Effect of Chronic Obstructive Pulmonary Disease (COPD) on Biventricular Mechanics in Patients Without Severe Airflow Obstruction
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Study Selection and Data Extraction
2.4. Risk of Bias Assessment
2.5. Statistical Analysis
3. Results
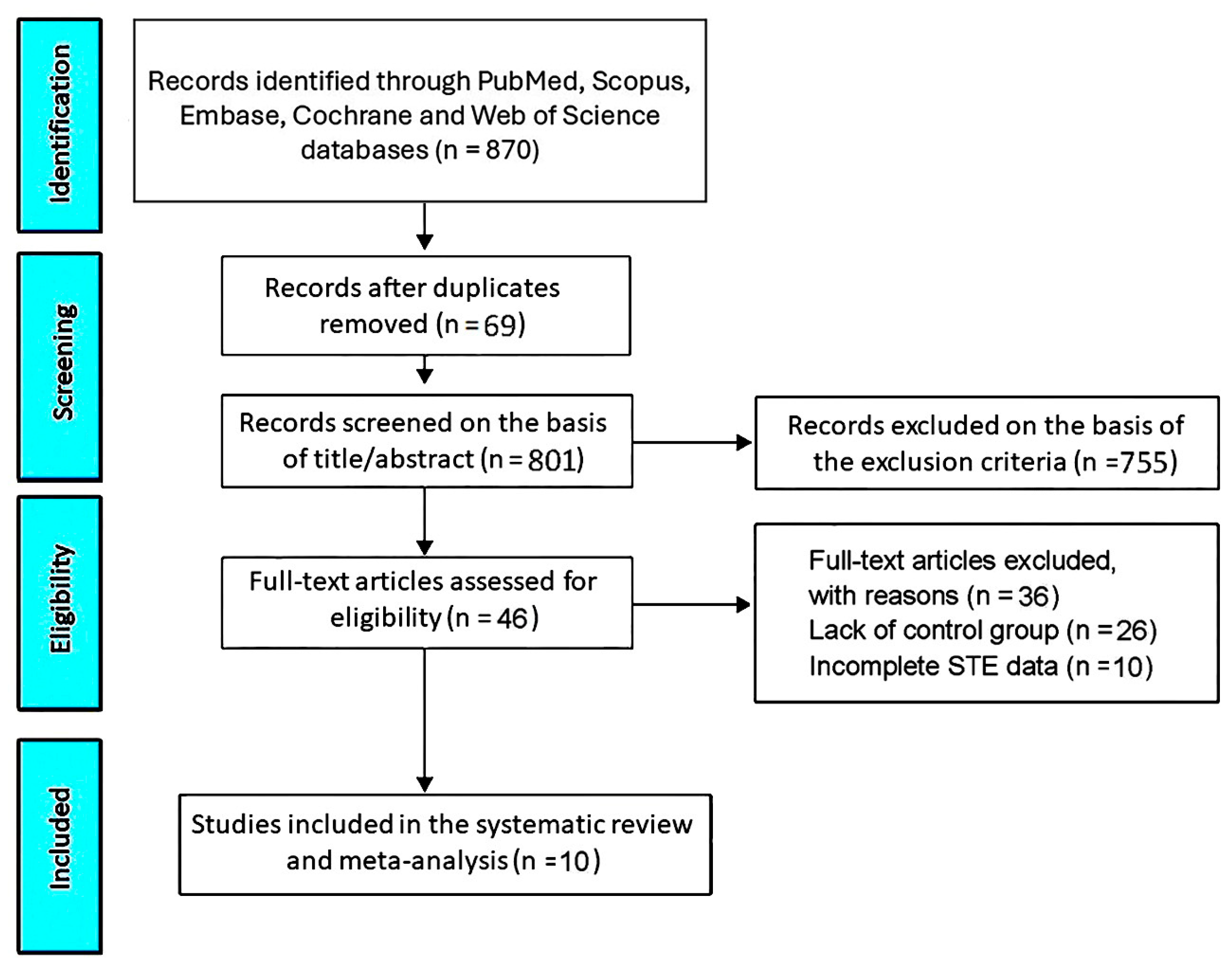
3.1. Study Selection
3.2. Clinical Findings
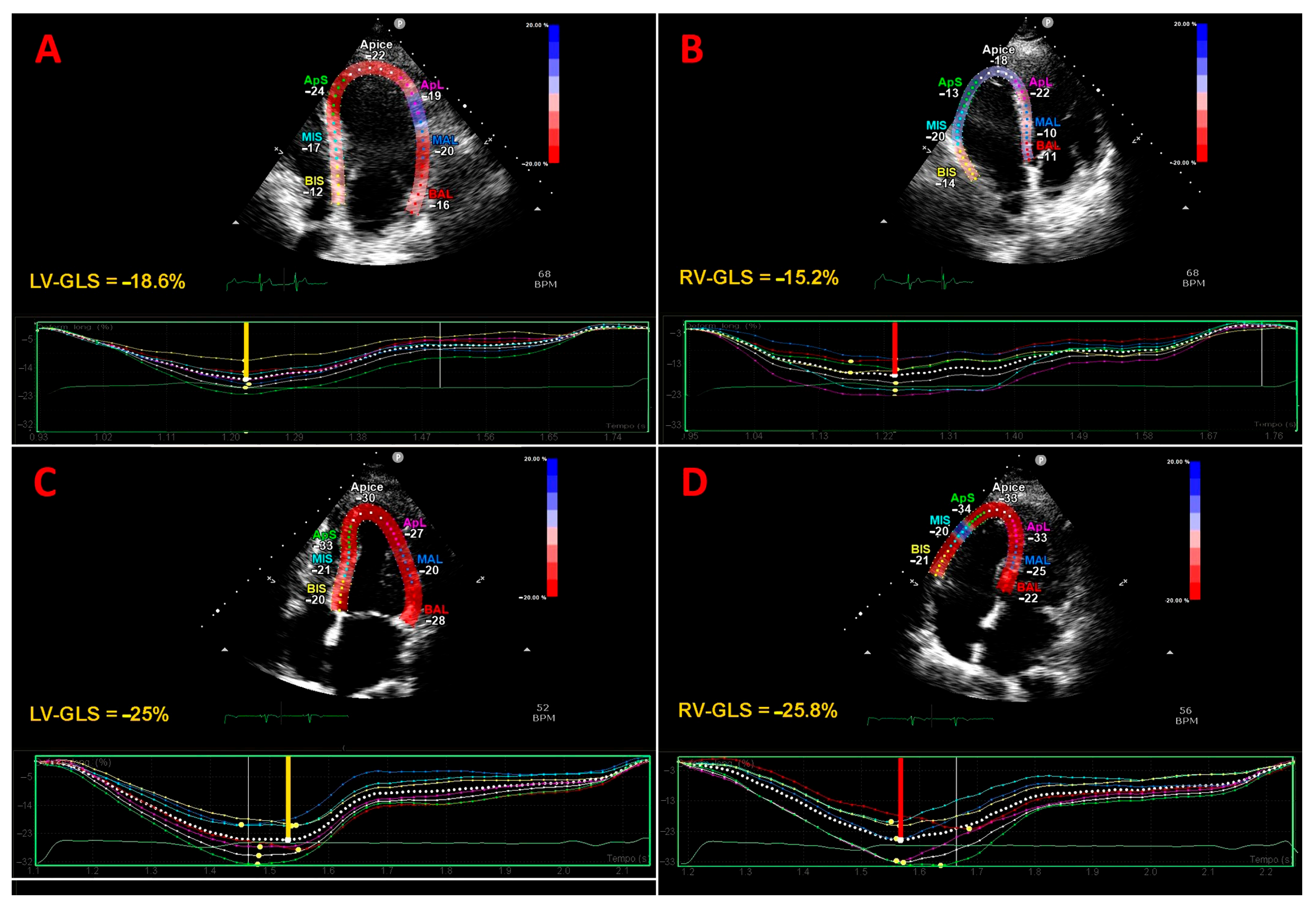
3.3. Traditional Echocardiography and Strain Imaging Findings
3.4. NIH Quality Rating
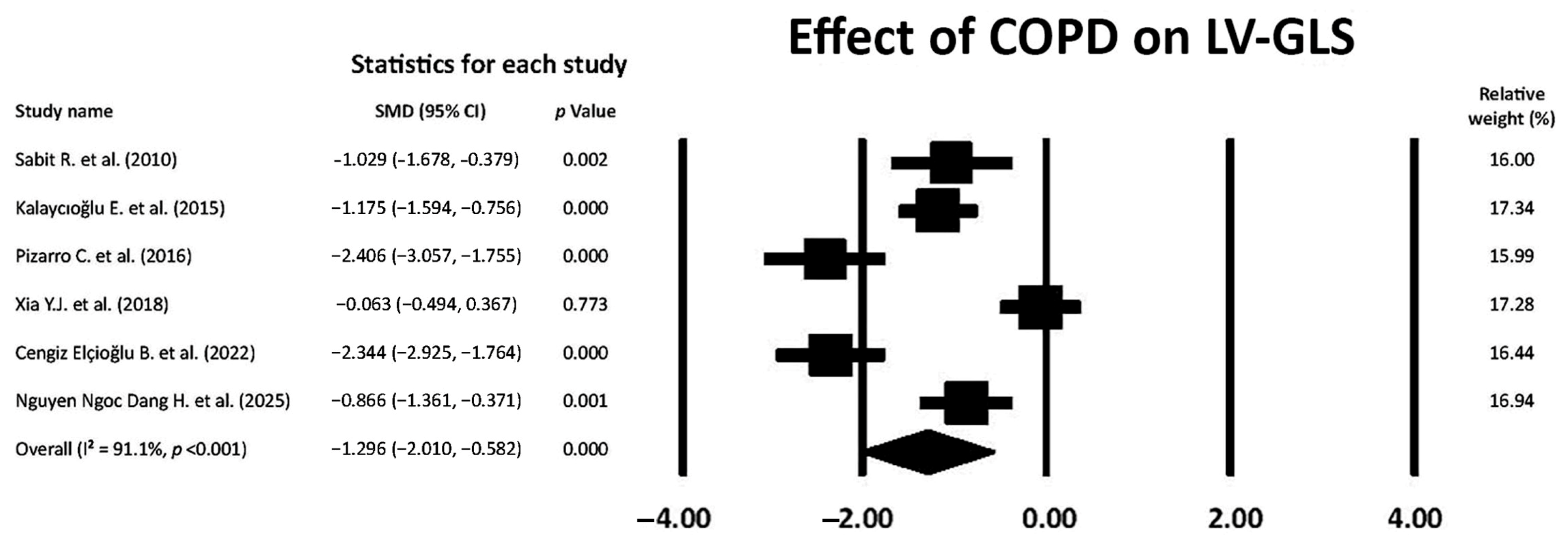
3.5. Effect of COPD on LV-GLS
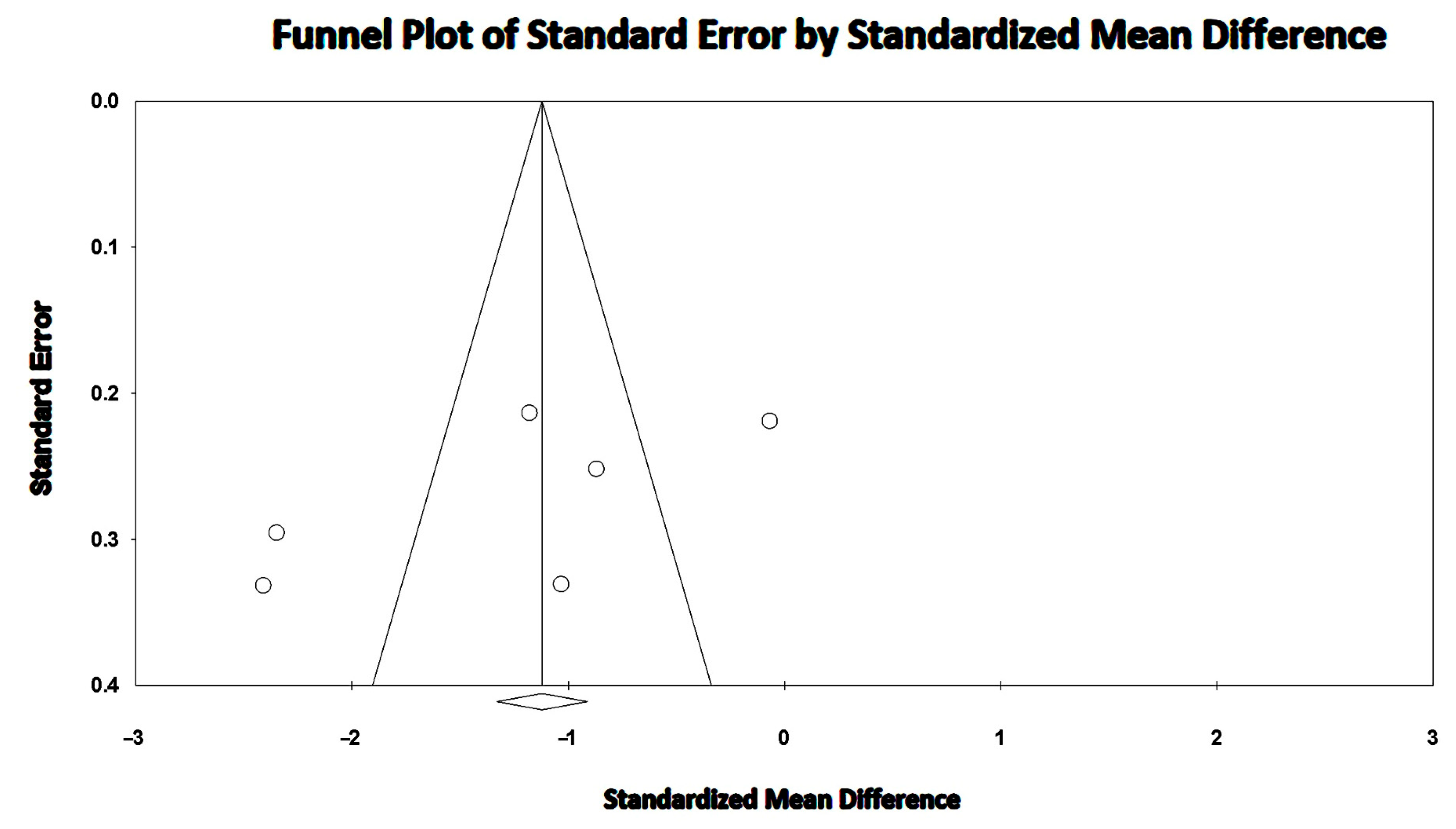
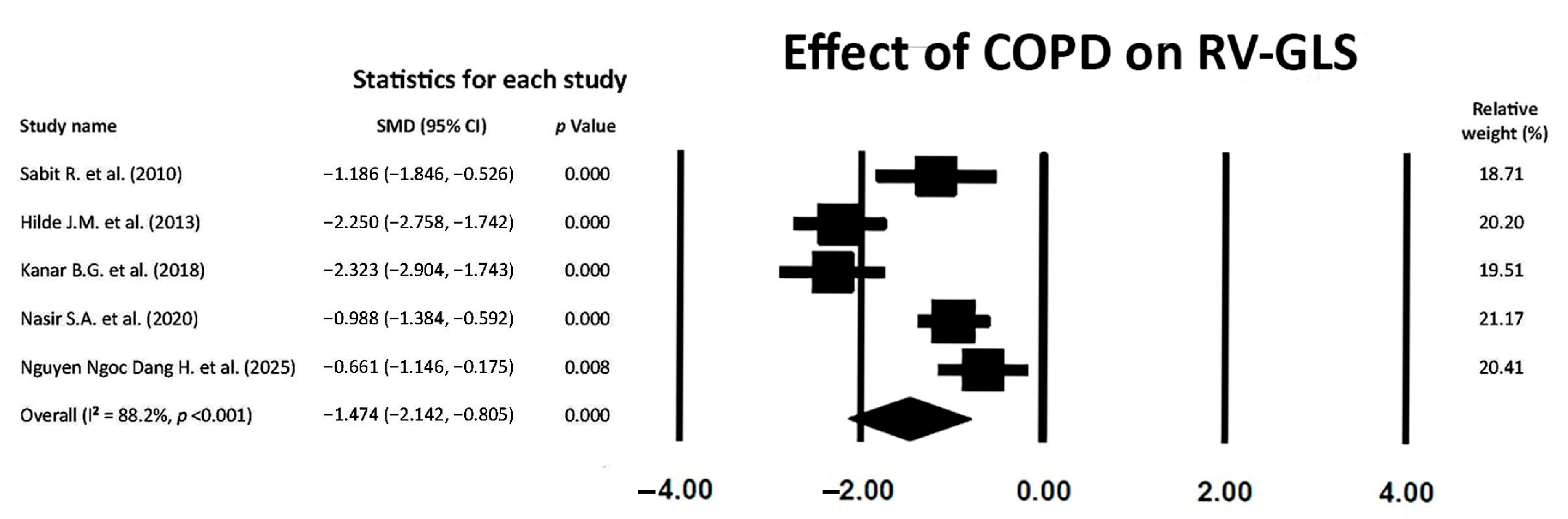
3.6. Effect of COPD on RV-GLS
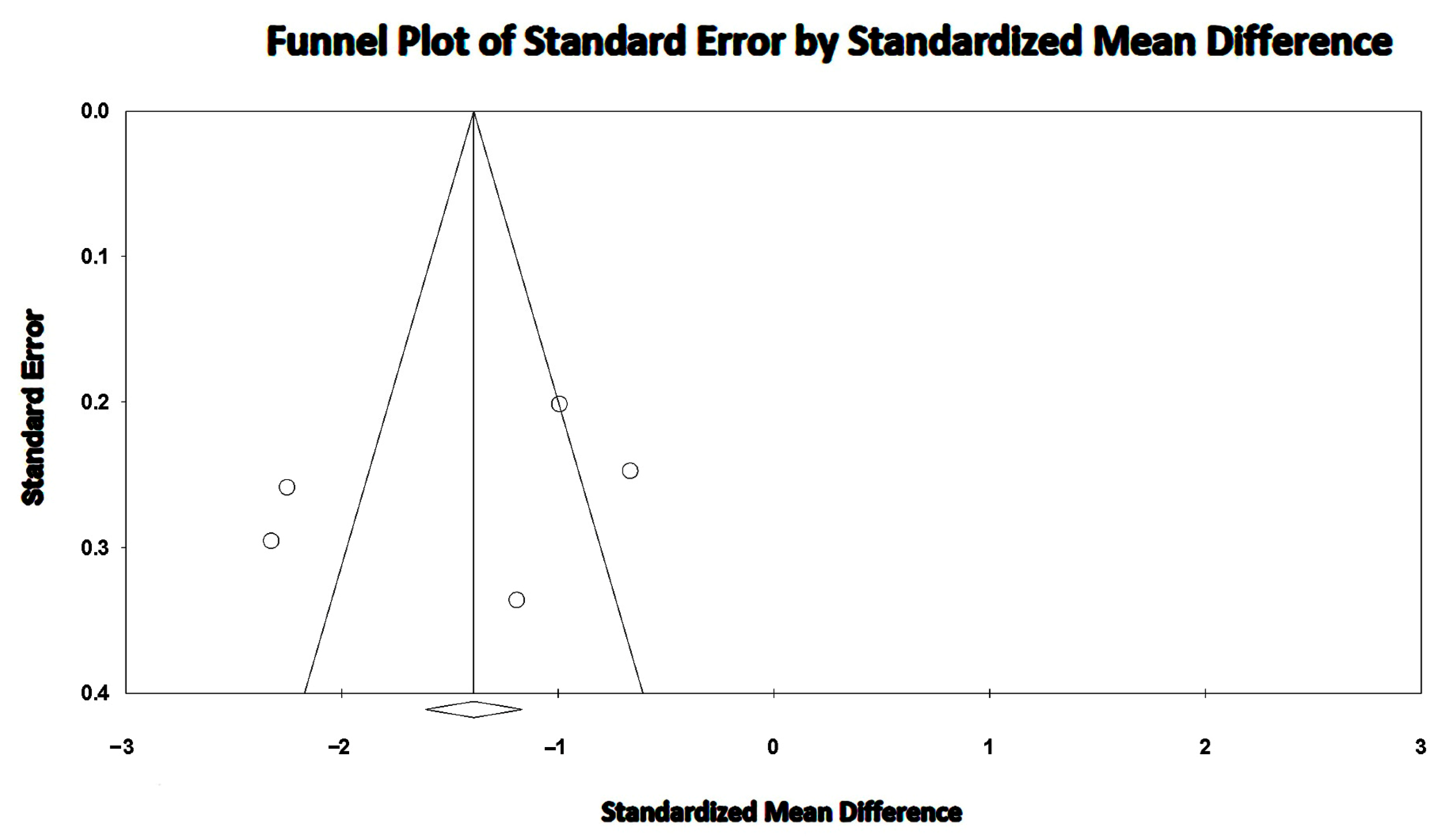
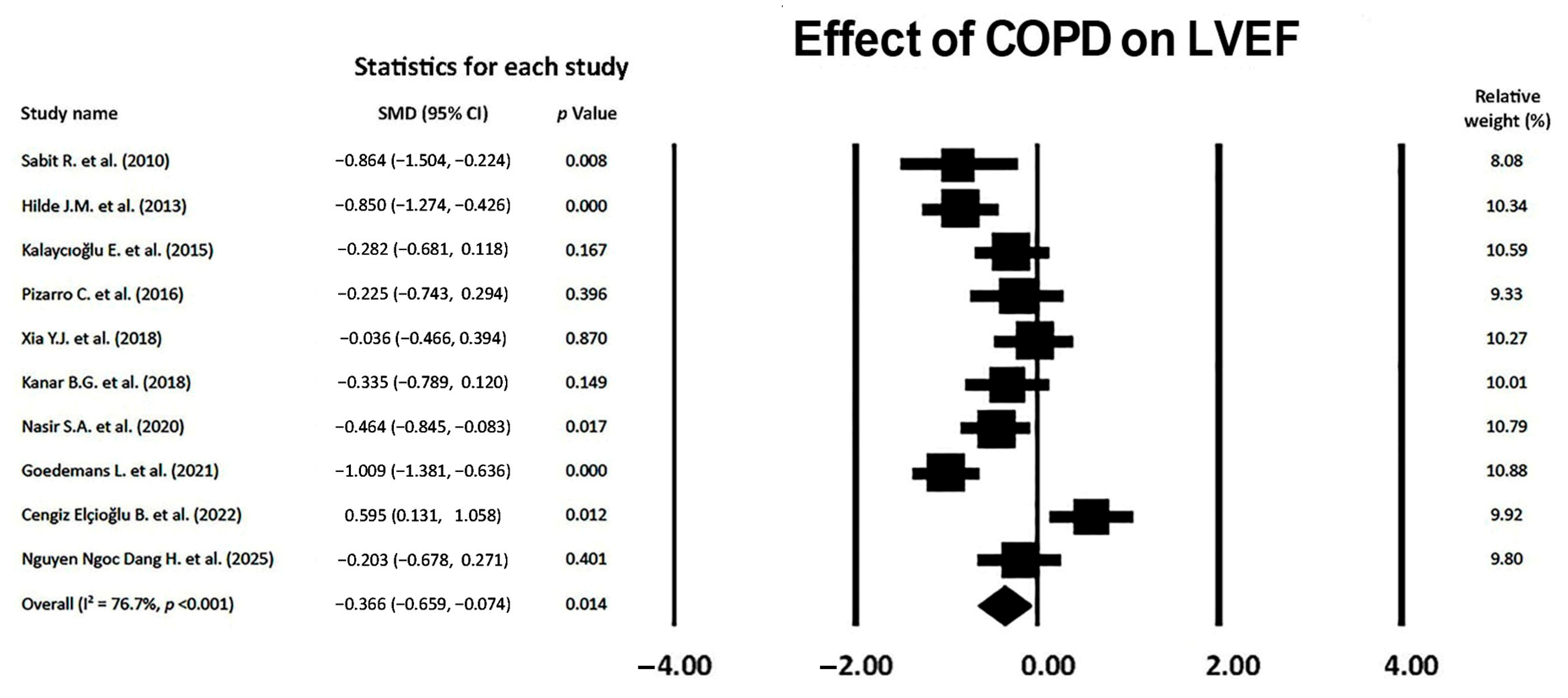
3.7. Effect of COPD on LVEF
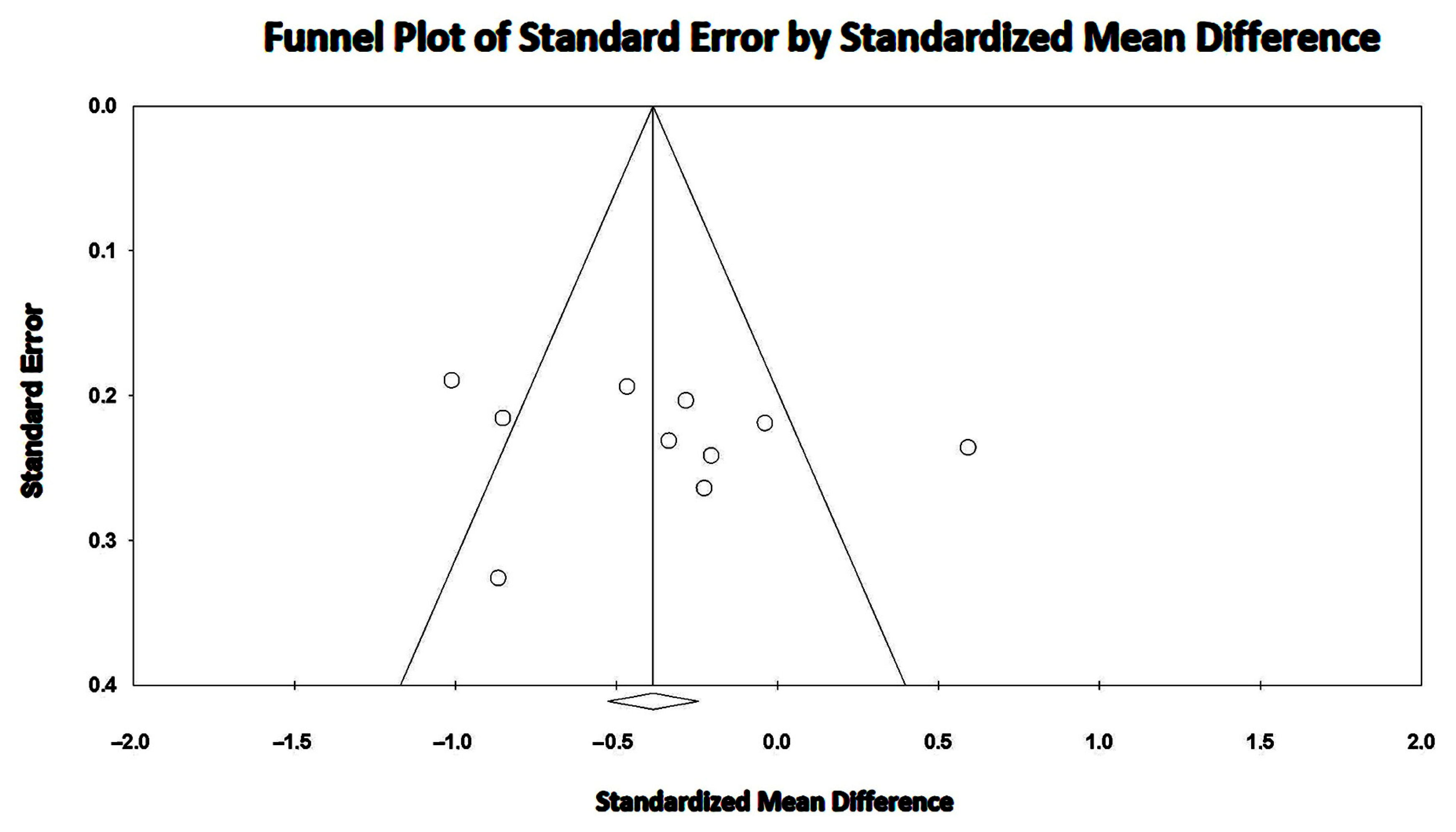
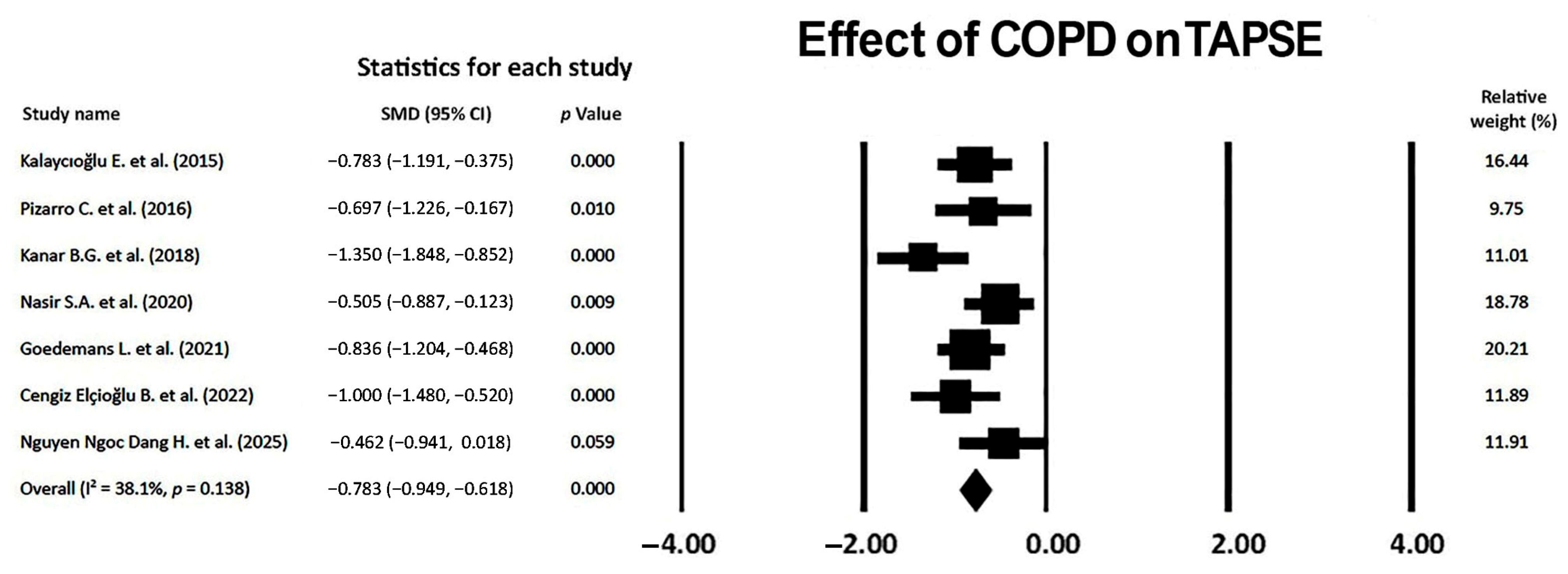
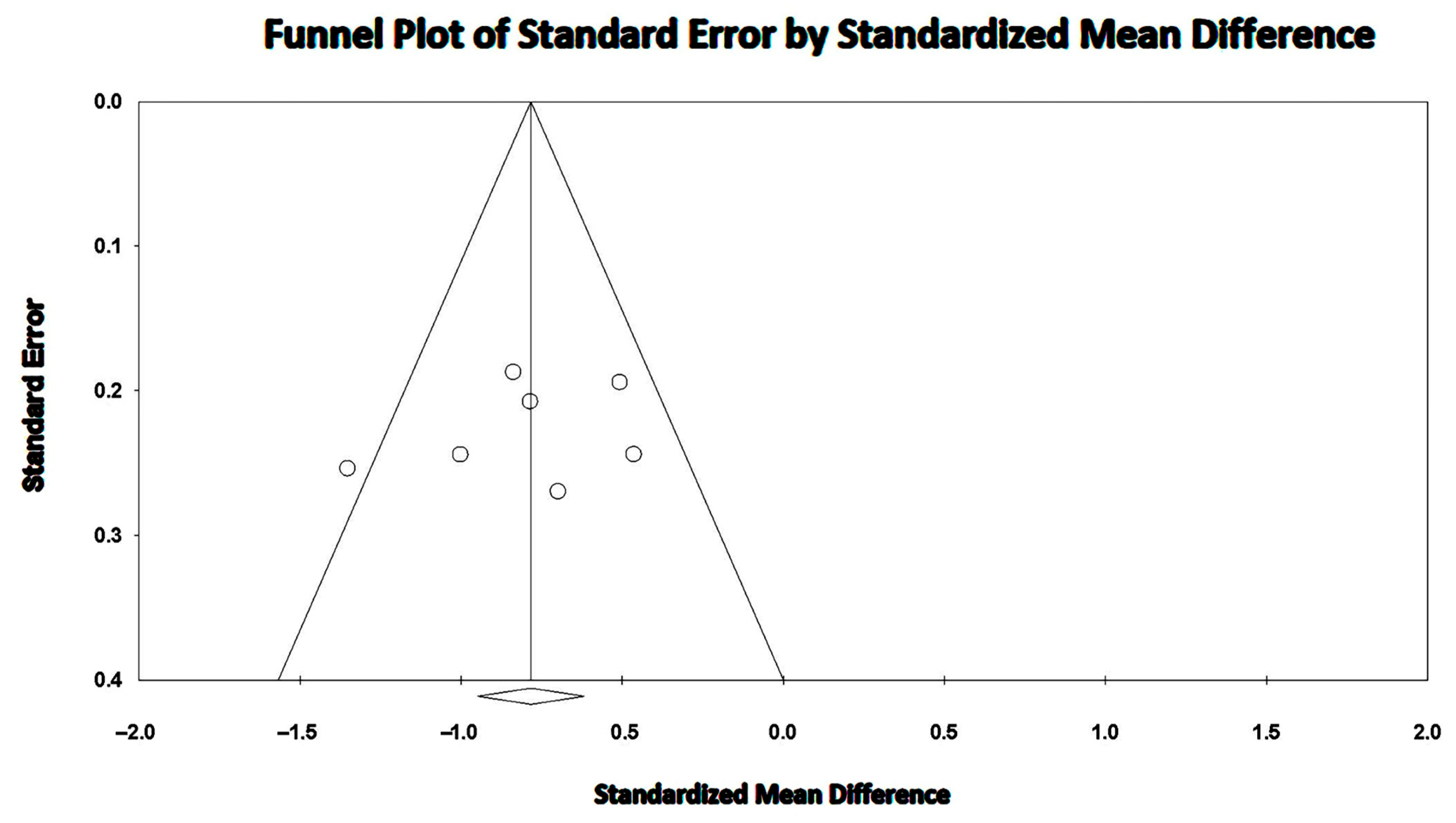
3.8. Effect of COPD on TAPSE
4. Discussion
4.1. Main Findings
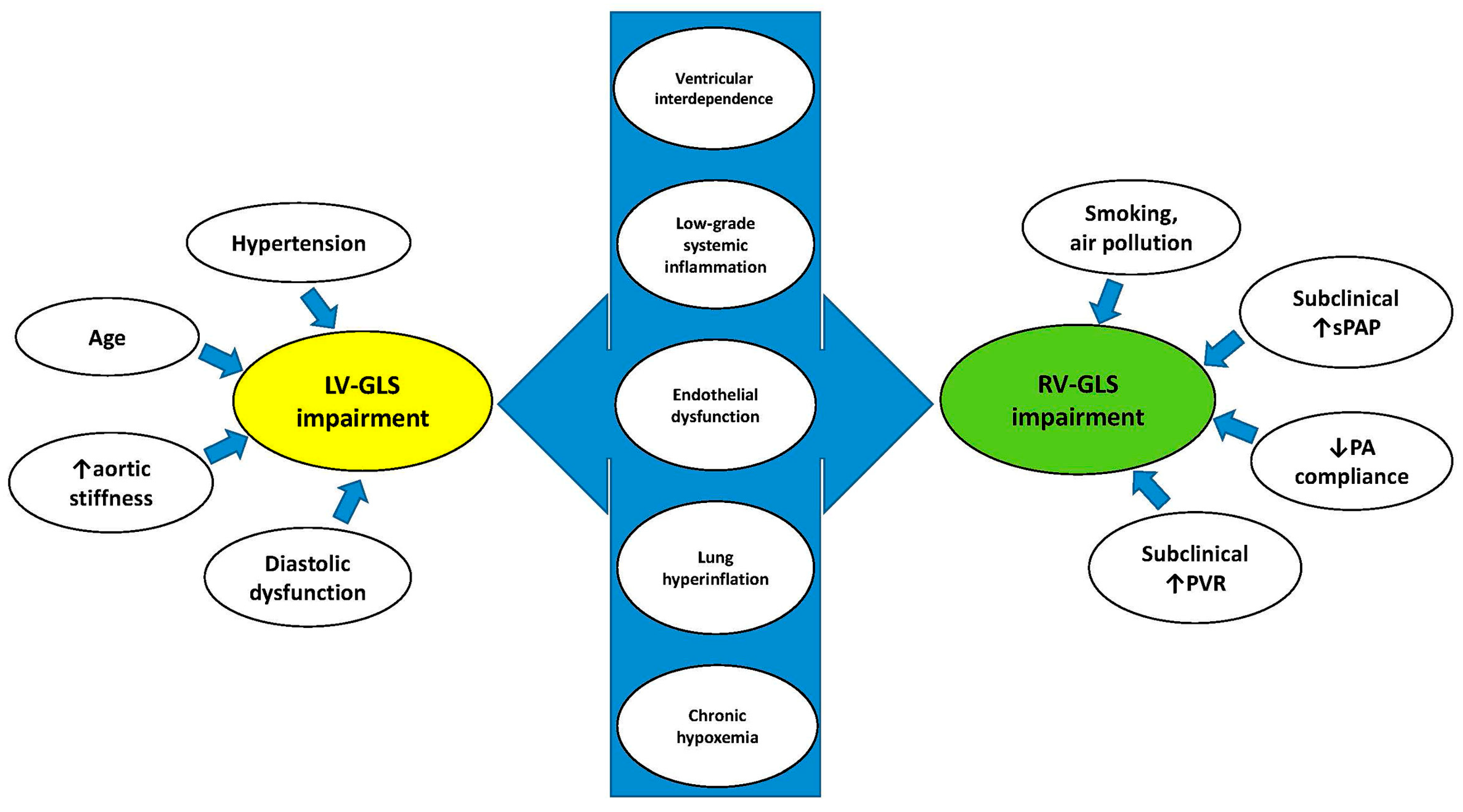
4.2. Pathophysiological Mechanisms Underpinning the Early Deterioration of Biventricular Mechanics in COPD Patients
4.3. Implications for Clinical Practice
4.4. Future Directions
4.5. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Celli, B.R.; Fabbri, L.M.; Aaron, S.D.; Agusti, A.; Brook, R.; Criner, G.J.; Franssen, F.M.E.; Humbert, M.; Hurst, J.R.; O’Donnell, D.; et al. An Updated Definition and Severity Classification of Chronic Obstructive Pulmonary Disease Exacerbations: The Rome Proposal. Am. J. Respir. Crit. Care Med. 2021, 204, 1251–1258. [Google Scholar] [CrossRef] [PubMed]
- Adeloye, D.; Chua, S.; Lee, C.; Basquill, C.; Papana, A.; Theodoratou, E.; Nair, H.; Gasevic, D.; Sridhar, D.; Campbell, H.; et al. Global and regional estimates of COPD prevalence: Systematic review and meta-analysis. J. Glob. Health 2015, 5, 020415. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, Z.; Li, S.; Xu, S. Association of Chronic Obstructive Pulmonary Disease With Arrhythmia Risks: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2021, 8, 732349. [Google Scholar] [CrossRef]
- Zheng, Y.; Hu, Z.; Seery, S.; Li, C.; Yang, J.; Wang, W.; Qi, Y.; Shao, C.; Fu, Y.; Xiao, H.; et al. Global Insights into Chronic Obstructive Pulmonary Disease and Coronary Artery Disease: A Systematic Review and Meta-Analysis of 6,400,000 Patients. Rev. Cardiovasc. Med. 2024, 25, 25. [Google Scholar] [CrossRef] [PubMed]
- Papaporfyriou, A.; Bartziokas, K.; Gompelmann, D.; Idzko, M.; Fouka, E.; Zaneli, S.; Bakakos, P.; Loukides, S.; Papaioannou, A.I. Cardiovascular Diseases in COPD: From Diagnosis and Prevalence to Therapy. Life 2023, 13, 1299. [Google Scholar] [CrossRef]
- Brassington, K.; Selemidis, S.; Bozinovski, S.; Vlahos, R. New frontiers in the treatment of comorbid cardiovascular disease in chronic obstructive pulmonary disease. Clin. Sci. 2019, 133, 885–904. [Google Scholar] [CrossRef]
- Macchia, A.; Rodriguez Moncalvo, J.J.; Kleinert, M.; Comignani, P.D.; Gimeno, G.; Arakaki, D.; Laffaye, N.; Fuselli, J.J.; Massolin, H.P.; Gambarte, J.; et al. Unrecognised ventricular dysfunction in COPD. Eur. Respir. J. 2012, 39, 51–58. [Google Scholar] [CrossRef]
- Potter, E.; Marwick, T.H. Assessment of Left Ventricular Function by Echocardiography: The Case for Routinely Adding Global Longitudinal Strain to Ejection Fraction. JACC Cardiovasc. Imaging. 2018, 11, 260–274. [Google Scholar] [CrossRef]
- Grenne, B.; Eek, C.; Sjøli, B.; Dahlslett, T.; Uchto, M.; Hol, P.K.; Skulstad, H.; Smiseth, O.A.; Edvardsen, T.; Brunvand, H. Acute coronary occlusion in non-ST-elevation acute coronary syndrome: Outcome and early identification by strain echocardiography. Heart 2010, 96, 1550–1556. [Google Scholar] [CrossRef]
