Long-Term Outcomes of Sacral Neuromodulation for Refractory Interstitial Cystitis/Bladder Pain Syndrome: A Retrospective Cohort Study
Abstract
1. Introduction
Role of Sacral Neuromodulation in IC/BPS and Mechanisms of Action
2. Materials and Methods
Data Analysis
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AUA | American Urological Association |
| BTX-A | Botulinum Toxin A |
| BPS | Bladder Pain Syndrome |
| CGIC | Clinical Global Impression of Change |
| CUA | Canadian Urological Association |
| CUAJ | Canadian Urological Association Journal |
| EQ-5D-5L | EuroQol Five-Dimension, Five-Level Questionnaire |
| ESSIC | International Society for the Study of Bladder Pain Syndrome |
| HLD | Hunner Lesion Disease |
| IC | Interstitial Cystitis |
| IC/BPS | Interstitial Cystitis/Bladder Pain Syndrome |
| MME | Morphine Milligram Equivalent |
| NRS | Numeric Rating Scale |
| NSAIDs | Non-Steroidal Anti-Inflammatory Drugs |
| RCTs | Randomized Controlled Trials |
| SNM | Sacral Neuromodulation |
| SUFU | Society for Urodynamics and Female Urology |
References
- Hanno, P.; Cervigni, M.; Choo, M.S.; Clemens, J.Q.; Lee, M.-H.; Malde, S.; Meijlink, J.; Samarinas, M.; Ueda, T.; Gold, D. Summary of the 2023 report of the international consultation on incontinence interstitial cystitis/bladder pain syndrome (IC/BPS) committee. Continence 2023, 8, 101056. [Google Scholar] [CrossRef]
- Nitti, V.W. Urodynamics, Incontinence, and Neurourology: Highlights from the Society for Urodynamics and Female Urology Annual Winter Meeting, February 28-March 2, 2008, Miami, FL. Rev. Urol. 2008, 10, 229–231. [Google Scholar] [PubMed]
- Malde, S.; Palmisani, S.; Al-Kaisy, A.; Sahai, A. Guideline of guidelines: Bladder pain syndrome. BJU Int. 2018, 122, 729–743. [Google Scholar] [CrossRef]
- Clemens, J.Q.; Erickson, D.R.; Varela, N.P.; Lai, H.H. Diagnosis and Treatment of Interstitial Cystitis/Bladder Pain Syndrome. J. Urol. 2022, 208, 34–42. [Google Scholar] [CrossRef]
