Impact of Surgical Stabilization of Flail Chest Injuries on Postoperative Computed Tomography Lung Volumes
Abstract
1. Introduction
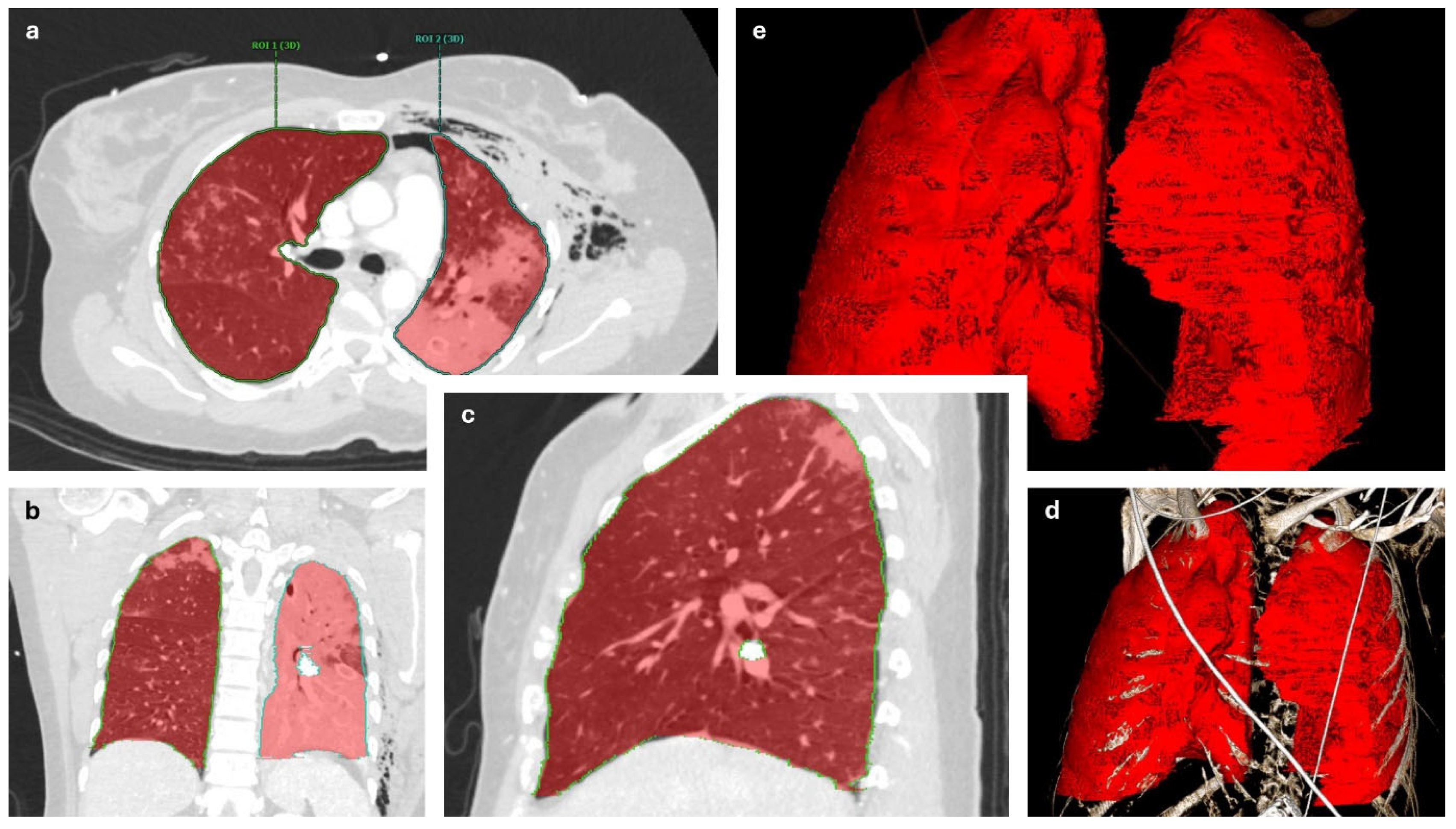
2. Methods
3. Results
3.1. Injury Characteristics
3.2. Perioperative CT Lung Volume Changes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Last 72 h Preoperative | First 72 h Postoperative | |
|---|---|---|
| PIP [mbar] | 20.39 (IQR 12.92, 24.36) | 22.09 (IQR 13.4, 24.83) |
| PEEP [mbar] | 9.67 (IQR 9.45, 9.99) | 10.37 (IQR 8.79, 11.66) |
| FiO2 [%] | 43.25 (IQR 37.25, 49.58) | 45.38 (IQR 37.19, 50.6) |
| TV [mL] | 461.04 (IQR 321.77, 600.73) | 469.37 (IQR 317, 553.48) |
| RR [/min] | 15.76 (IQR 13.41, 22.16) | 17.34 (IQR 14.17, 18) |
| RMV [L/min] | 8.49 (IQR 3.73, 9.2) | 8.37 (IQR 5.04, 9.27) |
References
- World Health Organization. Injuries and Violence: The Facts. 2014. Available online: https://apps.who.int/iris/bitstream/handle/10665/149798/9789241508018_eng.pdf (accessed on 2 August 2023).
- National Center for Injury Prevention and Control C for DC and P. 10 Leading Causes of Death. 2022. Available online: https://wisqars.cdc.gov/fatal-leading (accessed on 4 April 2023).
- Reith, G.; Lefering, R.; Wafaisade, A.; Hensel, K.O.; Paffrath, T.; Bouillon, B.; Probst, C. Injury pattern, outcome and characteristics of severely injured pedestrian. Scand. J. Trauma Resusc. Emerg. Med. 2015, 23, 56. [Google Scholar] [CrossRef] [PubMed]
- Benhamed, A.; Ndiaye, A.; Emond, M.; Lieutaud, T.; Boucher, V.; Gossiome, A.; Laumon, B.; Gadegbeku, B.; Tazarourte, K. Road traffic accident-related thoracic trauma: Epidemiology, injury pattern, outcome, and impact on mortality—A multicenter observational study. PLoS ONE 2022, 17, e0268202. [Google Scholar] [CrossRef] [PubMed]
- Lundin, A.; Akram, S.K.; Berg, L.; Göransson, K.E.; Enocson, A. Thoracic injuries in trauma patients: Epidemiology and its influence on mortality. Scand. J. Trauma Resusc. Emerg. Med. 2022, 30, 69. [Google Scholar] [CrossRef]
- American College of Surgeons. National Trauma Data Bank 2016—Annual Report; American College of Surgeons: Chicago, IL, USA, 2016. [Google Scholar]
- Sirmali, M.; Türüt, H.; Topçu, S.; Gülhan, E.; Yazici, Ü.; Kaya, S.; Taştepe, I. A comprehensive analysis of traumatic rib fractures: Morbidity, mortality and management. Eur. J. Cardio-Thoracic Surg. 2003, 24, 133–138. [Google Scholar] [CrossRef]
