Traumatic Spinal Cord Injury: Review of the Literature
Abstract
1. Introduction
2. Epidemiology and Complications
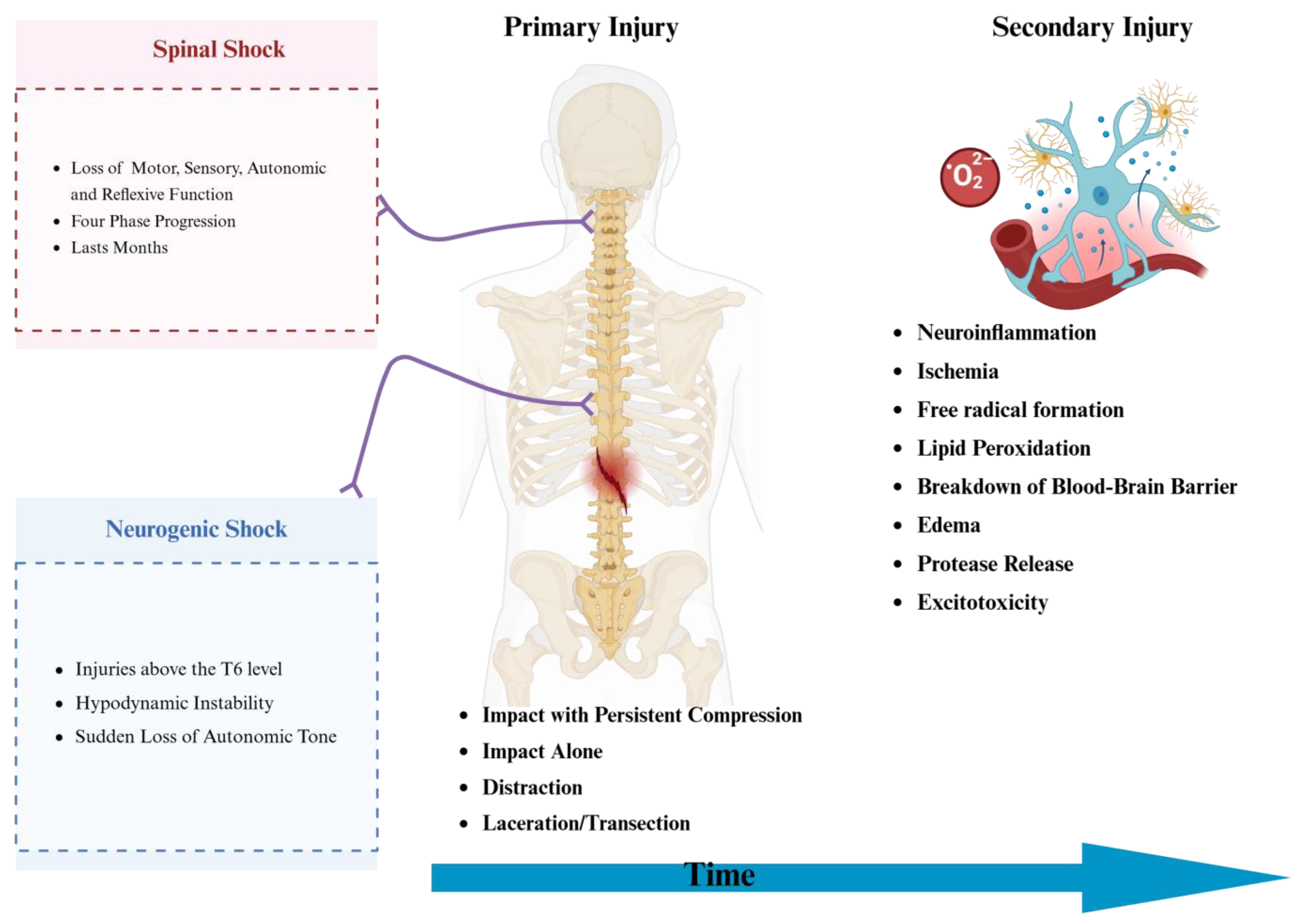
3. Pathophysiology of Presentation and Shock
4. Evaluation
5. Medical Management
5.1. Role of Corticosteroids
5.2. Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)
5.3. Monosialotetrahexosylganglioside (GM-1)
5.4. Anti-CD11d Antibodies
5.5. Hepatocyte Growth Factor (HGF)
5.6. Macrophage Transplantation
5.7. Fibroblast Growth Factors (FGFs)
5.8. Granulocyte Colony-Stimulating Factor (G-CSF)
5.9. Minocycline
5.10. Chondroitinase ABC (ChABC) Enzyme
5.11. Neuroimmunophilin Ligands
5.12. Anti-Nogo-A Antibodies (ATI-355)
5.13. Rho/ROCK Inhibitors (VX-210/Cethrin/BA-210, C3 Transferase, Fasudil, Y27632)
5.14. B-Cell Depletion Therapies
5.15. Riluzole
5.16. Stem Cell Therapy for tSCI
5.17. Extracellular Vesicle Therapy for tSCI
6. Surgical Management
6.1. Spinal Cord Cooling
6.2. Functional Electrical Stimulation (FES)
6.3. Spinal Cord Stimulation (SCS)
6.4. Cerebrospinal Fluid Drainage (CSFD)
6.5. Tissue Scaffolding
7. Bowel Management and Diet
8. Urological Considerations
9. VTE Prophylaxis
10. Mental Health
11. Mobility and Discharge Disposition
12. Summary of Recommendations
Author Contributions
Funding
Conflicts of Interest
References
- Ding, W.; Hu, S.; Wang, P.M.; Kang, H.; Peng, R.; Dong, Y.; Li, F. Spinal cord injury: The global incidence, prevalence, and disability from the global burden of disease study 2019. Spine 2022, 47, 1532–1540. [Google Scholar] [CrossRef] [PubMed]
- Eli, I.; Lerner, D.P.; Ghogawala, Z. Acute traumatic spinal cord injury. Neurol. Clin. 2021, 39, 471–488. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.B.; Ayers, G.D.; Peterson, E.N.; Harris, M.B.; Morse, L.; O’connor, K.C.; Garshick, E. Traumatic spinal cord injury in the United States, 1993–2012. JAMA 2015, 313, 2236–2243. [Google Scholar] [CrossRef] [PubMed]
- Safdarian, M.; Trinka, E.; Rahimi-Movaghar, V.; Thomschewski, A.; Aali, A.; Abady, G.G.; Abate, S.M.; Abd-Allah, F.; Abedi, A.; Adane, D.E.; et al. Global, regional, and national burden of spinal cord injury, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2023, 22, 1026–1047. [Google Scholar] [CrossRef]