- Suskind, A.M.; Berry, S.H.; Ewing, B.; Elliott, M.N.; Booth, M.; Clemens, J.Q. The prevalence and overlap of interstitial cystitis/bladder pain syndrome and chronic prostatitis/chronic pelvic pain syndrome in men: Results of the RAND Interstitial Cystitis Epidemiology male study. J. Urol. 2013, 189, 141–145. [Google Scholar] [CrossRef]
- Simon, L.J.; Landis, J.R.; Erickson, D.R.; Nyberg, L.M. The Interstitial Cystitis Data Base Study: Concepts and preliminary baseline descriptive statistics. Urology 1997, 49 (Suppl. S5A), 64–75. [Google Scholar] [CrossRef]
- Whitmore, K.E.; Fall, M.; Sengiku, A.; Tomoe, H.; Logadottir, Y.; Kim, Y.H. Hunner lesion versus non-Hunner lesion interstitial cystitis/bladder pain syndrome. Int. J. Urol. 2019, 26 (Suppl. S1), 26–34. [Google Scholar] [CrossRef]
- Fall, M.; Nordling, J.; Cervigni, M.; Oliveira, P.D.; Fariello, J.; Hanno, P.; Kåbjörn-Gustafsson, C.; Logadottir, Y.; Meijlink, J.; Mishra, N.; et al. Hunner lesion disease differs in diagnosis, treatment and outcome from bladder pain syndrome: An ESSIC working group report. Scand. J. Urol. 2020, 54, 91–98. [Google Scholar] [CrossRef]
- Cox, A.; Golda, N.; Nadeau, G.; Nickel, J.C.; Carr, L.; Corcos, J.; Teichman, J. CUA guideline: Diagnosis and treatment of interstitial cystitis/bladder pain syndrome. Can. Urol. Assoc. J. 2016, 10, e136–e155. [Google Scholar] [CrossRef]
- Van de Merwe, J.P.; Nordling, J.; Bouchelouche, P.; Bouchelouche, K.; Cervigni, M.; Daha, L.K.; Elneil, S.; Fall, M.; Hohlbrugger, G.; Irwin, P.; et al. Diagnostic criteria, classification, and nomenclature for painful bladder syndrome/interstitial cystitis: An ESSIC proposal. Eur. Urol. 2008, 53, 60–67. [Google Scholar] [CrossRef]