- Ingoe, H.M.; Eardley, W.; McDaid, C.; Rangan, A.; Lawrence, T.; Hewitt, C. Epidemiology of adult rib fracture and factors associated with surgical fixation: Analysis of a chest wall injury dataset from England and Wales. Injury 2020, 51, 218–223. [Google Scholar] [CrossRef]
- Ziegler, D.W.; Agarwal, N.N. The morbidity and mortality of rib fractures. J. Trauma 1994, 37, 975–979. [Google Scholar] [CrossRef] [PubMed]
- Graef, F.; Doll, C.; Niemann, M.; Tsitsilonis, S.; Stöckle, U.; Braun, K.F.; Wüster, J.; Märdian, S. Epidemiology, Injury Severity, and Pattern of Standing E-Scooter Accidents: 6-Month Experience from a German Level I Trauma Center. Clin. Orthop. Surg. 2021, 13, 443–448. [Google Scholar] [CrossRef]
- Edwards, J.G.; Clarke, P.; Pieracci, F.M.; Bemelman, M.; Black, E.A.; Doben, A.; Gasparri, M.; Gross, R.; Jun, W.; Long, W.B.; et al. Taxonomy of multiple rib fractures: Results of the chest wall injury society international consensus survey. J. Trauma Acute Care Surg. 2020, 88, E40–E45. [Google Scholar] [CrossRef]
- Veysi, V.T.; Nikolaou, V.S.; Paliobeis, C.; Efstathopoulos, N.; Giannoudis, P.V. Prevalence of chest trauma, associated injuries and mortality: A level I trauma centre experience. Int. Orthop. 2009, 33, 1425–1433. [Google Scholar] [CrossRef]
- Bakir, M.S.; Langenbach, A.; Pinther, M.; Lefering, R.; Krinner, S.; Grosso, M.; Ekkernkamp, A.; Schulz-Drost, S.; TraumaRegister DGU. The significance of a concomitant clavicle fracture in flail chest patients: Incidence, concomitant injuries, and outcome of 12,348 polytraumata from the TraumaRegister DGU®. Eur. J. Trauma Emerg. Surg. 2022, 48, 3623–3634. [Google Scholar] [CrossRef]
- Dehghan, N.; de Mestral, C.; McKee, M.D.; Schemitsch, E.H.; Nathens, A. Flail chest injuries: A review of outcomes and treatment practices from the National Trauma Data Bank. J. Trauma Acute Care Surg. 2014, 76, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Peek, J.; Beks, R.B.; Hietbrink, F.; De Jong, M.B.; Heng, M.; Beeres, F.J.P.; Ijpma, F.F.A.; Leenen, L.P.H.; Groenwold, R.H.H.; Houwert, R.M. Epidemiology and outcome of rib fractures: A nationwide study in the Netherlands. Eur. J. Trauma Emerg. Surg. 2022, 48, 265–271. [Google Scholar] [CrossRef]
- Bulger, E.M.; Arneson, M.A.; Mock, C.N.; Jurkovich, G.J. Rib fractures in the elderly. J. Trauma 2000, 48, 1040–1046. [Google Scholar] [CrossRef]
