Evaluation of the Efficacy and Safety of CollaSel PRO® Type I and Type III Hydrolyzed Collagen Peptides in the Treatment of Osteoarthritis: A Double-Blind, Placebo-Controlled, Randomized Clinical Trial
Abstract
1. Introduction
2. Patients and Methods
2.1. Manufacturing and Quality Evaluation of Collagen
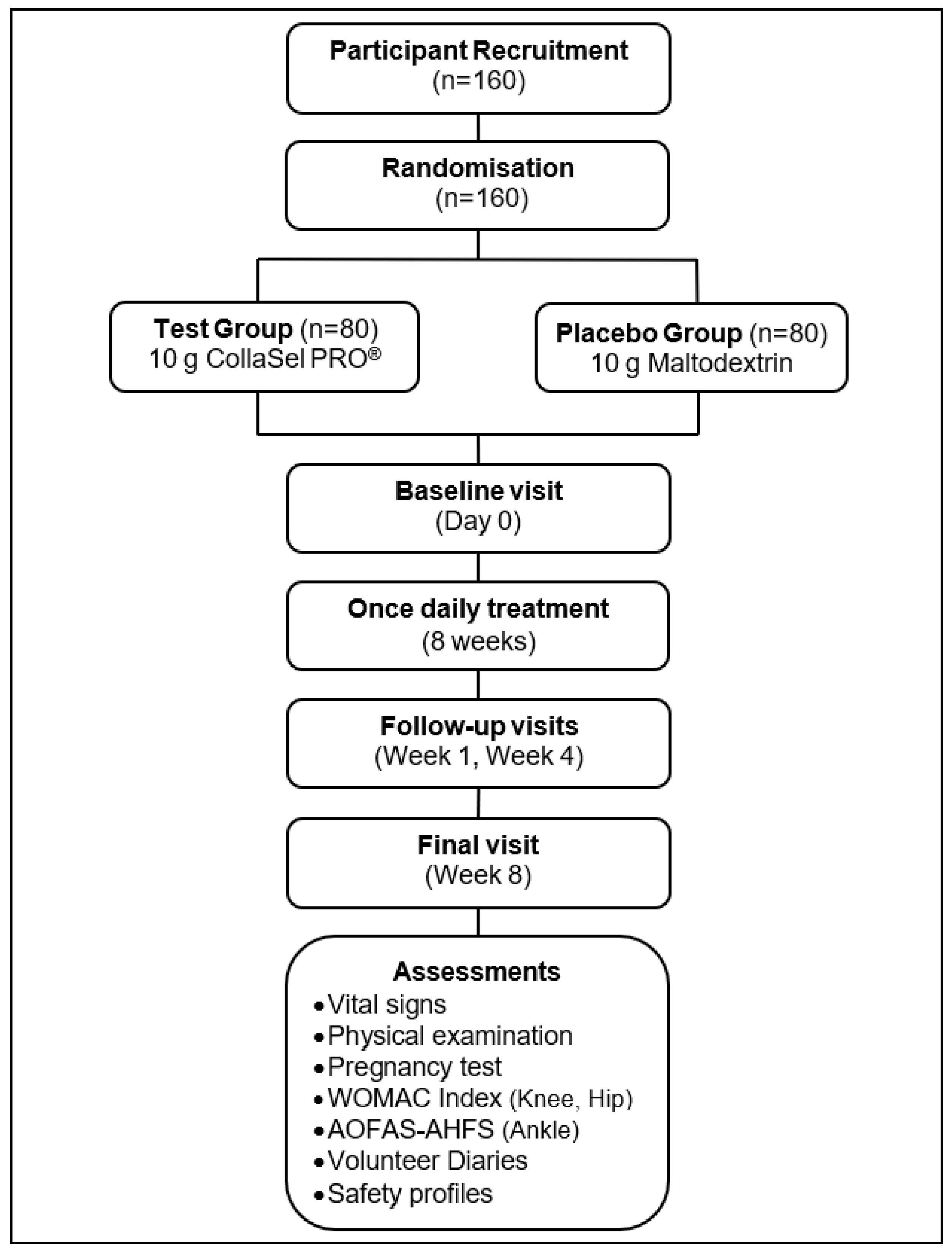
2.2. Study Design
2.3. Inclusion Criteria
- -
- Patients with OA aged between 45 and 60 years,
- -
- Patients diagnosed with knee or hip OA who were not drug-naïve to OA and related conditions,
- -
- Patients with normal blood pressure (systolic: 110–140 mmHg, diastolic: 60–90 mmHg) and heart rate (50–100 bpm) measured after 5 min of rest during the screening visit,
- -
- Patients who communicated adequately with the investigator, complied with the study requirements, and agreed to participate.