- Homma, Y.; Ueda, T.; Tomoe, H.; Lin, A.T.L.; Kuo, H.-C.; Lee, M.-H.; Lee, J.G.; Kim, D.Y.; Lee, K.-S.; The interstitial cystitis guideline committee. Clinical guidelines for interstitial cystitis and hypersensitive bladder syndrome. Int. J. Urol. 2009, 16, 597–615. [Google Scholar] [CrossRef] [PubMed]
- Homma, Y. Interstitial cystitis, bladder pain syndrome, hypersensitive bladder, and interstitial cystitis/bladder pain syndrome-clarification of definitions and relationships. Int. J. Urol. 2019, 26 (Suppl. S1), 20–24. [Google Scholar] [CrossRef]
- Akiyama, Y.; Luo, Y.; Hanno, P.M.; Maeda, D.; Homma, Y. Interstitial cystitis/bladder pain syndrome: The evolving landscape, animal models and future perspectives. Int. J. Urol. 2020, 27, 491–503. [Google Scholar] [CrossRef]
- Peters, K.M.; Konstandt, D. Sacral neuromodulation decreases narcotic requirements in refractory interstitial cystitis. BJU Int. 2004, 93, 777–779. [Google Scholar] [CrossRef]
- Feloney, M.P.; Stauss, K.; Leslie, S.W. Sacral Neuromodulation. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Wang, J.; Chen, Y.; Chen, J.; Zhang, G.; Wu, P. Sacral Neuromodulation for Refractory Bladder Pain Syndrome/Interstitial Cystitis: A Global Systematic Review and Meta-analysis. Sci. Rep. 2017, 7, 11031. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Y.; Chen, J.; Zhang, G.; Wu, P. Sacral neuromodulation treating chronic pelvic pain: A meta-analysis and systematic review of the literature. Int. Urogynecol. J. 2019, 30, 1023–1035. [Google Scholar]
- Srivastava, D. Efficacy of sacral neuromodulation in treating chronic pain related to painful bladder syndrome/interstitial cystitis in adults. J. Anaesthesiol. Clin. Pharmacol. 2012, 28, 428–435. [Google Scholar] [CrossRef]
- Kendall, H.J.; Schrijvers, J.; Heesakkers, J. Current position of neuromodulation for bladder pain syndrome/interstitial cystitis. Curr. Opin. Urol. 2024, 34, 64–68. [Google Scholar] [CrossRef]