- Schulz-Drost, S.; Krinner, S.; Langenbach, A.; Oppel, P.; Lefering, R.; Taylor, D.; Hennig, F.F.; Mauerer, A.; Schulz-Drost, S.; TraumaRegister DGU. Concomitant Sternal Fracture in Flail Chest: An Analysis of 21,741 Polytrauma Patients from the TraumaRegister DGU®. Thorac. Cardiovasc. Surg. 2017, 65, 551–559. [Google Scholar] [CrossRef]
- Wang, Z.; Jia, Y.; Li, M. The effectiveness of early surgical stabilization for multiple rib fractures: A multicenter randomized controlled trial. J. Cardiothorac. Surg. 2023, 18, 118. [Google Scholar] [CrossRef]
- Tanaka, H.; Tajimi, K.; Endoh, Y.; Kobayashi, K. Pneumatic stabilization for flail chest injury: An 11-year study. Surg. Today 2001, 31, 12–17. [Google Scholar] [CrossRef]
- Brown, S.D.; Walters, M.R. Patients With Rib Fractures. J. Trauma Nurs. 2012, 19, 89–91. [Google Scholar] [CrossRef]
- Todd, S.R.; McNally, M.M.; Holcomb, J.B.; Kozar, R.A.; Kao, L.S.; Gonzalez, E.A.; Cocanour, C.S.; Vercruysse, G.A.; Lygas, M.H.; Brasseaux, B.K.; et al. A multidisciplinary clinical pathway decreases rib fracture–associated infectious morbidity and mortality in high-risk trauma patients. Am. J. Surg. 2006, 192, 806–811. [Google Scholar] [CrossRef]
- Lucena-Amaro, S.; Cole, E.; Zolfaghari, P. Long term outcomes following rib fracture fixation in patients with major chest trauma. Injury 2022, 53, 2947–2952. [Google Scholar] [CrossRef]
- Ingoe, H.M.A.; Coleman, E.; Eardley, W.; Rangan, A.; Hewitt, C.; McDaid, C. Systematic review of systematic reviews for effectiveness of internal fixation for flail chest and rib fractures in adults. BMJ Open 2019, 9, e023444. [Google Scholar] [CrossRef]
- Liang, Y.S.; Yu, K.C.; Wong, C.S.; Kao, Y.; Tiong, T.Y.; Tam, K.W. Does Surgery Reduce the Risk of Complications among Patients with Multiple Rib Fractures? A Meta-analysis. Clin. Orthop. Relat. Res. 2019, 477, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Long, R.; Tian, J.; Wu, S.; Li, Y.; Yang, X.; Fei, J. Clinical efficacy of surgical versus conservative treatment for multiple rib fractures: A meta-analysis of randomized controlled trials. Int. J. Surg. 2020, 83, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, E.; Wullschleger, M.; Muller, N.; Muller, M. Surgical Rib Fixation of Multiple Rib Fractures and Flail Chest: A Systematic Review and Meta-Analysis. J. Surg. Res. 2022, 276, 221–234. [Google Scholar] [CrossRef]