2.4. Exclusion Criteria
- -
- Patients with an atopic constitution or asthma and/or a known allergy to collagen products or any of the excipients of the product,
- -
- Patients with hereditary conditions such as galactose intolerance, Lapp lactase deficiency, or glucose–galactose malabsorption,
- -
- Patients with diabetes mellitus,
- -
- Patients with serious or life-threatening conditions affecting the cardiovascular, neurological, hematological, hepatic, gastrointestinal, renal, pulmonary, endocrinological, metabolic, or psychiatric systems,
- -
- Patients with any porphyria,
- -
- Patients with swallowing difficulties, malabsorption, or a history of gastrointestinal surgery (except appendectomy or herniotomy),
- -
- Patients with active rheumatoid arthritis or any other inflammatory arthritic condition deemed inappropriate by the researchers,
- -
- Patients with a history of planned or prior joint-related reconstructive surgery,
- -
- Patients who have used oral retinoids or oral steroids within six months before the start of the study,
- -
- Patients currently or regularly taking NSAIDs, contraceptives, hormones, obesity drugs, absorption inhibitors, or antidepressants,
- -
- Patients deemed unlikely to comply with the study procedures or complete the study based on the investigator’s judgment.
2.5. Assessments
2.6. Western Ontario and McMaster Universities Arthritis Index (WOMAC)
2.7. American Orthopedic Foot and Ankle Society Ankle-Hindfoot Scale (AOFAS-AHFS)
2.8. Test Product and Placebo
2.9. Statistical Analysis
3. Results
3.1. Baseline Characteristics
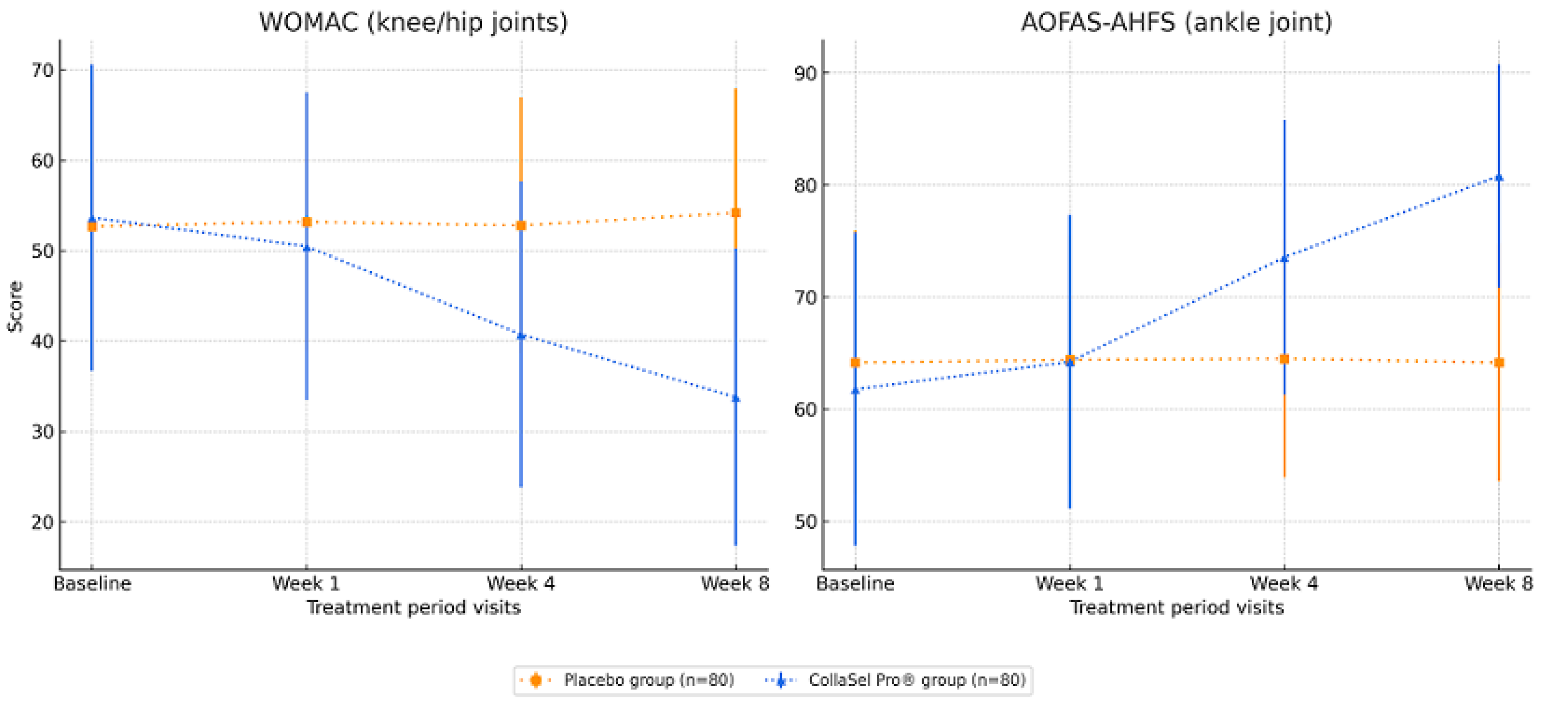
3.2. WOMAC (for Knee and Hip Joints) Scores in Test Product and Placebo Groups
3.3. AOFAS-AHFS (for Ankle Joint) Scores in Test Product and Placebo Groups
3.4. Safety
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Litwic, A.; Edwards, M.H.; Dennison, E.M.; Cooper, C. Epidemiology and burden of osteoarthritis. Br. Med. Bull. 2013, 105, 185–199. [Google Scholar] [CrossRef]
- Ma, W.; Chen, H.; Yuan, Q.; Chen, X.; Li, H. Global, regional, and national epidemiology of osteoarthritis in working-age individuals: Insights from the global burden of disease study 1990–2021. Sci. Rep. 2025, 15, 7907. [Google Scholar] [CrossRef]
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef]
- Mandl, L.A. Osteoarthritis year in review 2018: Clinical. Osteoarthr. Cartil. 2019, 27, 359–364. [Google Scholar] [CrossRef]
- Palazzo, C.; Nguyen, C.; Lefevre-Colau, M.M.; Rannou, F.; Poiraudeau, S. Risk factors and burden of osteoarthritis. Ann. Phys. Rehabil. Med. 2016, 59, 134–138. [Google Scholar] [CrossRef]