- Tahseen, S. Role of sacral neuromodulation in modern urogynaecology practice: A review of recent literature. Int. Urogynecol. J. 2018, 29, 1081–1091. [Google Scholar] [CrossRef]
- Weissbart, S.J.; Bhavsar, R.; Rao, H.; Wein, A.J.; Detre, J.A.; Arya, L.A.; Smith, A.L. Specific Changes in Brain Activity during Urgency in Women with Overactive Bladder after Successful Sacral Neuromodulation: A Functional Magnetic Resonance Imaging Study. J. Urol. 2018, 200, 382–388. [Google Scholar] [CrossRef]
- Gill, B.C.; Pizarro-Berdichevsky, J.; Bhattacharyya, P.K.; Brink, T.S.; Marks, B.K.; Quirouet, A.; Vasavada, S.P.; Jones, S.E.; Goldman, H.B. Real-Time Changes in Brain Activity during Sacral Neuromodulation for Overactive Bladder. J. Urol. 2017, 198, 1379–1385. [Google Scholar] [CrossRef] [PubMed]
- Melzack, R.; Wall, P.D. Pain mechanisms: A new theory. Science 1965, 150, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, J.N. Visceral pain: The neurophysiological mechanism. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2009; pp. 31–74. [Google Scholar]
- Birder, L.A.; de Groat, W.C. Induction of c-fos expression in spinal neurons by nociceptive and nonnociceptive stimulation of LUT. Am. J. Physiol. 1993, 265 Pt 2, R326–R333. [Google Scholar] [CrossRef]
- Schmidt, R.A.; Senn, E.; Tanagho, E.A. Functional evaluation of sacral nerve root integrity. Report of a technique. Urology 1990, 35, 388–392. [Google Scholar] [CrossRef]
- Janknegt, R.A.; Weil, E.H.; Eerdmans, P.H. Improving neuromodulation technique for refractory voiding dysfunctions: Two-stage implant. Urology 1997, 49, 358–362. [Google Scholar] [CrossRef]
- Spinelli, M.; Weil, E.; Ostardo, E.; Del Popolo, G.; Ruiz-Cerdá, J.L.; Kiss, G.; Heesakkers, J. New tined lead electrode in sacral neuromodulation: Experience from a multicentre European study. World J. Urol. 2005, 23, 225–229. [Google Scholar] [CrossRef]