- Sonaglioni, A.; Albini, A.; Fossile, E.; Pessi, M.A.; Nicolosi, G.L.; Lombardo, M.; Anzà, C.; Ambrosio, G. Speckle-Tracking Echocardiography for Cardioncological Evaluation in Bevacizumab-Treated Colorectal Cancer Patients. Cardiovasc. Toxicol. 2020, 20, 581–592. [Google Scholar] [CrossRef]
- Purwowiyoto, S.L.; Halomoan, R. Highlighting the role of global longitudinal strain assessment in valvular heart disease. Egypt. Heart J. 2022, 74, 46. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. Open Med. 2009, 3, e123-30. [Google Scholar] [PubMed]
- Global Initiative for Chronic Obstructive Lung Disease [Homepage on the Internet]. Global Strategy for Prevention; Diagnosis and Management of COPD: 2025 Report. Available online: https://goldcopd.org/2025-gold-report/ (accessed on 30 April 2025).
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Respir. J. 2023, 61, 2200879. [Google Scholar] [CrossRef] [PubMed]
- Celli, B.R.; Cote, C.G.; Marin, J.M.; Casanova, C.; Montes de Oca, M.; Mendez, R.A.; Pinto Plata, V.; Cabral, H.J. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N. Engl. J. Med. 2004, 350, 1005–1012. [Google Scholar] [CrossRef]
- Ma, L.L.; Wang, Y.Y.; Yang, Z.H.; Huang, D.; Weng, H.; Zeng, X.T. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: What are they and which is better? Mil. Med. Res. 2020, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochem. Med. 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Sabit, R.; Bolton, C.E.; Fraser, A.G.; Edwards, J.M.; Edwards, P.H.; Ionescu, A.A.; Cockcroft, J.R.; Shale, D.J. Sub-clinical left and right ventricular dysfunction in patients with COPD. Respir. Med. 2010, 104, 1171–1178. [Google Scholar] [CrossRef]
- Hilde, J.M.; Skjørten, I.; Grøtta, O.J.; Hansteen, V.; Melsom, M.N.; Hisdal, J.; Humerfelt, S.; Steine, K. Right ventricular dysfunction and remodeling in chronic obstructive pulmonary disease without pulmonary hypertension. J. Am. Coll. Cardiol. 2013, 62, 1103–1111. [Google Scholar] [CrossRef]
- Kalaycıoğlu, E.; Gökdeniz, T.; Aykan, A.Ç.; Hatem, E.; Gürsoy, M.O.; Toksoy, F.; Dursun, I.; Çelik, S. Evaluation of Left Ventricular Function and its Relationship With Multidimensional Grading System (BODE Index) in Patients with COPD. COPD 2015, 12, 568–574. [Google Scholar] [CrossRef]
- Pizarro, C.; van Essen, F.; Linnhoff, F.; Schueler, R.; Hammerstingl, C.; Nickenig, G.; Skowasch, D.; Weber, M. Speckle tracking echocardiography in chronic obstructive pulmonary disease and overlapping obstructive sleep apnea. Int. J. Chron. Obstruct. Pulmon. Dis. 2016, 11, 1823–1834. [Google Scholar] [CrossRef]
- Xia, Y.J.; Sun, H.Y.; Jiang, L.; Liu, B.; Wang, Y.Y. Evaluation of the effects of right ventricular pressure load on left ventricular myocardial mechanics in patients with chronic obstructive pulmonary disease by ultrasound speckle tracking imaging. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 4949–4955. [Google Scholar] [CrossRef] [PubMed]
- Kanar, B.G.; Ozmen, I.; Yildirim, E.O.; Ozturk, M.; Sunbul, M. Right Ventricular Functional Improvement after Pulmonary Rehabilitation Program in Patients with COPD Determined by Speckle Tracking Echocardiography. Arq. Bras. Cardiol. 2018, 111, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Nasir, S.A.; Singh, S.; Fotedar, M.; Chaudhari, S.K.; Sethi, K.K. Echocardiographic Evaluation of Right Ventricular Function and its Role in the Prognosis of Chronic Obstructive Pulmonary Disease. J. Cardiovasc. Echogr. 2020, 30, 125–130. [Google Scholar] [CrossRef]
