prematurity Eye Study. Retina 2022, 42, 1716–1728. [Google Scholar] [CrossRef]
- Fieß, A.; Fauer, A.; Mildenberger, E.; Urschitz, M.S.; Elflein, H.M.; Zepp, F.; Stoffelns, B.; Pfeiffer, N.; Schuster, A.K. Refractive error, accommodation and lens opacification in adults born preterm and full-term: Results from the Gutenberg Prematurity Eye Study (GPES). Acta Ophthalmol. 2022, 100, e1439–e1450. [Google Scholar] [CrossRef]
- Fieß, A.; Schäffler, A.; Mildenberger, E.; Urschitz, M.S.; Wagner, F.M.; Hoffmann, E.M.; Zepp, F.; Pfeiffer, N.; Schuster, A.K. Peripapillary Retinal Nerve Fiber Layer Thickness in Adults Born Extremely, Very, and Moderately Preterm with and Without Retinopathy of Prematurity: Results From the Gutenberg Prematurity Eye Study (GPES). Am. J. Ophthalmol. 2022, 244, 88–97. [Google Scholar] [CrossRef]
- Fieß, A.; Gißler, S.; Mildenberger, E.; Urschitz, M.S.; Zepp, F.; Hoffmann, E.M.; Brockmann, M.A.; Stoffelns, B.; Pfeiffer, N.; Schuster, A.K. Optic Nerve Head Morphology in Adults Born Extreme, Very, and Moderate Preterm With and Without Retinopathy of Prematurity: Results From the Gutenberg Prematurity Eye Study. Am. J. Ophthalmol. 2022, 239, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Fieß, A.; Nauen, H.; Mildenberger, E.; Zepp, F.; Urschitz, M.S.; Pfeiffer, N.; Schuster, A.K. Ocular geometry in adults born extremely, very and moderately preterm with and without retinopathy of prematurity: Results from the Gutenberg Prematurity Eye Study. Br. J. Ophthalmol. 2023, 107, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Fieß, A.; Gißler, S.; Mildenberger, E.; Urschitz, M.S.; Laspas, P.; Stoffelns, B.; Pfeiffer, N.; Hartmann, A.; Schuster, A.K. Macular morphology is not affected by low or high birthweight in individuals born at term-Results from the Gutenberg Prematurity Eye Study. Acta Ophthalmol. 2024, 102, e657–e658. [Google Scholar] [CrossRef] [PubMed]
- Fieß, A.; Volmering, C.; Gißler, S.; Mildenberger, E.; Urschitz, M.S.; Laspas, P.; Stoffelns, B.; Pfeiffer, N.; Schuster, A.K. Macular Curvature in Adults Born Preterm with and Without ROP: Results from the Gutenberg Prematurity Eye Study. Investig. Ophthalmol. Vis. Sci. 2024, 65, 39. [Google Scholar] [CrossRef]
- Fieß, A.; Grabitz, S.D.; Mildenberger, E.; Urschitz, M.S.; Fauer, A.; Hampel, U.; Wasielica-Poslednik, J.; Zepp, F.; Pfeiffer, N.; Schuster, A.K. A lower birth weight percentile is associated with central corneal thickness thinning: Results from the Gutenberg Prematurity Eye Study (GPES). J. Optom. 2023, 16, 143–150. [Google Scholar] [CrossRef]
- Fieß, A.; Zange, M.; Gißler, S.; Mildenberger, E.; Urschitz, M.S.; Laspas, P.; Stoffelns, B.; Pfeiffer, N.; Schuster, A.K. Foveal avascular zone in adults born preterm with and without retinopathy of prematurity—Results from the Gutenberg Prematurity Eye Study. Retina 2024, 44, 1431–1440. [Google Scholar] [CrossRef]
- Fieß, A.; Schulze, K.; Grabitz, S.D.; Gißler, S.; Mildenberger, E.; Urschitz, M.S.; Stoffelns, B.; Pfeiffer, N.; Schuster, A.K. Foveal and Peripapillary Choroidal Thickness in Adults Born Extremely, Very, and Moderately Preterm with and Without ROP-Results From the Gutenberg Prematurity Eye Study. Transl. Vis. Sci. Technol. 2022, 11, 4. [Google Scholar] [CrossRef]