- Cataneo, A.J.M.; Cataneo, D.C.; de Oliveira, F.H.; Arruda, K.A.; El Dib, R.; Carvalho, P.E.d.O. Surgical versus nonsurgical interventions for flail chest. Cochrane Database Syst. Rev. 2015, 2015, CD009919. [Google Scholar] [CrossRef]
- Qiu, M.; Shi, Z.; Xiao, J.; Zhang, X.; Ling, S.; Ling, H. Potential Benefits of Rib Fracture Fixation in Patients with Flail Chest and Multiple Non-flail Rib Fractures. Indian J. Surg. 2016, 78, 458–463. [Google Scholar] [CrossRef]
- Taghavi, S.; Ali, A.; Green, E.; Schmitt, K.; Jackson-Weaver, O.; Tatum, D.; Harris, C.; Guidry, C.; McGrew, P.; Schroll, R.; et al. Surgical stabilization of rib fractures is associated with improved survival but increased acute respiratory distress syndrome. Surgery 2021, 169, 1525–1531. [Google Scholar] [CrossRef]
- Majak, P.; Ness, P.A. Rib fractures in trauma patients: Does operative fixation improve outcome? Curr. Opin. Crit. Care 2016, 22, 572–577. [Google Scholar] [CrossRef]
- Kane, E.D.; Jeremitsky, E.; Pieracci, F.M.; Majercik, S.; Doben, A.R. Quantifying and exploring the recent national increase in surgical stabilization of rib fractures. J. Trauma Acute Care Surg. 2017, 83, 1047–1052. [Google Scholar] [CrossRef]
- Liu, T.; Liu, P.; Chen, J.; Xie, J.; Yang, F.; Liao, Y. A Randomized Controlled Trial of Surgical Rib Fixation in Polytrauma Patients With Flail Chest. J. Surg. Res. 2019, 242, 223–230. [Google Scholar] [CrossRef]
- Tanaka, H.; Yukioka, T.; Yamaguti, Y.; Shimizu, S.; Goto, H.; Matsuda, H.; Shimazaki, S. Surgical stabilization of internal pneumatic stabilization? A prospective randomized study of management of severe flail chest patients. J. Trauma 2002, 52, 727–732; discussion 732. [Google Scholar] [CrossRef]
- Marasco, S.F.; Davies, A.R.; Cooper, J.; Varma, D.; Bennett, V.; Nevill, R.; Lee, G.; Bailey, M.; Fitzgerald, M. Prospective randomized controlled trial of operative rib fixation in traumatic flail chest. J. Am. Coll. Surg. 2013, 216, 924–932. [Google Scholar] [CrossRef] [PubMed]
- Marasco, S.F.; Balogh, Z.J.M.; Wullschleger, M.E.M.; Hsu, J.M.; Patel, B.M.; Fitzgerald, M.M.; Martin, K.M.; Summerhayes, R.B.; Bailey, M. Rib fixation in non–ventilator-dependent chest wall injuries: A prospective randomized trial. J. Trauma Acute Care Surg. 2022, 92, 1047–1053. [Google Scholar] [CrossRef]
- Sedaghat, N.; Chiong, C.; Tjahjono, R.; Hsu, J. Early Outcomes of Surgical Stabilisation of Traumatic Rib Fractures: Single-Center Review With a Real-World Evidence Perspective. J. Surg. Res. 2021, 264, 222–229. [Google Scholar] [CrossRef]