- Goedemans, L.; Leung, M.; van der Bijl, P.; Abou, R.; Vo, N.M.; Ajmone Marsan, N.; Delgado, V.; Bax, J.J. Influence of Chronic Obstructive Pulmonary Disease on Atrial Mechanics by Speckle Tracking Echocardiography in Patients With Atrial Fibrillation. Am. J. Cardiol. 2021, 143, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Cengiz Elçioğlu, B.; Kamat, S.; Yurdakul, S.; Şahin, Ş.T.; Sarper, A.; Yıldız, P.; Aytekin, S. Assessment of subclinical left ventricular systolic dysfunction and structural changes in patients with chronic obstructive pulmonary disease. Intern. Med. J. 2022, 52, 1791–1798. [Google Scholar] [CrossRef]
- Nguyen Ngoc Dang, H.; Viet Luong, T.; Thi Y Nguyen, N.; Khanh Tran, H.; Thi Nguyen Tran, H.; Minh Vu, H.; Van Ho, T.; Thi Minh Vo, N.; Thien Tran, T.; Song Do, T.; et al. Assessment of the right ventricular strain; left ventricular strain and left atrial strain using speckle tracking echocardiography in patients with chronic obstructive pulmonary disease. BMJ Open Respir. Res. 2025, 12, e002706. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F., 3rd; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging. 2016, 17, 1321–1360. [Google Scholar] [CrossRef]
- Galderisi, M.; Cosyns, B.; Edvardsen, T.; Cardim, N.; Delgado, V.; Di Salvo, G.; Donal, E.; Sade, L.E.; Ernande, L.; Garbi, M.; et al. Standardization of adult transthoracic echocardiography reporting in agreement with recent chamber quantification; diastolic function; and heart valve disease recommendations: An expert consensus document of the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging. 2017, 18, 1301–1310. [Google Scholar] [CrossRef]
- Muraru, D.; Onciul, S.; Peluso, D.; Soriani, N.; Cucchini, U.; Aruta, P.; Romeo, G.; Cavalli, G.; Iliceto, S.; Badano, L.P. Sex- and Method-Specific Reference Values for Right Ventricular Strain by 2-Dimensional Speckle-Tracking Echocardiography. Circ. Cardiovasc. Imaging. 2016, 9, e003866. [Google Scholar] [CrossRef]
- Hung, C.L.; Gonçalves, A.; Shah, A.M.; Cheng, S.; Kitzman, D.; Solomon, S.D. Age- and Sex-Related Influences on Left Ventricular Mechanics in Elderly Individuals Free of Prevalent Heart Failure: The ARIC Study (Atherosclerosis Risk in Communities). Circ. Cardiovasc. Imaging 2017, 10, e004510. [Google Scholar] [CrossRef] [PubMed]
- Soufi Taleb Bendiab, N.; Meziane-Tani, A.; Ouabdesselam, S.; Methia, N.; Latreche, S.; Henaoui, L.; Monsuez, J.J.; Benkhedda, S. Factors associated with global longitudinal strain decline in hypertensive patients with normal left ventricular ejection fraction. Eur. J. Prev. Cardiol. 2017, 24, 1463–1472. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Tajiri, K.; Suzuki, A.; Nagata, H.; Kojima, M. Influence of cigarette smoking on biventricular systolic function independent of respiratory function: A cross-sectional study. BMC Cardiovasc. Disord. 2020, 20, 451. [Google Scholar] [CrossRef] [PubMed]
- Sin, D.D.; Doiron, D.; Agusti, A.; Anzueto, A.; Barnes, P.J.; Celli, B.R.; Criner, G.J.; Halpin, D.; Han, M.K.; Martinez, F.J.; et al. Air pollution and COPD: GOLD 2023 committee report. Eur. Respir. J. 2023, 61, 2202469. [Google Scholar] [CrossRef]
- Badesch, D.B.; Champion, H.C.; Gomez Sanchez, M.A.; Hoeper, M.M.; Loyd, J.E.; Manes, A.; McGoon, M.; Naeije, R.; Olschewski, H.; Oudiz, R.J.; et al. Diagnosis and assessment of pulmonary arterial hypertension. J. Am. Coll. Cardiol. 2009, 54, S55–S66. [Google Scholar] [CrossRef]
- Hermann, E.A.; Sun, Y.; Hoffman, E.A.; Allen, N.B.; Ambale-Venkatesh, B.; Bluemke, D.A.; Carr, J.J.; Kawut, S.M.; Prince, M.R.; Shah, S.J.; et al. Lung structure and longitudinal change in cardiac structure and function: The MESA COPD Study. Eur. Respir. J. 2024, 64, 2400820. [Google Scholar] [CrossRef]