- Fieß, A.; Hufschmidt-Merizian, C.; Gißler, S.; Hampel, U.; Mildenberger, E.; Urschitz, M.S.; Zepp, F.; Stoffelns, B.; Pfeiffer, N.; Schuster, A.K. Dry Eye Parameters and Lid Geometry in Adults Born Extremely, Very, and Moderately Preterm with and without ROP: Results from the Gutenberg Prematurity Eye Study. J. Clin. Med. 2022, 11, 2702. [Google Scholar] [CrossRef]
- Fieß, A.; Gißler, S.; Mildenberger, E.; Urschitz, M.S.; Fauer, A.; Elflein, H.M.; Zepp, F.; Stoffelns, B.; Pfeiffer, N.; Schuster, A.K. Anterior Chamber Angle in Adults Born Extremely, Very, and Moderately Preterm with and without Retinopathy of Prematurity-Results of the Gutenberg Prematurity Eye Study. Children 2022, 9, 281. [Google Scholar] [CrossRef]
- Kulmala, M.; Jørgensen, A.P.M.; Aakvik, K.A.D.; Jussinniemi, L.; Benum, S.D.; Ingvaldsen, S.H.; Austeng, D.; Kajantie, E.; Evensen, K.A.I.; Majander, A.; et al. Visual function in adults born preterm with very low birth weight-A two-country birth cohort study. Acta Ophthalmol. 2024, 102, 49–57. [Google Scholar] [CrossRef]
- Smith, B.T.; Tasman, W.S. Retinopathy of prematurity: Late complications in the baby boomer generation (1946–1964). Trans. Am. Ophthalmol. Soc. 2005, 103, 225–234, discussion 234–226. [Google Scholar] [PubMed]
- Jain, S.; Sim, P.Y.; Beckmann, J.; Ni, Y.; Uddin, N.; Unwin, B.; Marlow, N. Functional Ophthalmic Factors Associated with Extreme Prematurity in Young Adults. JAMA Netw. Open 2022, 5, e2145702. [Google Scholar] [CrossRef] [PubMed]
- Al-Abaiji, H.A.; Nissen, K.R.; Slidsborg, C.; La Cour, M.; Kessel, L. Tracking visual outcomes—Follow-up on patients born preterm with childhood-onset visual impairment. Acta Ophthalmol. 2025. [Google Scholar] [CrossRef] [PubMed]
- Ferrone, P.J.; Trese, M.T.; Williams, G.A.; Cox, M.S. Good visual acuity in an adult population with marked posterior segment changes secondary to retinopathy of prematurity. Retina 1998, 18, 335–338. [Google Scholar] [CrossRef]
- Buffenn, A.N. The impact of strabismus on psychosocial heath and quality of life: A systematic review. Surv. Ophthalmol. 2021, 66, 1051–1064. [Google Scholar] [CrossRef]
- Lingham, G.; Mackey, D.A.; Sanfilippo, P.G.; Mountain, J.; Hewitt, A.W.; Newnham, J.P.; Yazar, S. Influence of prenatal environment and birth parameters on amblyopia, strabismus, and anisometropia. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2020, 24, e71–e74. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, Y.; Li, B.; Zhu, D.; Sang, T.; Du, X.; Shi, W.; Yang, L. Analysis of risk factors associated with the high incidence of amblyopia in preterm infants at the corrected gestational age of 12 months. BMC Pediatr. 2023, 23, 136. [Google Scholar] [CrossRef]
- van Leeuwen, R.; Eijkemans, M.J.; Vingerling, J.R.; Hofman, A.; de Jong, P.T.; Simonsz, H.J. Risk of bilateral visual impairment in individuals with amblyopia: The Rotterdam study. Br. J. Ophthalmol. 2007, 91, 1450–1451. [Google Scholar] [CrossRef]