- Bannuru, R.R.; Osani, M.C.; Vaysbrot, E.E.; Arden, N.K.; Bennell, K.; Bierma-Zeinstra, S.M.A.; Kraus, V.B.; Lohmander, L.S.; Abbott, J.H.; Bhandari, M.; et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr. Cartil. 2019, 27, 1578–1589. [Google Scholar] [CrossRef]
- Kolasinski, S.L.; Neogi, T.; Hochberg, M.C.; Oatis, C.; Guyatt, G.; Block, J.; Callahan, L.; Copenhaver, C.; Dodge, C.; Felson, D.; et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthritis Care Res. 2020, 72, 149–162. [Google Scholar] [CrossRef]
- Zeng, C.; Wei, J.; Persson, M.S.M.; Sarmanova, A.; Doherty, M.; Xie, D.; Wang, Y.; Li, X.; Li, J.; Long, H.; et al. Relative efficacy and safety of topical non-steroidal anti-inflammatory drugs for osteoarthritis: A systematic review and network meta-analysis of randomised controlled trials and observational studies. Br. J. Sports Med. 2018, 52, 642–650. [Google Scholar] [CrossRef]
- Garcia-Coronado, J.M.; Martinez-Olvera, L.; Elizondo-Omana, R.E.; Acosta-Olivo, C.A.; Vilchez-Cavazos, F.; Simental-Mendia, L.E.; Simental-Mendia, M. Effect of collagen supplementation on osteoarthritis symptoms: A meta-analysis of randomized placebo-controlled trials. Int. Orthop. 2019, 43, 531–538. [Google Scholar] [CrossRef]
- Van Vijven, J.P.; Luijsterburg, P.A.; Verhagen, A.P.; van Osch, G.J.; Kloppenburg, M.; Bierma-Zeinstra, S.M. Symptomatic and chondroprotective treatment with collagen derivatives in osteoarthritis: A systematic review. Osteoarthr. Cartil. 2012, 20, 809–821. [Google Scholar] [CrossRef]
- Oliviero, F.; Ramonda, R.; Hoxha, A.; Scanu, A.; Galozzi, P.; Favero, M.; Frallonardo, P.; Punzi, L. Effect of an oral preparation containing hyaluronic acid, chondroitin sulfate, hydrolyzed collagen type II and hydrolyzed keratin on synovial fluid features and clinical indices in knee osteoarthritis: A pilot study. Reumatismo 2020, 72, 125–130. [Google Scholar] [CrossRef]
- Liu, X.; Machado, G.C.; Eyles, J.P.; Ravi, V.; Hunter, D.J. Dietary supplements for treating osteoarthritis: A systematic review and meta-analysis. Br. J. Sports Med. 2018, 52, 167–175. [Google Scholar] [CrossRef]
- Zdzieblik, D.; Brame, J.; Oesser, S.; Gollhofer, A.; Konig, D. The Influence of Specific Bioactive Collagen Peptides on Knee Joint Discomfort in Young Physically Active Adults: A Randomized Controlled Trial. Nutrients 2021, 13, 523. [Google Scholar] [CrossRef]
- Honvo, G.; Lengele, L.; Charles, A.; Reginster, J.Y.; Bruyere, O. Role of Collagen Derivatives in Osteoarthritis and Cartilage Repair: A Systematic Scoping Review with Evidence Mapping. Rheumatol. Ther. 2020, 7, 703–740. [Google Scholar] [CrossRef]