- Hilde, J.M.; Skjørten, I.; Hansteen, V.; Melsom, M.N.; Hisdal, J.; Humerfelt, S.; Steine, K. Haemodynamic responses to exercise in patients with COPD. Eur. Respir. J. 2013, 41, 1031–1041. [Google Scholar] [CrossRef]
- Tello, K.; Ghofrani, H.A.; Heinze, C.; Krueger, K.; Naeije, R.; Raubach, C.; Seeger, W.; Sommer, N.; Gall, H.; Richter, M.J. A simple echocardiographic estimate of right ventricular-arterial coupling to assess severity and outcome in pulmonary hypertension on chronic lung disease. Eur. Respir. J. 2019, 54, 1802435. [Google Scholar] [CrossRef]
- He, Q.; Lin, Y.; Zhu, Y.; Gao, L.; Ji, M.; Zhang, L.; Xie, M.; Li, Y. Clinical Usefulness of Right Ventricle-Pulmonary Artery Coupling in Cardiovascular Disease. J. Clin. Med. 2023, 12, 2526. [Google Scholar] [CrossRef]
- Sin, D.D.; Man, S.F. Chronic obstructive pulmonary disease: A novel risk factor for cardiovascular disease. Can. J. Physiol. Pharmacol. 2005, 83, 8–13. [Google Scholar] [CrossRef]
- Cavaillès, A.; Brinchault-Rabin, G.; Dixmier, A.; Goupil, F.; Gut-Gobert, C.; Marchand-Adam, S.; Meurice, J.C.; Morel, H.; Person-Tacnet, C.; Leroyer, C.; et al. Comorbidities of COPD. Eur. Respir. Rev. 2013, 22, 454–475. [Google Scholar] [CrossRef] [PubMed]
- Ozben, B.; Eryüksel, E.; Tanrikulu, A.M.; Papila-Topal, N.; Celikel, T.; Başaran, Y. Acute exacerbation impairs endothelial function in patients with chronic obstructive pulmonary disease. Turk. Kardiyol. Dern. Ars. 2010, 38, 1–7. [Google Scholar] [PubMed]
- Maclay, J.D.; MacNee, W. Cardiovascular disease in COPD: Mechanisms. Chest 2013, 143, 798–807. [Google Scholar] [CrossRef]
- Ukena, C.; Mahfoud, F.; Kindermann, M.; Kindermann, I.; Bals, R.; Voors, A.A.; van Veldhuisen, D.J.; Böhm, M. The cardiopulmonary continuum systemic inflammation as ’common soil’ of heart and lung disease. Int. J. Cardiol. 2010, 145, 172–176. [Google Scholar] [CrossRef]
- Seta, Y.; Shan, K.; Bozkurt, B.; Oral, H.; Mann, D.L. Basic mechanisms in heart failure: The cytokine hypothesis. J. Card. Fail. 1996, 2, 243–249. [Google Scholar] [CrossRef]
- Sonaglioni, A.; Nicolosi, G.L.; Trevisan, R.; Granato, A.; Zompatori, M.; Lombardo, M. Modified Haller index validation and correlation with left ventricular strain in a cohort of subjects with obesity and without overt heart disease. Intern. Emerg. Med. 2022, 17, 1907–1919. [Google Scholar] [CrossRef] [PubMed]
- Sonaglioni, A.; Nicolosi, G.L.; Granato, A.; Lombardo, M.; Anzà, C.; Ambrosio, G. Reduced Myocardial Strain Parameters in Subjects With Pectus Excavatum: Impaired Myocardial Function or Methodological Limitations Due to Chest Deformity? Semin. Thorac. Cardiovasc. Surg. 2021, 33, 251–262. [Google Scholar] [CrossRef]
- Niki, K.; Sugawara, M.; Kayanuma, H.; Takamisawa, I.; Watanabe, H.; Mahara, K.; Sumiyoshi, T.; Ida, T.; Takanashi, S.; Tomoike, H. Associations of increased arterial stiffness with left ventricular ejection performance and right ventricular systolic pressure in mitral regurgitation before and after surgery: Wave intensity analysis. Int. J. Cardiol. Heart Vasc. 2017, 16, 7–13. [Google Scholar] [CrossRef]
- Chaouat, A.; Naeije, R.; Weitzenblum, E. Pulmonary hypertension in COPD. Eur. Respir. J. 2008, 32, 1371–1385. [Google Scholar] [CrossRef]
- van der Woude, H.J.; Zaagsma, J.; Postma, D.S.; Winter, T.H.; van Hulst, M.; Aalbers, R. Detrimental effects of beta-blockers in COPD: A concern for nonselective beta-blockers. Chest 2005, 127, 818–824. [Google Scholar] [CrossRef]
- Hjalmarson, A.; Goldstein, S.; Fagerberg, B.; Wedel, H.; Waagstein, F.; Kjekshus, J.; Wikstrand, J.; El Allaf, D.; Vítovec, J.; Aldershvile, J.; et al. Effects of controlled-release metoprolol on total mortality; hospitalizations; and well-being in patients with heart failure: The Metoprolol CR/XL Randomized Intervention Trial in congestive heart failure (MERIT-HF). MERIT-HF Study Group. JAMA 2000, 283, 1295–1302. [Google Scholar] [CrossRef]