- Plotnikov, D.; Williams, C.; Guggenheim, J.A. Association between birth weight and refractive error in adulthood: A Mendelian randomisation study. Br. J. Ophthalmol. 2020, 104, 214–219. [Google Scholar] [CrossRef]
- Ghoraba, H.H.; Ludwig, C.A.; Moshfeghi, D.M. Biometric Variations in High Myopia Associated with Different Underlying Ocular and Genetic Conditions. Ophthalmol. Sci. 2023, 3, 100236. [Google Scholar] [CrossRef]
- Kong, Q.; Ming, W.K.; Mi, X.S. Refractive outcomes after intravitreal injection of antivascular endothelial growth factor versus laser photocoagulation for retinopathy of prematurity: A meta-analysis. BMJ Open 2021, 11, e042384. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Y.; Liao, Y.; Zeng, R.; Zeng, P.; Lan, Y. Comparison of efficacy between anti-vascular endothelial growth factor (VEGF) and laser treatment in Type-1 and threshold retinopathy of prematurity (ROP). BMC Ophthalmol. 2018, 18, 19. [Google Scholar] [CrossRef] [PubMed]
- Scoville, N.M.; Legocki, A.T.; Touch, P.; Ding, L.; Moshiri, Y.; Bays-Muchmore, C.; Qiao, E.; Zhou, K.; Zhong, J.; Tarczy-Hornoch, K.; et al. Vitreous opacities in infants born full-term and preterm by handheld swept-source optical coherence tomography. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2022, 26, 20.e1–20.e7. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.; Robinson, J.; Trese, M.T.; Williams, G.A. Long-term Glaucoma Outcomes in Adult Retinopathy of Prematurity. Investig. Ophthalmol. Vis. Sci. 2015, 56, 4319. [Google Scholar]
- Hendrickson, A.E.; Yuodelis, C. The morphological development of the human fovea. Ophthalmology 1984, 91, 603–612. [Google Scholar] [CrossRef]
- Baker, P.S.; Tasman, W. Optical coherence tomography imaging of the fovea in retinopathy of prematurity. Ophthalmic Surg. Lasers Imaging 2010, 41, 201–206. [Google Scholar] [CrossRef]
- Nilsson, M.; Hellström, A.; Jacobson, L. Retinal Sequelae in Adults Treated with Cryotherapy for Retinopathy of Prematurity. Investig. Ophthalmol. Vis. Sci. 2016, 57, Oct550–Oct555. [Google Scholar] [CrossRef]
- Thanos, A.; Yonekawa, Y.; Todorich, B.; Huang, N.; Drenser, K.A.; Williams, G.A.; Trese, M.T.; Capone, A., Jr. Spectral-Domain Optical Coherence Tomography in Older Patients with History of Retinopathy of Prematurity. Ophthalmic Surg. Lasers Imaging Retin. 2016, 47, 1086–1094. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Beckmann, J.; Mehta, H.; Sadda, S.R.; Chanwimol, K.; Nassisi, M.; Tsui, I.; Marlow, N.; Jain, S. Relationship between retinal thickness profiles and visual outcomes in young adults born extremely preterm: The EPICure@19 Study. Ophthalmology 2019, 126, 107–112. [Google Scholar] [CrossRef]
- Jørgensen, A.P.M.; Kulmala, M.; Austeng, D.; Evensen, K.A.I.; Kajantie, E.; Majander, A.; Morken, T.S. Foveal thickness and its association with visual acuity in adults born preterm with very low birth weight: A two-country birth cohort study. Acta Ophthalmol. 2024, 102, 942–952. [Google Scholar] [CrossRef]