- Peek, J.B.; Beks, R.B.; Hietbrink, F.; Heng, M.M.; De Jong, M.B.; Beeres, F.J.; Leenen, L.P.; Groenwold, R.H.; Houwert, R.M. Complications and outcome after rib fracture fixation: A systematic review. J. Trauma Acute Care Surg. 2020, 89, 411–418. [Google Scholar] [CrossRef]
- Sarani, B.; Allen, R.; Pieracci, F.M.; Doben, A.R.; Eriksson, E.; Bauman, Z.M.; Gupta, P.; Semon, G.; Greiffenstein, P.; Chapman, A.J.; et al. Characteristics of hardware failure in patients undergoing surgical stabilization of rib fractures: A Chest Wall Injury Society multicenter study. J. Trauma Acute Care Surg. 2019, 87, 1277–1281. [Google Scholar] [CrossRef]
- MatrixRIB™ RIB™Fixation System. Reference Guide. Available online: https://p1.aprimocdn.net/jjamp/en/depuy-synthes/surgical-technique-guide/matrixrib-fixation-system-dsuscmf04140060.pdf (accessed on 15 May 2025).
- RibLoc® U Plus 90—Surgical Technique. Available online: https://www.acumed.net/wp-content/uploads/2023/12/Acumed-Surgical-Technique-EN-Ribloc-U-Plus-90-Instrumentation-Implantmed-RBL7017-N.pdf (accessed on 22 August 2023).
- De La Santa Barajas, P.M.; Otero, M.D.P.; Sánchez-Gracián, C.D.; Gómez, M.L.; Novella, A.T.; Del Prado, J.C.M.; Ruiloba, S.L.; Durán, M.L.C. Surgical fixation of rib fractures with clips and titanium bars (STRATOS System). Preliminary experience. Cir. Esp. 2010, 88, 180–186. [Google Scholar] [CrossRef]
- Delaplain, P.T.; Schubl, S.D.; Pieracci, F.M.; Shen, A.; Brabender, D.E.; Loftus, J.; Towe, C.W.; White, T.W.; Gross, R.I.; Doben, A.R.; et al. Chest Wall Injury Society Guideline for SSRF Indications, Contraindications and Timing. 2020. Available online: https://cwisociety.org/wp-content/uploads/2020/05/CWIS-SSRF-Guideline-01102020.pdf (accessed on 25 November 2024).
- Brink, M.; Deunk, J.; Dekker, H.M.; Edwards, M.J.R.; Kool, D.R.; van Vugt, A.B.; van Kuijk, C.; Blickman, J.G. Criteria for the selective use of chest computed tomography in blunt trauma patients. Eur. Radiol. 2010, 20, 818–828. [Google Scholar] [CrossRef][Green Version]
- Deutsche Gesellschaft für Unfallchirurgie, Deutsche Gesellschaft für Orthopädie und Unfallchirurgie. Polytrauma/Schwerverletzten-Behandlung—S3-Leitlinie. 2022. Available online: https://www.awmf.org/leitlinien/detail/ll/187-023.html (accessed on 31 March 2023).