- Choi, F.D.; Sung, C.T.; Juhasz, M.L.; Mesinkovsk, N.A. Oral Collagen Supplementation: A Systematic Review of Dermatological Applications. J. Drugs Dermatol. 2019, 18, 9–16. [Google Scholar]
- Sato, K. The presence of food-derived collagen peptides in human body-structure and biological activity. Food Funct. 2017, 8, 4325–4330. [Google Scholar] [CrossRef]
- Proksch, E.; Segger, D.; Degwert, J.; Schunck, M.; Zague, V.; Oesser, S. Oral supplementation of specific collagen peptides has beneficial effects on human skin physiology: A double-blind, placebo-controlled study. Ski. Pharmacol. Physiol. 2013, 27, 47–55. [Google Scholar] [CrossRef]
- Konig, D.; Oesser, S.; Scharla, S.; Zdzieblik, D.; Gollhofer, A. Specific Collagen Peptides Improve Bone Mineral Density and Bone Markers in Postmenopausal Women-A Randomized Controlled Study. Nutrients 2018, 10, 97. [Google Scholar] [CrossRef]
- McConnell, S.; Kolopack, P.; Davis, A.M. The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC): A review of its utility and measurement properties. Arthrit Care Res. 2001, 45, 453–461. [Google Scholar] [CrossRef]
- Basaran, S.; Guzel, R.; Seydaoglu, G.; Guler-Uysal, F. Validity, reliability, and comparison of the WOMAC osteoarthritis index and Lequesne algofunctional index in Turkish patients with hip or knee osteoarthritis. Clin. Rheumatol. 2010, 29, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Kitaoka, H.B.; Alexander, I.J.; Adelaar, R.S.; Nunley, J.A.; Myerson, M.S.; Sanders, M.; Lutter, L.D. Clinical Rating Systems for the Ankle-Hindfoot, Midfoot, Hallux, and Lesser Toes. Foot Ankle Int. 1997, 18, 187–188. [Google Scholar] [CrossRef]
- Analay Akbaba, Y.; Celik, D.; Ogut, R.T. Translation, Cross-Cultural Adaptation, Reliability, and Validity of Turkish Version of the American Orthopaedic Foot and Ankle Society Ankle-Hindfoot Scale. J. Foot Ankle Surg. 2016, 55, 1139–1142. [Google Scholar] [CrossRef]
- Angst, F.; Aeschlimann, A.; Stucki, G. Smallest detectable and minimal clinically important differences of rehabilitation intervention with their implications for required sample sizes using WOMAC and SF-36 quality of life measurement instruments in patients with osteoarthritis of the lower extremities. Arthrit Care Res. 2001, 45, 384–391. [Google Scholar] [CrossRef]
- SooHoo, N.F.; Shuler, M.; Fleming, L.L.; American Orthopaedic, F.; Ankle, S. Evaluation of the validity of the AOFAS Clinical Rating Systems by correlation to the SF-36. Foot Ankle Int. 2003, 24, 50–55. [Google Scholar] [CrossRef]