- Müller, P.L.; Kihara, Y.; Olvera-Barrios, A.; Warwick, A.N.; Egan, C.; Williams, K.M.; Lee, A.Y.; Tufail, A.; Eyes, F.T.U.B.; Consortium, V. Quantification and Predictors of OCT-Based Macular Curvature and Dome-Shaped Configuration: Results From the UK Biobank. Investig. Ophthalmol. Vis. Sci. 2022, 63, 28. [Google Scholar] [CrossRef] [PubMed]
- Fieß, A.; Brandt, M.; Mildenberger, E.; Urschitz, M.S.; Wagner, F.M.; Grabitz, S.D.; Hoffmann, E.M.; Pfeiffer, N.; Schuster, A.K. Adults Born Small for Gestational Age at Term Have Thinner Peripapillary Retinal Nerve Fiber Layers Than Controls. Eye Brain 2022, 14, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Fieß, A.; Nickels, S.; Urschitz, M.S.; Münzel, T.; Wild, P.S.; Beutel, M.E.; Lackner, K.J.; Hoffmann, E.M.; Pfeiffer, N.; Schuster, A.K. Association of Birth Weight with Peripapillary Retinal Nerve Fiber Layer Thickness in Adulthood—Results from a Population-Based Study. Investig. Ophthalmol. Vis. Sci. 2020, 61, 4. [Google Scholar] [CrossRef] [PubMed]
- Kulmala, M.K.; Jørgensen, A.; Austeng, D.; Evensen, K.A.I.; Kajantie, E.; Morken, T.S.; Majander, A. Imprint of preterm birth with very low birth weight on optic disc OCT in adulthood-A two-country birth cohort study. Acta Ophthalmol. 2025, 103, 50–60. [Google Scholar] [CrossRef]
- Tong, A.Y.; El-Dairi, M.; Maldonado, R.S.; Rothman, A.L.; Yuan, E.L.; Stinnett, S.S.; Kupper, L.; Cotten, C.M.; Gustafson, K.E.; Goldstein, R.F.; et al. Evaluation of optic nerve development in preterm and term infants using handheld spectral-domain optical coherence tomography. Ophthalmology 2014, 121, 1818–1826. [Google Scholar] [CrossRef]
- Jacobson, L.; Lundin, S.; Flodmark, O.; Ellström, K.G. Periventricular leukomalacia causes visual impairment in preterm children. A study on the aetiologies of visual impairment in a population-based group of preterm children born 1989–1995 in the county of Värmland, Sweden. Acta Ophthalmol. Scand. 1998, 76, 593–598. [Google Scholar] [CrossRef]
- Baum, J.D. Retinal artery tortuosity in ex-premature infants. 18-year follow-up on eyes of premature infants. Arch. Dis. Child. 1971, 46, 247–252. [Google Scholar] [CrossRef][Green Version]
- Kistner, A.; Jacobson, L.; Jacobson, S.H.; Svensson, E.; Hellstrom, A. Low gestational age associated with abnormal retinal vascularization and increased blood pressure in adult women. Pediatr. Res. 2002, 51, 675–680. [Google Scholar] [CrossRef]
- Liew, G.; Wang, J.J.; Duncan, B.B.; Klein, R.; Sharrett, A.R.; Brancati, F.; Yeh, H.-C.; Mitchell, P.; Wong, T.Y. Low Birthweight Is Associated with Narrower Arterioles in Adults. Hypertension 2008, 51, 933–938. [Google Scholar] [CrossRef]
- Chui, T.Y.; Zhong, Z.; Song, H.; Burns, S.A. Foveal avascular zone and its relationship to foveal pit shape. Optom. Vis. Sci. 2012, 89, 602–610. [Google Scholar] [CrossRef]
- Tick, S.; Rossant, F.; Ghorbel, I.; Gaudric, A.; Sahel, J.-A.; Chaumet-Riffaud, P.; Paques, M. Foveal Shape and Structure in a Normal Population. Investig. Ophthalmol. Vis. Sci. 2011, 52, 5105–5110. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.T.; Knapp, A.N.; Chen, C.; Baynes, K.; Burton, D.; Kaiser, P.K.; Srivastava, S.K.; Rachitskaya, A.V. Macular Changes in Adults with History of Premature Birth. Retina 2025, 45, 171–177. [Google Scholar] [CrossRef]