- Gottlieb, S.S.; McCarter, R.J.; Vogel, R.A. Effect of beta-blockade on mortality among high-risk and low-risk patients after myocardial infarction. N. Engl. J. Med. 1998, 339, 489–497. [Google Scholar] [CrossRef]
- Andell, P.; Erlinge, D.; Smith, J.G.; Sundström, J.; Lindahl, B.; James, S.; Koul, S. β-blocker use and mortality in COPD patients after myocardial infarction: A Swedish nationwide observational study. J. Am. Heart Assoc. 2015, 4, e001611. [Google Scholar] [CrossRef]
- Wade, C.; Wells, J.M. Practical recommendations for the use of beta-blockers in chronic obstructive pulmonary disease. Expert Rev. Respir. Med. 2020, 14, 671–678. [Google Scholar] [CrossRef]
- Chen, X.; Hu, F.; Chai, F.; Chen, X. Effect of statins on pulmonary function in patients with chronic obstructive pulmonary disease: A systematic review and meta-analysis of randomized controlled trials. J. Thorac. Dis. 2023, 15, 3944–3952. [Google Scholar] [CrossRef]
- Sade, L.E.; Joshi, S.S.; Cameli, M.; Cosyns, B.; Delgado, V.; Donal, E.; Edvardsen, T.; Carvalho, R.F.; Manka, R.; Podlesnikar, T.; et al. Current clinical use of speckle-tracking strain imaging: Insights from a worldwide survey from the European Association of Cardiovascular Imaging (EACVI). Eur. Heart J. Cardiovasc. Imaging. 2023, 24, 1583–1592. [Google Scholar] [CrossRef]
- Rao, A.; Huynh, E.; Royston, T.J.; Kornblith, A.; Roy, S. Acoustic Methods for Pulmonary Diagnosis. IEEE Rev. Biomed. Eng. 2019, 12, 221–239. [Google Scholar] [CrossRef]
- Farsalinos, K.E.; Daraban, A.M.; Ünlü, S.; Thomas, J.D.; Badano, L.P.; Voigt, J.U. Head-to-Head Comparison of Global Longitudinal Strain Measurements among Nine Different Vendors: The EACVI/ASE Inter-Vendor Comparison Study. J. Am. Soc. Echocardiogr. 2015, 28, 1171–1181.e2. [Google Scholar] [CrossRef]
- Negishi, T.; Negishi, K.; Thavendiranathan, P.; Cho, G.Y.; Popescu, B.A.; Vinereanu, D.; Kurosawa, K.; Penicka, M.; Marwick, T.H.; SUCCOUR Investigators. Effect of Experience and Training on the Concordance and Precision of Strain Measurements. JACC Cardiovasc. Imaging 2017, 10, 518–522. [Google Scholar] [CrossRef]
- Rösner, A.; Barbosa, D.; Aarsæther, E.; Kjønås, D.; Schirmer, H.; D’hooge, J. The influence of frame rate on two-dimensional speckle-tracking strain measurements: A study on silico-simulated models and images recorded in patients. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 1137–1147. [Google Scholar] [CrossRef]
- Sonaglioni, A.; Fagiani, V.; Nicolosi, G.L.; Lombardo, M. The influence of pectus excavatum on biventricular mechanics: A systematic review and meta-analysis. Minerva Cardiol. Angiol. 2024; Online ahead of print. [Google Scholar] [CrossRef]
| Study Name and Country | Number of Patients | Mean Age (yrs) | Males (%) | Study Design | Ultrasound System | Main Echocardiographic Findings in COPD Patients vs. Healthy Controls |
|---|---|---|---|---|---|---|
| Sabit, R., et al. (2010) [18], United Kingdom | COPD = 36 Controls = 14 | COPD = 66.5 Controls = 67 | COPD = 52.8 Controls = 64.3 | Prospective | GE | ↑IVRT, ↔E/A ratio, ↑E/e’ ratio ↔LVEF, ↑aortic PWV ↓LV-GLS, ↓GLSRs ↔sPAP, ↑Tei index, ↓RV-FWLS |
| Hilde, J.M., et al. (2013) [19], Norway | COPD = 72 Controls = 34 | COPD = 63 Controls = 64 | COPD = 52.8 Controls = 44.1 | Prospective | GE | ↔E/A ratio, ↔E/e’ ratio, ↓LVEF ↑RV wall thickness, ↑RV size ↔sPAP, ↑RV-MPI, ↓RV basal s’ ↓TAPSE, ↓RVEF, ↓RV-GLS |