- Kumar, S.; Sugihara, F.; Suzuki, K.; Inoue, N.; Venkateswarathirukumara, S. A double-blind, placebo-controlled, randomised, clinical study on the effectiveness of collagen peptide on osteoarthritis. J. Sci. Food Agric. 2015, 95, 702–707. [Google Scholar] [CrossRef]
- Puigdellivol, J.; Comellas Berenger, C.; Perez Fernandez, M.A.; Cowalinsky Millan, J.M.; Carreras Vidal, C.; Gil Gil, I.; Martinez Pagan, J.; Ruiz Nieto, B.; Jimenez Gomez, F.; Comas Figuerola, F.X.; et al. Effectiveness of a Dietary Supplement Containing Hydrolyzed Collagen, Chondroitin Sulfate, and Glucosamine in Pain Reduction and Functional Capacity in Osteoarthritis Ptients. J. Diet. Suppl. 2019, 16, 379–389. [Google Scholar] [CrossRef]
- Benito-Ruiz, P.; Camacho-Zambrano, M.M.; Carrillo-Arcentales, J.N.; Mestanza-Peralta, M.A.; Vallejo-Flores, C.A.; Vargas-Lopez, S.V.; Villacis-Tamayo, R.A.; Zurita-Gavilanes, L.A. A randomized controlled trial on the efficacy and safety of a food ingredient, collagen hydrolysate, for improving joint comfort. Int. J. Food Sci. Nutr. 2009, 60, 99–113. [Google Scholar] [CrossRef]
- McAlindon, T.E.; Nuite, M.; Krishnan, N.; Ruthazer, R.; Price, L.L.; Burstein, D.; Griffith, J.; Flechsenhar, K. Change in knee osteoarthritis cartilage detected by delayed gadolinium enhanced magnetic resonance imaging following treatment with collagen hydrolysate: A pilot randomized controlled trial. Osteoarthr. Cartil. 2011, 19, 399–405. [Google Scholar] [CrossRef]
- Trc, T.; Bohmova, J. Efficacy and tolerance of enzymatic hydrolysed collagen (EHC) vs. glucosamine sulphate (GS) in the treatment of knee osteoarthritis (KOA). Int. Orthop. 2011, 35, 341–348. [Google Scholar] [CrossRef]
- Schadow, S.; Simons, V.S.; Lochnit, G.; Kordelle, J.; Gazova, Z.; Siebert, H.C.; Steinmeyer, J. Metabolic Response of Human Osteoarthritic Cartilage to Biochemically Characterized Collagen Hydrolysates. Int. J. Mol. Sci. 2017, 18, 207. [Google Scholar] [CrossRef]
- Boonmaleerat, K.; Wanachewin, O.; Phitak, T.; Pothacharoen, P.; Kongtawelert, P. Fish Collagen Hydrolysates Modulate Cartilage Metabolism. Cell Biochem. Biophys. 2018, 76, 279–292. [Google Scholar] [CrossRef]
- Jiang, J.X.; Yu, S.; Huang, Q.R.; Zhang, X.L.; Zhang, C.Q.; Zhou, J.L.; Prawitt, J. Collagen peptides improve knee osteoarthritis in elderly women A 6-month randomized, double-blind, placebo-controlled study. Agro Food Ind. Hi Tec. 2014, 25, 19–23. [Google Scholar]
- Schauss, A.G.; Stenehjem, J.; Park, J.; Endres, J.R.; Clewell, A. Effect of the novel low molecular weight hydrolyzed chicken sternal cartilage extract, BioCell Collagen, on improving osteoarthritis-related symptoms: A randomized, double-blind, plcebo-controlled trial. J. Agric. Food Chem. 2012, 60, 4096–4101. [Google Scholar] [CrossRef]