- Finn, M.; Baldwin, G.; Garg, I.; Wescott, H.E.; Koch, T.; Vingopoulos, F.; Zeng, R.; Choi, H.; Sayah, D.; Husain, D.; et al. Comparative study of widefield swept-source optical coherence tomography angiography in eyes with concomitant age-related macular degeneration and diabetic retinopathy. Br. J. Ophthalmol. 2024, 108, 963. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.; Shivitz, I. Angle-closure glaucoma in adults with cicatricial retinopathy of prematurity. Arch. Ophthalmol. 1984, 102, 371–372. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, R.S.; Trese, M.T.; Williams, G.A.; Cox, M.S., Jr. Adult retinopathy of prematurity: Outcomes of rhegmatogenous retinal detachments and retinal tears. Ophthalmology 2001, 108, 1647–1653. [Google Scholar] [CrossRef]
- Kaiser, R.S.; Fenton, G.L.; Tasman, W.; Trese, M.T. Adult retinopathy of prematurity: Retinal complications from cataract surgery. Am. J. Ophthalmol. 2008, 145, 729–735. [Google Scholar] [CrossRef]
- Quan, A.V.; Pineles, S.L.; Tsui, I.; Velez, F.G. Phthisis bulbi after lensectomy in retinopathy of prematurity eyes previously treated with laser photocoagulation. Retin. Cases Brief Rep. 2015, 9, 67–71. [Google Scholar] [CrossRef]
- Pan, C.W.; Ikram, M.K.; Cheung, C.Y.; Choi, H.W.; Cheung, C.M.; Jonas, J.B.; Saw, S.M.; Wong, T.Y. Refractive errors and age-related macular degeneration: A systematic review and meta-analysis. Ophthalmology 2013, 120, 2058–2065. [Google Scholar] [CrossRef]
- Markopoulou, P.; Papanikolaou, E.; Analytis, A.; Zoumakis, E.; Siahanidou, T. Preterm Birth as a Risk Factor for Metabolic Syndrome and Cardiovascular Disease in Adult Life: A Systematic Review and Meta-Analysis. J. Pediatr. 2019, 210, 69–80.e65. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fieß, A.; Gißler, S.; Mildenberger, E.; Pfeiffer, N.; Hartmann, A.; Schuster, A.K. Long-Term Ocular Outcomes of Prematurity: Morphological Alterations, Visual Aspects and Implications for Age-Related Ocular Diseases. J. Clin. Med. 2025, 14, 3667. https://doi.org/10.3390/jcm14113667
Fieß A, Gißler S, Mildenberger E, Pfeiffer N, Hartmann A, Schuster AK. Long-Term Ocular Outcomes of Prematurity: Morphological Alterations, Visual Aspects and Implications for Age-Related Ocular Diseases. Journal of Clinical Medicine. 2025; 14(11):3667. https://doi.org/10.3390/jcm14113667
Chicago/Turabian StyleFieß, Achim, Sandra Gißler, Eva Mildenberger, Norbert Pfeiffer, Alica Hartmann, and Alexander K. Schuster. 2025. "Long-Term Ocular Outcomes of Prematurity: Morphological Alterations, Visual Aspects and Implications for Age-Related Ocular Diseases" Journal of Clinical Medicine 14, no. 11: 3667. https://doi.org/10.3390/jcm14113667
APA StyleFieß, A., Gißler, S., Mildenberger, E., Pfeiffer, N., Hartmann, A., & Schuster, A. K. (2025). Long-Term Ocular Outcomes of Prematurity: Morphological Alterations, Visual Aspects and Implications for Age-Related Ocular Diseases. Journal of Clinical Medicine, 14(11), 3667. https://doi.org/10.3390/jcm14113667