- Feler, C.A.; Whitworth, L.A.; Brookoff, D.; Powell, R. Recent Advances: Sacral Nerve Root Stimulation Using a Retrograde Method of Lead Insertion for the Treatment of Pelvic Pain due to Interstitial Cystitis. Neuromodulation 1999, 2, 211–216. [Google Scholar] [CrossRef]
- Zermann, D.-H.; Weirich, T.; Wunderlich, H.; Reichelt, O.; Schubert, J. Sacral nerve stimulation for pain relief in interstitial cystitis. Urol. Int. 2000, 65, 120–121. [Google Scholar] [CrossRef]
- Kütükoğlu, M.U.; Altuntaş, T.; Şahin, B.; Onur, A.R. Sacral neuromodulation treatment for urinary voiding dysfunctions: Results of treatment with the largest single-center series in a tertiary referral center in Turkey. Turk. J. Med. Sci. 2023, 53, 206–210. [Google Scholar] [CrossRef]
- Van Kerrebroeck, P.E.; Marcelissen, T.A. Sacral neuromodulation for lower urinary tract dysfunction. World J. Urol. 2012, 30, 445–450. [Google Scholar] [CrossRef]
- Cappellano, F.; Bertapelle, P.; Spinelli, M.; Catanzaro, F.; Carone, R.; Zanollo, A.; de Seta, F.; Giardiello, G.; For the Italian Group of Sacral Neuromodulation (GINS). Quality of life assessment in patients who undergo sacral neuromodulation implantation for urge incontinence: An additional tool for evaluating outcome. J. Urol. 2001, 166, 2277–2280. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Hernández, D.; Padilla-Fernández, B.; Romera, M.C.; Medler, S.H.; Castro-Díaz, D. Long-term Outcomes of Sacral Nerve Stimulation in Pelvic Floor Dysfunctions. Int. Neurourol. J. 2021, 25, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhang, J.Z.; Wu, L.Y.; Niu, H.Q.; Yang, Y.B.; Zhang, X.D. Short-term outcome of sacral neuromodulation on refractory interstitial cystitis/pelvic pain syndrome. Zhonghua Yi Xue Za Zhi 2016, 96, 3875–3878. [Google Scholar]
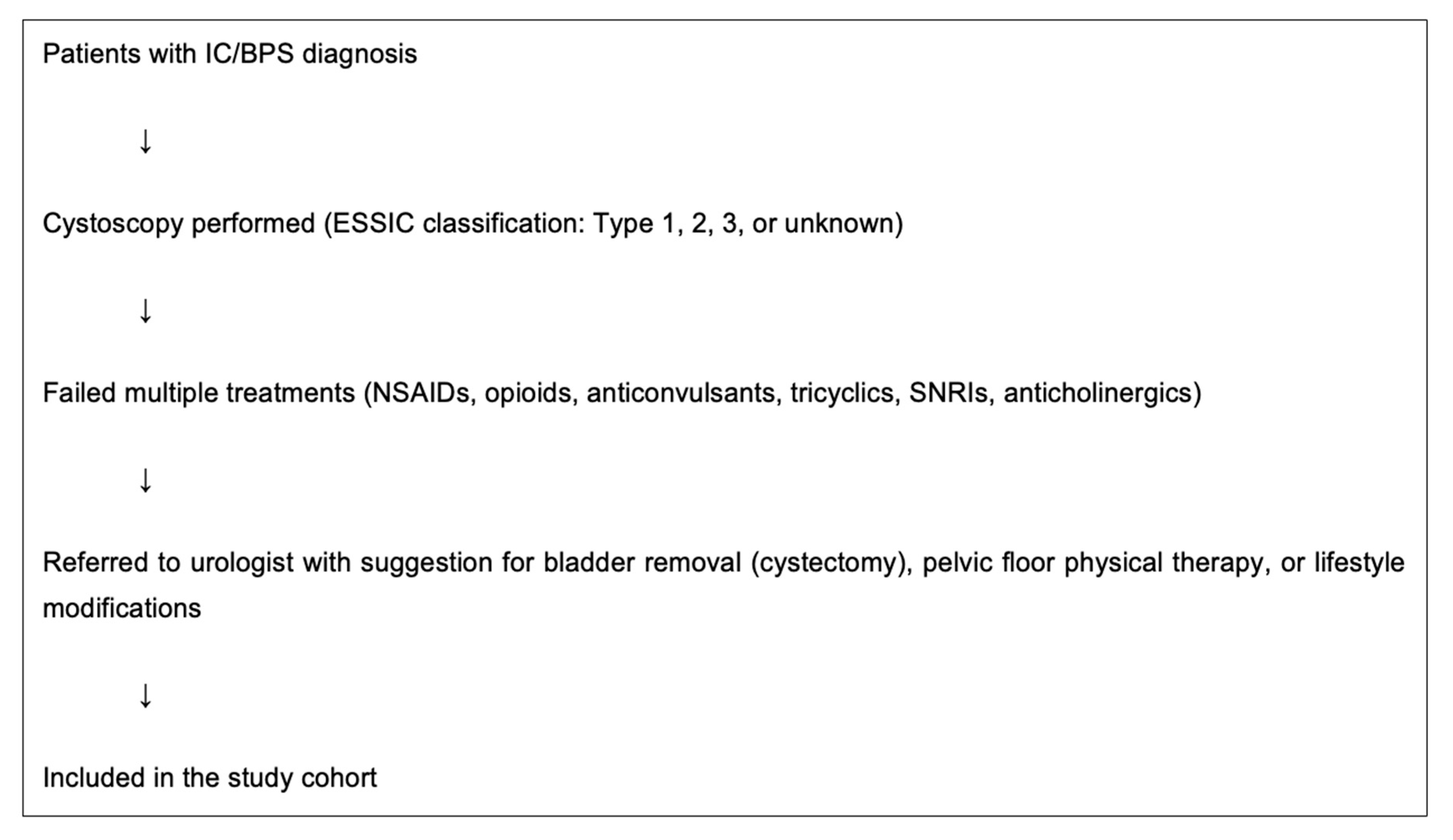


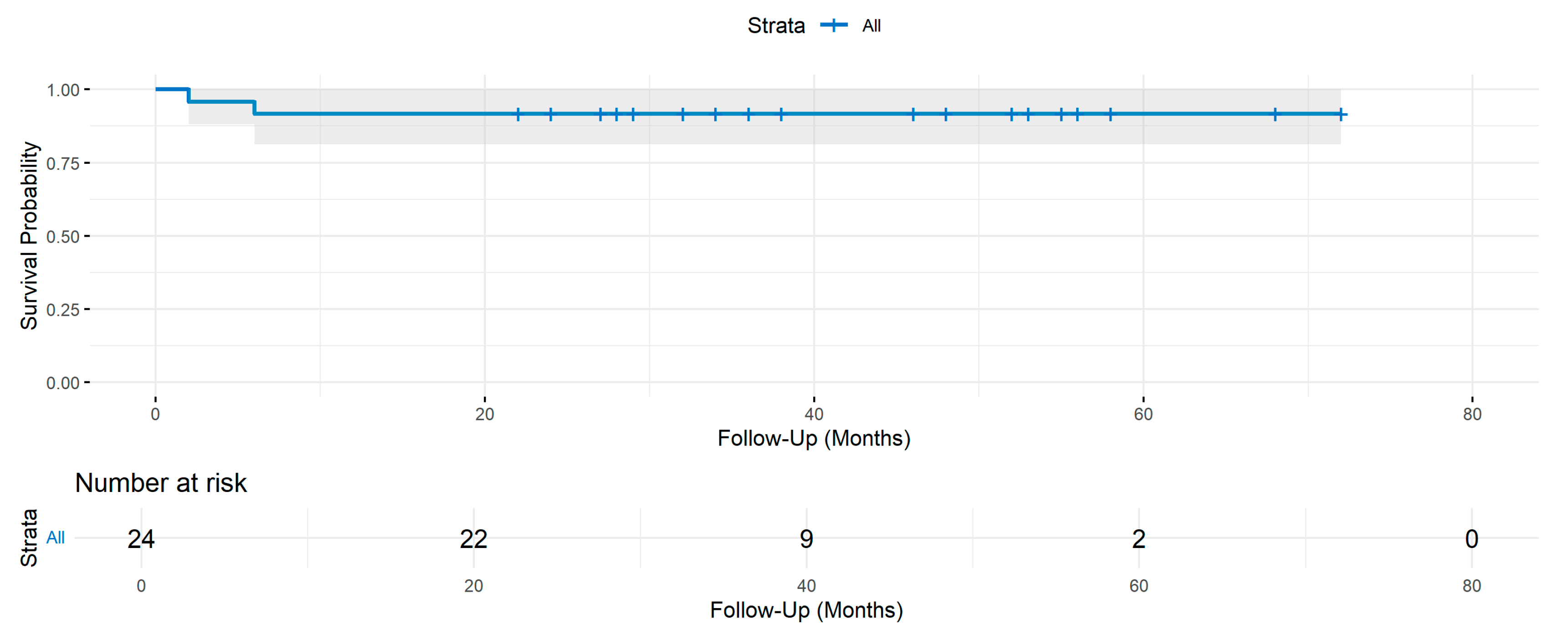
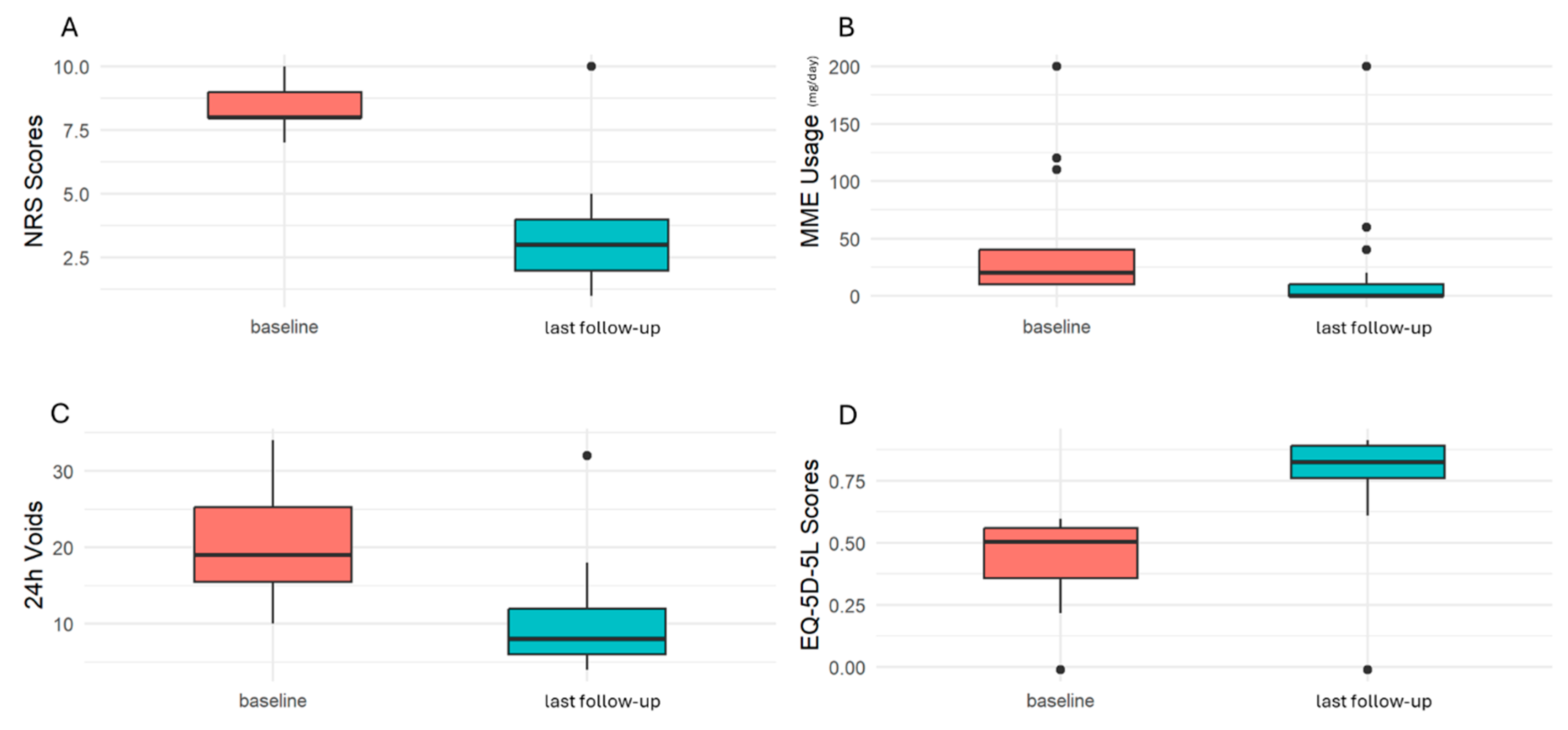

| Variable | IC/BPS Group, n = 24, n (%) |