- Crowley, D.C.; Lau, F.C.; Sharma, P.; Evans, M.; Guthrie, N.; Bagchi, M.; Bagchi, D.; Dey, D.K.; Raychaudhuri, S.P. Safety and efficacy of undenatured type II collagen in the treatment of osteoarthritis of the knee: A clinical trial. Int. J. Med. Sci. 2009, 6, 312–321. [Google Scholar] [CrossRef]
- Scarpellini, M.; Lurati, A.; Vignati, G.; Marrazza, M.G.; Telese, F.; Re, K.; Bellistri, A. Biomarkers, type II collagen, glucosamine and chondroitin sulfate in osteoarthritis follow-up: The “Magenta osteoarthritis study”. J. Orthop. Traumatol. 2008, 9, 81–87. [Google Scholar] [CrossRef]
- Dai, M.; Sui, B.; Xue, Y.; Liu, X.; Sun, J. Cartilage repair in degenerative osteoarthritis mediated by squid type II collagen via immunomodulating activation of M2 macrophages, inhibiting apoptosis and hypertrophy of chondrocytes. Biomaterials 2018, 180, 91–103. [Google Scholar] [CrossRef]
- Dar, Q.A.; Schott, E.M.; Catheline, S.E.; Maynard, R.D.; Liu, Z.Y.; Kamal, F.; Farnsworth, C.W.; Ketz, J.P.; Mooney, R.A.; Hilton, M.J.; et al. Daily oral consumption of hydrolyzed type 1 collagen is chondroprotective and anti-inflammatory in murine posttraumatic osteoarthritis. PLoS ONE 2017, 12, e0174705. [Google Scholar] [CrossRef]
- Di Cesare Mannelli, L.; Micheli, L.; Zanardelli, M.; Ghelardini, C. Low dose native type II collagen prevents pain in a rat osteoarthritis model. BMC Musculoskelet. Disord. 2013, 14, 228. [Google Scholar] [CrossRef]
- Ohara, H.; Iida, H.; Ito, K.; Takeuchi, Y.; Nomura, Y. Effects of Pro-Hyp, a collagen hydrolysate-derived peptide, on hyaluronic acid synthesis using in vitro cultured synovium cells and oral ingestion of collagen hydrolysates in a guinea pig model of osteoarthritis. Biosci. Biotechnol. Biochem. 2010, 74, 2096–2099. [Google Scholar] [CrossRef]
- Iwai, K.; Hasegawa, T.; Taguchi, Y.; Morimatsu, F.; Sato, K.; Nakamura, Y.; Higashi, A.; Kido, Y.; Nakabo, Y.; Ohtsuki, K. Identification of food-derived collagen peptides in human blood after oral ingestion of gelatin hydrolysates. J. Agric. Food Chem. 2005, 53, 6531–6536. [Google Scholar] [CrossRef]
- Demir-Dora, D.; Ozsoy, U.; Yildirim, Y.; Yilmaz, O.; Aytac, P.; Yilmaz, B.; Dogan Kurtoglu, E.; Akman, A.; Tezman, S.; Inaloz, H.S.; et al. The Efficacy and Safety of CollaSel PRO® Hydrolyzed Collagen Peptide Supplementation without Addons in Improving Skin Health in Adult Females: A Double Blind, Randomized, Placebo-Controlled Clinical Study Using Biophysical and Skin Imaging Techniques. J. Clin. Med. 2024, 13, 5370. [Google Scholar] [CrossRef]