- Nicolaou, S.; Eftekhari, A.; Sedlic, T.; Hou, D.; Mudri, M.; Aldrich, J.; Louis, L. The utilization of dual source CT in imaging of polytrauma. Eur. J. Radiol. 2008, 68, 398–408. [Google Scholar] [CrossRef]
- Campbell, D.; Arnold, N.; Wake, E.; Grieve, J.; Provenzano, S.; Wullschleger, M.; Patel, B. Three-dimensional volume-rendered computed tomography application for follow-up fracture healing and volume measurements pre–surgical rib fixation and post–surgical rib fixation. J. Trauma Acute Care Surg. 2021, 91, 961–965. [Google Scholar] [CrossRef]
- Caragounis, E.C.; Olsén, M.F.; Granhed, H.; Norrlund, R.R. CT-lung volume estimates in trauma patients undergoing stabilizing surgery for flail chest. Injury 2019, 50, 101–108. [Google Scholar] [CrossRef]
- Büyükkarabacak, Y.B.; Gürz, S.; Pirzirenli, M.G.; Şengül, A.T.; Başoğlu, A.; Şahïn, B. Quantitative assessment of the posttreatment lung volume changes in patients with flail chest on computed chest tomography images. J. Exp. Clin. Med. 2021, 38, 132–137. [Google Scholar] [CrossRef]
- Niemann, M.; Graef, F.; Tsitsilonis, S.; Stöckle, U.; Märdian, S. Retrospective Analysis of the Clinical Outcome in a Matched Case-Control Cohort of Polytrauma Patients Following an Osteosynthetic Flail Chest Stabilization. J. Clin. Med. 2020, 9, 2379. [Google Scholar] [CrossRef]
- Baker, S.P.; O’Neill, B.; Haddon, W., Jr.; Long, W.B. The injury severity score: A method for describing patients with multiple injuries and evaluating emergency care. J. Trauma 1974, 14, 187–196. [Google Scholar] [CrossRef]
- Li, P.; Stuart, E.A.; Allison, D.B. Multiple Imputation: A Flexible Tool for Handling Missing Data. JAMA 2015, 314, 1966. [Google Scholar] [CrossRef]
- Graef, F.; Walter, S.; Baur, A.; Tsitsilonis, S.; Moroder, P.; Kempfert, J.; Märdian, S. Traumatic cardiac dislocation—A case report and review of the literature including a new classification system. J. Trauma Acute Care Surg. 2019, 87, 944–953. [Google Scholar] [CrossRef]
- Vaccaro, A.R.; Oner, C.; Kepler, C.K.; Dvorak, M.; Schnake, K.; Bellabarba, C.; Reinhold, M.; Aarabi, B.; Kandziora, F.; Chapman, J.; et al. AOSpine thoracolumbar spine injury classification system: Fracture description, neurological status, and key modifiers. Spine 2013, 38, 2028–2037. [Google Scholar] [CrossRef]
- Nyland, B.A.; Spilman, S.K.; Halub, M.E.; Lamb, K.D.; Jackson, J.A.; Oetting, T.W.; Sahr, S.M. A Preventative Respiratory Protocol to Identify Trauma Subjects at Risk for Respiratory Compromise on a General In-Patient Ward. Respir. Care 2016, 61, 1580–1587. [Google Scholar] [CrossRef]
- Kerr-Valentic, M.A.; Arthur, M.; Mullins, R.J.; Pearson, T.E.; Mayberry, J.C. Rib fracture pain and disability: Can we do better? J. Trauma 2003, 54, 1058–1064. [Google Scholar] [CrossRef]
- Çlnar, H.U.; Çelik, B. Comparison of Surgical Stabilization Time in Patients with Flail Chest. Thorac. Cardiovasc. Surg. 2020, 68, 743–751. [Google Scholar] [CrossRef]