| Kalaycıoğlu, E., et al. (2015) [20], Turkey | COPD = 125 Controls = 30 | COPD = 70 Controls = 67 | COPD = 92 Controls = 90 | Prospective | NS | ↔LV size ↑E/e’ ratio, ↔LVEF ↓LV-GLS, ↓GLSRs ↑sPAP, ↓RV basal s’, ↓TAPSE |
| Pizarro, C., et al. (2016) [21], Germany | COPD = 51 Controls = 20 | COPD = 64.1 Controls = 61.3 | COPD = 54.1 Controls = 55 | Prospective | GE | ↔E/A ratio ↔LVEF ↔sPAP, ↔TAPSE ↓LV-GLS, ↓LV apical septal strain |
| Xia, Y.J., et al. (2018) [22], China | COPD = 41 Controls = 42 | COPD = 49.1 Controls = 48.9 | COPD = 53.7 Controls = 61.9 | Prospective | Philips | ↔LV size ↔E/A ratio, ↔E/e’ ratio, ↔LVEF ↓LV-GLS, ↓LV-GCS, ↓LV-GRS ↓LV peak rotation angle |
| Kanar, B.G., et al. (2018) [23], Turkey | COPD = 46 Controls = 32 | COPD = 60.8 Controls = 58.5 | COPD = 61 Controls = 41 | Prospective | Philips | ↑RV size, ↔RV thickness ↔LVEF, ↔RVEF, ↔RV basal s’ ↑sPAP, ↓TAPSE ↓RV-GLS, ↓RV-FWLS |
| Nasir, S.A., et al. (2020) [24], India | COPD = 84 Controls = 40 | COPD = 63.9 Controls = 61.1 | NS | Prospective | GE | ↓LVEF ↑sPAP, ↓TAPSE, ↓RV basal s’, ↓RV-FAC ↓RV-GLS |
| Goedemans, L., et al. (2021) [25], The Netherlands | COPD = 143 Controls = 38 | COPD = 69 Controls = 66 | COPD = 27 Controls = 53 | Retrospective | GE | ↔LV size, ↑LAVi ↑E/e’ ratio, ↓LVEF ↑RA size, ↑sPAP, ↓TAPSE ↓LASr, ↓RASr |
| Cengiz Elçioğlu, B., et al. (2022) [26], Turkey | COPD = 52 Controls = 29 | COPD = 60.2 Controls = 57.7 | COPD = 100 Controls = 100 | Prospective | Philips | ↔LV size, ↔LA size, ↔RV size ↔E/A ratio, ↔E/e’ ratio, ↔LVEF ↑sPAP, ↓TAPSE, ↓RV basal s’ ↓LV-GLS, ↓GLSRs |
| Nguyen Ngoc Dang, H., et al. (2025) [27], Vietnam | COPD = 32 Controls = 37 | COPD = 68 Controls = 65 | COPD = 91 Controls = 81 | Prospective | Philips | ↔LVMi, ↔LVEF ↔sPAP, ↔TAPSE ↔LASr, ↓LV-GLS ↓RV-GLS, ↓RV-FWLS |
| Number of Studies for Parameters Assessed (%) | Sample Size COPD vs. Controls | COPD | Controls | p-Value | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age (yrs) | 10 (100) | 682 vs. 316 | 63.5 (49.1−70) | 61.6 (48.9−67) | <0.05 |
| Males (%) | 9 (90) | 598 vs. 276 | 64.9 (27−100) | 65.6 (41−100) | NS |
| Anthropometrics | |||||
| BSA (m2) | 4 (40) | 331 vs. 149 | 1.75 (1.52−2) | 1.77 (1.56−1.9) | NS |
| BMI (Kg/m2) | 7 (70) | 455 vs. 212 | 25.1 (20.3−28.2) | 25.6 (21.3−27.9) | <0.05 |
| Cardiovascular risk factors | |||||
| Hypertension (%) | 3 (30) | 319 vs. 88 | 58.2 (41−76) | 30 (0−50) | <0.05 |
| Current smoking (%) | 4 (40) | 391 vs. 122 | 32 (20−43) | 11.2 (0−23) | <0.05 |
| Pack—years of smoking | 5 (50) | 316 vs. 135 | 41.9 (30−52) | 10.9 (0−30) | <0.05 |
| Type 2 diabetes (%) | 3 (30) | 319 vs. 88 | 16.4 (14−21) | 5.3 (0−10) | <0.05 |
| Dyslipidemia (%) | 3 (30) | 266 vs. 92 | 44 (38.2−55) | 17.5 (0−35) | <0.05 |
| Cardiovascular comorbidities | |||||
| CAD (%) | 2 (20) | 194 vs. 58 | 39.1 (28.2−50) | 5 (0−10) | <0.05 |
| Noncardiovascular comorbidities | |||||
| OSAS (%) | 1 (10) | 143 vs. 38 | 4 | 0 | NS |
| Hemodynamics | |||||
| Sinus rhythm (%) | 10 (100) | 682 vs. 316 | 89.9 (0−100) | 100 | <0.05 |
| AF (%) | 10 (100) | 682 vs. 316 | 10.1 (0−100) | 0 | <0.05 |
| Heart rate (bpm) | 6 (60) | 371 vs. 172 | 80.3 (71.4−96.5) | 72.2 (60.3−80.4) | <0.05 |
| SBP (mmHg) | 6 (60) | 406 vs. 212 | 126 (114−139) | 121.6 (111−133.9) | <0.05 |
| DBP (mmHg) | 6 (60) | 406 vs. 212 | 74.5 (69−83.2) | 75 (68.1−80.9) | NS |
| COPD severity | |||||
| GOLD stage | 6 (60) | 428 | 1.9 (1.5−2.5) | / | / |
| BODE index | 3 (30) | 255 | 2.5 (2−3) | / | / |
| Pulmonary function tests | |||||
| FEV1 (% predicted) | 6 (60) | 371 vs. 172 | 51.9 (45−60.1) | 97.3 (89−103.6) | <0.05 |
| FVC (% predicted) | 3 (30) | 233 vs. 78 | 77.4 (74.6−81.7) | 98.3 (85.4−105) | <0.05 |
| FEV1/FVC (%) | 7 (70) | 455 vs. 212 | 56 (49−60.5) | 82.6 (76−96.8) | <0.05 |