| Placebo Group (n = 80) Mean ± SD n (%) | Test Group (n = 80) Mean ± SD n (%) | p | 95% CI | ||
|---|---|---|---|---|---|
| Age | 52.91 ± 0.47 | 51.84 ± 0.48 | 0.149 | 0.143; 0.157 | |
| BMI | 28.04 ± 0.39 | 27.71 ± 0.33 | 0.897 | 0.887; 0.899 | |
| Sex | Female | 51 (63.8%) | 60 (75%) | 0.170 | |
| Male | 29 (36.2%) | 20 (25%) | |||
| OA | Knee/Hip | 80 (100%) | 80 (100%) | 0.256 | |
| Ankle | 53 (66.3%) | 45 (56.3%) | |||
| WOMAC | n = 80 | n = 80 | |||
| Day 0 | 52.7 ± 15.8 | 53.7 ± 16.9 | 0.707 | −6.095; 4.145 | |
| Week 1 | 53.2 ± 14.2 | 50.5 ± 17.0 | 0.278 | −2.196; 7.596 | |
| Week 4 | 52.8 ± 14.1 | 40.7 ± 16.9 | <0.001 | 7.180; 16.920 | |
| Week 8 | 54.2 ± 13.7 | 33.8 ± 16.5 | <0.001 | 15.637; 25.143 | |
| AOFAS-AHFS | n = 53 | n = 45 | |||
| Day 0 | 64.2 ± 11.8 | 61.8 ± 13.9 | 0.360 | −2.772; 7.556 | |
| Week 1 | 64.4 ± 11.1 | 64.2 ± 13.1 | 0.943 | −4.682; 5.030 | |
| Week 4 | 64.5 ± 10.6 | 73.6 ± 12.3 | <0.001 | −13.627; −4.465 | |
| Week 8 | 64.2 ± 10.6 | 80.8 ± 9.9 | <0.001 | −20.801; −12.504 | |
| Placebo Group (n = 80) | Test Product (CollaSel Pro®) Group (n = 80) | |||||||
|---|---|---|---|---|---|---|---|---|
| Visits | Day 0 | Week 1 | Week 4 | Week 8 | Day 0 | Week 1 | Week 4 | Week 8 |
| WOMAC (n) | 80 | 80 | 80 | 79 | 80 | 80 | 80 | 80 |
| Mean ± SD | 52.7 ± 15.8 | 53.2 ± 14.2 | 52.8 ± 14.1 | 54.2 ± 13.7 | 53.7 ± 16.9 | 50.5 ± 17.0 | 40.7 ± 16.9 | 33.8 ± 16.5 |
| Day 0 p (95% CI) | 0.540 (−2.116; 1.116) | 0.920 (−2.068; 1.868) | 0.224 (−3.819; 0.908) | <0.001 (1.625; 4.725) | <0.001 (10.57; 15.28) | <0.001 (17.027; 22.698) | ||
| Week 1 p (95% CI) | 0.553 (−0.936; 1.736) | 0.368 (−2.431; 0.912) | <0.001 (8.175; 11.325) | <0.001 (14.642; 18.733) | ||||
| Week 4 p (95% CI) | 0.024 (−1.865; −0.135) | <0.001 (5.924; 7.951) | ||||||
| AOFAS-AHFS (n) | 53 | 53 | 53 | 53 | 45 | 45 | 45 | 45 |
| Mean ± SD | 64.2 ± 11.8 | 64.4 ± 11.1 | 64.5 ± 10.6 | 64.2 ± 10.6 | 61.8 ± 13.9 | 64.2 ± 13.1 | 73.6 ± 12.3 | 80.8 ± 9.9 |
| Day 0 p (95% CI) | 0.699 (−1.395; 0.943) | 0.704 (−2.127; 1.447) | 1.000 (−1.801; 1.801) | <0.001 (−3.717; −1.172) | <0.001 (−14.038; −9.518) | <0.001 (−21.615; −16.474) | ||
| Week 1 p (95% CI) | 0.867 (−1.466; 1.24) | 0.770 (−1.318; 1.771) | <0.001 (−11.122; −7.544) | <0.001 (−18.718; −14.482) | ||||
| Week 4 p (95% CI) | 0.552 (−0.798; 1.477) | <0.001 (−8.591; −5.942) | ||||||
| Overall Adverse Events (AEs) Summary | n (%) | |||
|---|---|---|---|---|
| Patients with Adverse Events | 24 (52.2%) | |||
| Total Number of Adverse Events | 46 (100%) | |||
| Severity Level of AEs | Mild | 45 (97.8%) | 46 (100%) | |
| Moderate | 1 (2.2%) | |||
| Severe | 0 (0%) | |||