- Simmonds, A.; Smolen, J.; Ciurash, M.; Alexander, K.; Alwatari, Y.; Wolfe, L.; Whelan, J.F.M.; Bennett, J.M.; Leichtle, S.W.M.; Aboutanos, M.B.M.; et al. Early surgical stabilization of rib fractures for flail chest is associated with improved patient outcomes: An ACS-TQIP review. J. Trauma Acute Care Surg. 2023, 94, 532–537. [Google Scholar] [CrossRef]
- Zhu, R.C.; de Roulet, A.; Ogami, T.; Khariton, K.D. Rib fixation in geriatric trauma: Mortality benefits for the most vulnerable patients. J. Trauma Acute Care Surg. 2020, 89, 103–110. [Google Scholar] [CrossRef]
- Granhed, H.P.; Pazooki, D. A feasibility study of 60 consecutive patients operated for unstable thoracic cage. J. Trauma Manag. Outcomes 2014, 8, 20. [Google Scholar] [CrossRef] [PubMed]
- Pieracci, F.M.; Lin, Y.; Rodil, M.; Synder, M.; Herbert, B.; Tran, D.K.; Stoval, R.T.; Johnson, J.L.; Biffl, W.L.; Barnett, C.C.; et al. A prospective, controlled clinical evaluation of surgical stabilization of severe rib fractures. J. Trauma Acute Care Surg. 2016, 80, 187–194. [Google Scholar] [CrossRef]
- Granetzny, A.; El-Aal, M.A.; Emam, E.; Shalaby, A.; Boseila, A. Surgical versus conservative treatment of flail chest. Evaluation of the pulmonary status. Interact. Cardiovasc. Thorac. Surg. 2005, 4, 583–587. [Google Scholar] [CrossRef]
- Slobogean, G.P.; Kim, H.; Russell, J.P.; Stockton, D.J.; Hsieh, A.H.; O’toole, R.V. Rib Fracture Fixation Restores Inspiratory Volume and Peak Flow in a Full Thorax Human Cadaveric Breathing Model. Arch. Trauma Res. 2015, 4, e28018. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Kayali, M.K.D.; Böning, G.; Mewes, M.G.; Braun, K.F.; Steinecke, K.; Neumann, K.; Stöckle, U.; Jaecker, V.; Niemann, M. Impact of Surgical Stabilization of Flail Chest Injuries on Postoperative Computed Tomography Lung Volumes. J. Clin. Med. 2025, 14, 3644. https://doi.org/10.3390/jcm14113644
El Kayali MKD, Böning G, Mewes MG, Braun KF, Steinecke K, Neumann K, Stöckle U, Jaecker V, Niemann M. Impact of Surgical Stabilization of Flail Chest Injuries on Postoperative Computed Tomography Lung Volumes. Journal of Clinical Medicine. 2025; 14(11):3644. https://doi.org/10.3390/jcm14113644
Chicago/Turabian StyleEl Kayali, Moses K. D., Georg Böning, Moritz Günther Mewes, Karl F. Braun, Karin Steinecke, Konrad Neumann, Ulrich Stöckle, Vera Jaecker, and Marcel Niemann. 2025. "Impact of Surgical Stabilization of Flail Chest Injuries on Postoperative Computed Tomography Lung Volumes" Journal of Clinical Medicine 14, no. 11: 3644. https://doi.org/10.3390/jcm14113644
APA StyleEl Kayali, M. K. D., Böning, G., Mewes, M. G., Braun, K. F., Steinecke, K., Neumann, K., Stöckle, U., Jaecker, V., & Niemann, M. (2025). Impact of Surgical Stabilization of Flail Chest Injuries on Postoperative Computed Tomography Lung Volumes. Journal of Clinical Medicine, 14(11), 3644. https://doi.org/10.3390/jcm14113644