| RV (% predicted) | 2 (20) | 123 vs. 54 | 181.5 (171−192) | 115.2 (111.4−119) | <0.05 |
| DLCO (% predicted) | 2 (20) | 123 vs. 54 | 52.7 (48.5−57) | 89.1 (78.3−100) | <0.05 |
| 6MWD (m) | 2 (20) | 156 vs. 74 | 379 (343−415) | 494.5 (489−500) | <0.05 |
| Blood gas analysis | |||||
| SaO2 (%) | 4 (40) | 289 vs. 126 | 93.6 (92−96) | 96.1 (96−96.3) | <0.05 |
| PaO2 (mmHg) | 4 (40) | 289 vs. 126 | 69.4 (66.4−73.5) | 77.7 (63.7−92) | <0.05 |
| PaCO2 (mmHg) | 4 (40) | 289 vs. 126 | 37.9 (35.7−40) | 35 (34.1−36) | <0.05 |
| Biochemical parameters | |||||
| Hemoglobin (g/dL) | 4 (40) | 360 vs. 130 | 14.2 (13.2−15.8) | 14.2 (12.6−16.2) | NS |
| Creatinine (g/dL) | 2 (20) | 184 vs. 80 | 0.97 (0.75−1.18) | 0.80 (0.72−0.88) | <0.05 |
| Fasting glucose (mg/dL) | 2 (20) | 161 vs. 44 | 99.6 (95.6−103.6) | 97.1 (94.1−100.1) | <0.05 |
| LDL cholesterol (mg/dL) | 2 (20) | 193 vs. 81 | 129.8 (119.1−140.5) | 124.9 (124−125.9) | <0.05 |
| NT-proBNP (pg/mL) | 2 (20) | 123 vs. 54 | 268.4 (83.7−453.2) | 87.7 (78.6−96.8) | <0.05 |
| Nonrespiratory medical treatment | |||||
| ACE-i/ARBs (%) | 2 (20) | 268 vs. 68 | 45 (25−65) | 12 (0−24) | <0.05 |
| CCB (%) | 2 (20) | 268 vs. 68 | 14.5 (14−15) | 8.5 (0−17) | <0.05 |
| BB (%) | 1 (10) | 125 vs. 30 | 4 | 10 | NS |
| Statins (%) | 1 (10) | 125 vs. 30 | 17 | 13 | NS |
| Oral hypoglycemic agents (%) | 1 (10) | 125 vs. 30 | 11 | 3 | NS |
| Insulin (%) | 1 (10) | 125 vs. 30 | 4 | 3 | NS |
| Respiratory medical treatment | |||||
| Home oxygen therapy (%) | 1 (10) | 51 | 20 | / | / |
| Short-acting beta2-agonist (%) | 1 (10) | 143 | 15 | / | / |
| Long-acting beta2-agonist (%) | 2 (20) | 194 | 63.8 (57−70.6) | / | / |
| Long-acting anticholinergic (%) | 1 (10) | 51 | 60 | / | / |
| Inhaled glucocorticoid (%) | 2 (20) | 194 | 49 (34.1−64) | / | / |
| Systemic glucocorticoid (%) | 1 (10) | 51 | 4.7 | / | / |
| PDE-4 inhibitor (%) | 1 (10) | 51 | 17.6 | / | / |
| Echocardiographic Parameters | Number of Studies for Parameters Assessed (%) | Sample Size COPD vs. Controls | COPD | Controls | p-Value |
|---|---|---|---|---|---|
| TTE parameters | |||||
| IVS thickness (mm) | 2 (20) | 177 vs. 59 | 10.5 (10−11.1) | 10.4 (9.9−11) | NS |
| LV-PW thickness (mm) | 2 (20) | 177 vs. 59 | 10.2 (9.8−10.6) | 10 (9.8−10.2) | <0.05 |
| LV-EDD (mm) | 3 (30) | 218 vs. 101 | 46.9 (45.8−48) | 47.2 (46.3−48) | <0.04 |
| LV-ESD (mm) | 3 (30) | 218 vs. 101 | 29.6 (28.1−32) | 29.4 (27.7−32) | NS |
| RWT | 2 (20) | 177 vs. 59 | 0.44 (0.41−0.46) | 0.42 (0.41−0.44) | <0.05 |
| LVMi (g/m2) | 2 (20) | 68 vs. 51 | 95.5 (86.5−104.5) | 93.9 (84.1−103.8) | NS |
| LVEF (%) | 10 (100) | 682 vs. 316 | 60 (50−67.8) | 62.7 (59.4−69.1) | <0.05 |
| SV (mL) | 2 (20) | 113 vs. 76 | 58.7 (47.2−70.2) | 58.1 (47.8−68.4) | NS |
| CO (L/min) | 2 (20) | 113 vs. 76 | 4.4 (3.6−5.2) | 4.6 (3.6−5.6) | NS |
| E/A ratio | 4 (40) | 201 vs. 119 | 1.09 (0.8−1.43) | 1.14 (0.9−1.51) | NS |
| E/e’ ratio | 6 (60) | 469 vs. 187 | 9.7 (5.8−17) | 7.7 (6.1−11) | <0.05 |
| LAVi (ml/m2) | 2 (20) | 215 vs. 72 | 35.5 (24−47) | 22 (21−23) | <0.05 |
| RV-EDD (mm) | 4 (40) | 206 vs. 109 | 30.5 (24−38.1) | 26 (24−27.6) | <0.05 |
| TDI RV basal s’ (cm/s) | 6 (60) | 415 vs. 179 | 11.6 (10−12.9) | 13.3 (12−15.2) | <0.05 |
| RVEF (%) | 2 (20) | 118 vs. 66 | 52.4 (50−54.8) | 57.1 (56.3−58) | <0.05 |
| TAPSE (mm) | 7 (70) | 533 vs. 226 | 19.3 (16.6−23) | 22 (19.7−26) | <0.05 |
| sPAP (mmHg) | 9 (90) | 641 vs. 274 | 32.3 (22.9−46.7) | 23.2 (18−30) | <0.05 |
| STE parameters | |||||
| LV-GLS (%) | 6 (60) | 337 vs. 172 | 17.1 (13.9−18.9) | 19.9 (17.1−22.3) | <0.05 |
| LV-GLSRs (s−1) | 3 (30) | 213 vs. 73 | 1.28 (0.9−1.6) | 1.34 (1−1.7) | NS |
| LV-GCS (%) | 1 (10) | 41 vs. 42 | 18.9 (16.3−21.5) | 19.2 (16.6−21.8) | NS |