| Relation to Study Drug | Certain | 1 (2.2%) | 46 (100%) | |
| Probable/likely | 0 (0%) | |||
| Possible | 30 (65.2%) | |||
| Unlikely | 15 (32.6%) | |||
| Type of Adverse Events | Gastrointestinal | Nausea | 9 (19.5%) | 18 (39.1%) |
| Constipation | 4 (8.7%) | |||
| Dyspepsia | 2 (4.3%) | |||
| Stomachache | 1 (2.2%) | |||
| Acid reflux | 1 (2.2%) | |||
| Bloating | 1 (2.2%) | |||
| Skin | Itching (hands, arms, body) | 4 (8.7%) | 10 (21.8%) | |
| Redness (arms, legs) | 3 (6.5%) | |||
| Skin blistering on hands | 1 (2.2%) | |||
| Dry skin | 1 (2.2%) | |||
| Acne | 1 (2.2%) | |||
| Neurological | Headache | 4 (8.7%) | 6 (13%) | |
| Tingling | 1 (%) | |||
| Insomnia | 1 (%) | |||
| Other | Joint pain | 4 (8.7%) | 12 (26.1%) | |
| Edema | 2 (4,3%) | |||
| High blood pressure | 1 (2.2%) | |||
| Fatigue | 1 (2.2%) | |||
| Dry mouth and tongue with odor | 1 (2.2%) | |||
| Dry mouth with thirst | 1 (2.2%) | |||
| Bitterness in the mouth | 1 (2.2%) | |||
| Weight increase | 1 (2.2%) | |||
| Total | 46 (100%) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demir-Dora, D.; Tuna, S.; Kurtoglu, E.D.; Gursoy, S.; Balci, N.; Tezman, S.; Erenmemisoglu, A. Evaluation of the Efficacy and Safety of CollaSel PRO® Type I and Type III Hydrolyzed Collagen Peptides in the Treatment of Osteoarthritis: A Double-Blind, Placebo-Controlled, Randomized Clinical Trial. J. Clin. Med. 2025, 14, 3655. https://doi.org/10.3390/jcm14113655
Demir-Dora D, Tuna S, Kurtoglu ED, Gursoy S, Balci N, Tezman S, Erenmemisoglu A. Evaluation of the Efficacy and Safety of CollaSel PRO® Type I and Type III Hydrolyzed Collagen Peptides in the Treatment of Osteoarthritis: A Double-Blind, Placebo-Controlled, Randomized Clinical Trial. Journal of Clinical Medicine. 2025; 14(11):3655. https://doi.org/10.3390/jcm14113655
Chicago/Turabian StyleDemir-Dora, Devrim, Serpil Tuna, Emel Dogan Kurtoglu, Savas Gursoy, Nilufer Balci, Selim Tezman, and Aydin Erenmemisoglu. 2025. "Evaluation of the Efficacy and Safety of CollaSel PRO® Type I and Type III Hydrolyzed Collagen Peptides in the Treatment of Osteoarthritis: A Double-Blind, Placebo-Controlled, Randomized Clinical Trial" Journal of Clinical Medicine 14, no. 11: 3655. https://doi.org/10.3390/jcm14113655
APA StyleDemir-Dora, D., Tuna, S., Kurtoglu, E. D., Gursoy, S., Balci, N., Tezman, S., & Erenmemisoglu, A. (2025). Evaluation of the Efficacy and Safety of CollaSel PRO® Type I and Type III Hydrolyzed Collagen Peptides in the Treatment of Osteoarthritis: A Double-Blind, Placebo-Controlled, Randomized Clinical Trial. Journal of Clinical Medicine, 14(11), 3655. https://doi.org/10.3390/jcm14113655