|---|---|
| Age, median (IQR), years | 54.5 (47–61) |
| Gender | |
| Male | 2 (8%) |
| Female | 22 (92%) |
| IC/BPS subtype according to biopsy | |
| Type 1 (normal bladder wall) | 2 (8.3%) |
| Type 2 (glomerulations) | 11 (45.8%) |
| Type 3 (Hunner’s lesions) | 9 (37.6%) |
| Unknown type | 2 (8.3%) |
| Pain duration, median (IQR), years | 4.5 (3–7.3) |
| Comorbidities | |
| Irritable bowel syndrome | 8 (33.3%) |
| Migraine | 6 (25%) |
| Bacterial cystitis | 10 (41.7%) |
| Rheumatoid arthritis | 4 (16.7%) |
| Fibromyalgia | 4 (16.7%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rekatsina, M.; Leoni, M.L.G.; Visser-Vandewalle, V.; Mercieri, M.; Varrassi, G.; Matis, G. Long-Term Outcomes of Sacral Neuromodulation for Refractory Interstitial Cystitis/Bladder Pain Syndrome: A Retrospective Cohort Study. J. Clin. Med. 2025, 14, 3647. https://doi.org/10.3390/jcm14113647
Rekatsina M, Leoni MLG, Visser-Vandewalle V, Mercieri M, Varrassi G, Matis G. Long-Term Outcomes of Sacral Neuromodulation for Refractory Interstitial Cystitis/Bladder Pain Syndrome: A Retrospective Cohort Study. Journal of Clinical Medicine. 2025; 14(11):3647. https://doi.org/10.3390/jcm14113647
Chicago/Turabian StyleRekatsina, Martina, Matteo Luigi Giuseppe Leoni, Veerle Visser-Vandewalle, Marco Mercieri, Giustino Varrassi, and Georgios Matis. 2025. "Long-Term Outcomes of Sacral Neuromodulation for Refractory Interstitial Cystitis/Bladder Pain Syndrome: A Retrospective Cohort Study" Journal of Clinical Medicine 14, no. 11: 3647. https://doi.org/10.3390/jcm14113647
APA StyleRekatsina, M., Leoni, M. L. G., Visser-Vandewalle, V., Mercieri, M., Varrassi, G., & Matis, G. (2025). Long-Term Outcomes of Sacral Neuromodulation for Refractory Interstitial Cystitis/Bladder Pain Syndrome: A Retrospective Cohort Study. Journal of Clinical Medicine, 14(11), 3647. https://doi.org/10.3390/jcm14113647