| LV-GRS (%) | 1 (10) | 41 vs. 42 | 25.9 (20.4−31.4) | 26.5 (21−32) | NS |
| RV-GLS (%) | 5 (50) | 270 vs. 157 | 19.5 (14.6−22) | 25.4 (18.3−31) | <0.05 |
| RV-FWLS (%) | 2 (20) | 78 vs. 69 | 17.3 (16.5−18.1) | 24.6 (21.4−27.9) | <0.05 |
| LASr (%) | 2 (20) | 175 vs. 75 | 21.8 (14.2−29.5) | 30.6 (30.2−31.1) | <0.05 |
| RASr (%) | 1 (10) | 143 vs. 38 | 15.3 (9−25.1) | 42.8 (33.7−48.3) | <0.05 |
| NIH Quality Assessment Tool of Case-Control Studies Criteria Met | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study Name | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 | Q12 | Quality |
| Sabit R. et al. [18] | Yes | Yes | Yes | NS | Yes | Yes | NS | Yes | Yes | Yes | Yes | No | 9 (Good) |
| Hilde J.M. et al. [19] | Yes | Yes | No | Yes | Yes | Yes | NS | Yes | Yes | Yes | Yes | Yes | 10 (Good) |
| Kalaycıoğlu E. et al. [20] | Yes | Yes | No | Yes | Yes | Yes | NS | Yes | Yes | Yes | NS | No | 8 (Fair) |
| Pizarro C. et al. [21] | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | 10 (Good) |
| Xia Y.J. et al. [22] | Yes | Yes | No | Yes | Yes | Yes | NS | Yes | Yes | Yes | NS | No | 8 (Fair) |
| Kanar B.G. et al. [23] | Yes | Yes | Yes | NS | Yes | Yes | NS | Yes | Yes | Yes | NS | No | 8 (Fair) |
| Nasir S.A. et al. [24] | Yes | Yes | No | Yes | Yes | Yes | NS | Yes | Yes | Yes | NS | No | 8 (Fair) |
| Goedemans L. et al. [25] | Yes | Yes | No | Yes | Yes | Yes | NS | Yes | Yes | Yes | NS | Yes | 9 (Good) |
| Cengiz Elçioğlu B. et al. [26] | Yes | Yes | No | NS | Yes | Yes | NS | Yes | Yes | Yes | NS | No | 7 (Fair) |
| Nguyen Ngoc Dang H. et al. [27] | Yes | Yes | No | Yes | Yes | Yes | NS | Yes | Yes | Yes | NS | No | 8 (Fair) |
| Coefficient | Standard Error | 95%CI Lower | 95%CI Upper | p-Value | |
|---|---|---|---|---|---|
| Intercept | −3.7559 | 8.9379 | −21.274 | 13.7621 | 0.67 |
| Age | −0.1431 | 0.0978 | −0.3348 | 0.0485 | 0.14 |
| BMI | −0.0659 | 0.1855 | −0.4296 | 0.2978 | 0.72 |
| SBP | 0.1057 | 0.0866 | −0.0639 | 0.2754 | 0.22 |
| Coefficient | Standard Error | 95%CI Lower | 95%CI Upper | p-Value | |
|---|---|---|---|---|---|
| Intercept | −0.3641 | 3.9875 | −8.1795 | 7.4512 | 0.93 |
| NonGE ultrasound machine | 0.0817 | 1.0647 | −2.0051 | 2.1685 | 0.94 |
| sPAP | −0.0402 | 0.1424 | −0.3194 | 0.239 | 0.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sonaglioni, A.; Baravelli, M.; Caminati, A.; Tagariello, F.; De Cesco, F.; Nicolosi, G.L.; Lombardo, M.; Harari, S. Effect of Chronic Obstructive Pulmonary Disease (COPD) on Biventricular Mechanics in Patients Without Severe Airflow Obstruction. J. Clin. Med. 2025, 14, 3660. https://doi.org/10.3390/jcm14113660
Sonaglioni A, Baravelli M, Caminati A, Tagariello F, De Cesco F, Nicolosi GL, Lombardo M, Harari S. Effect of Chronic Obstructive Pulmonary Disease (COPD) on Biventricular Mechanics in Patients Without Severe Airflow Obstruction. Journal of Clinical Medicine. 2025; 14(11):3660. https://doi.org/10.3390/jcm14113660
Chicago/Turabian StyleSonaglioni, Andrea, Massimo Baravelli, Antonella Caminati, Federico Tagariello, Federico De Cesco, Gian Luigi Nicolosi, Michele Lombardo, and Sergio Harari. 2025. "Effect of Chronic Obstructive Pulmonary Disease (COPD) on Biventricular Mechanics in Patients Without Severe Airflow Obstruction" Journal of Clinical Medicine 14, no. 11: 3660. https://doi.org/10.3390/jcm14113660
APA StyleSonaglioni, A., Baravelli, M., Caminati, A., Tagariello, F., De Cesco, F., Nicolosi, G. L., Lombardo, M., & Harari, S. (2025). Effect of Chronic Obstructive Pulmonary Disease (COPD) on Biventricular Mechanics in Patients Without Severe Airflow Obstruction. Journal of Clinical Medicine, 14(11), 3660. https://doi.org/10.3390/jcm14113660