- Chen, Y.; He, Y.; DeVivo, M.J. Changing demographics and injury profile of new traumatic spinal cord injuries in the United States, 1972–2014. Arch. Phys. Med. Rehabil. 2016, 97, 1610–1619. [Google Scholar] [CrossRef]
- DeVivo, M.J.; Chen, Y.; Wen, H. Cause of death trends among persons with spinal cord injury in the United States: 1960–2017. Arch. Phys. Med. Rehabil. 2022, 103, 634–641. [Google Scholar] [CrossRef]
- Post, M.W.; Reinhardt, J.D.; Avellanet, M.; Escorpizo, R.; Schwegler, U.; Leiulfsrud, A.S.; Engkasan, J.P.; Middleton, J.W.; Stucki, G.; Brach, M.; et al. Employment Among People With Spinal Cord Injury in 22 Countries Across the World: Results From the International Spinal Cord Injury Community Survey. Arch. Phys. Med. Rehabil. 2020, 101, 2157–2166. [Google Scholar] [CrossRef]
- Wang, T.Y.; Park, C.; Zhang, H.; Rahimpour, S.; Murphy, K.R.; Goodwin, C.R.; Karikari, I.O.; Than, K.D.; Shaffrey, C.I.; Foster, N.; et al. Management of Acute Traumatic Spinal Cord Injury: A Review of the Literature. Front. Surg. 2021, 8, 698736. [Google Scholar] [CrossRef]
- El Masry, W.S.; Tsubo, M.; Katoh, S.; El Miligui, Y.H.S.; Khan, A. Validation of the American Spinal Injury Association (ASIA) Motor Score and the National Acute Spinal Cord Injury Study (NASCIS) Motor Score. Spine 1996, 21, 614–619. [Google Scholar] [CrossRef]
- Rupp, R.; Biering-Sørensen, F.; Burns, S.P.; Graves, D.E.; Guest, J.; Jones, L.; Read, M.S.; Rodriguez, G.M.; Schuld, C.; Tansey-Md, K.E.; et al. International standards for neurological classification of spinal cord injury: Revised 2019. Top. Spinal Cord Inj. Rehabil. 2021, 27, 1. [Google Scholar] [CrossRef]
- Ahuja, C.S.; Nori, S.; Tetreault, L.; Wilson, J.; Kwon, B.; Harrop, J.; Choi, D.; Fehlings, M.G. Traumatic spinal cord injury. Nat. Rev. Dis. Primers 2017, 3, 17018. [Google Scholar] [CrossRef] [PubMed]
- Roberts, T.T.; Leonard, G.R.; Cepela, D.J. Classifications In Brief: American Spinal Injury Association (ASIA) Impairment Scale. Clin. Orthop. Relat. Res. 2017, 475, 1499–1504. [Google Scholar] [CrossRef] [PubMed]
- Sherwood, A.M.; Dimitrijevic, M.R.; McKay, W.B. Evidence of subclinical brain influence in clinically complete spinal cord injury: Discomplete SCI. J. Neurol. Sci. 1992, 110, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Naglah, A.; Aslan, S.; Khalifa, F.; El-Baz, A.; Harkema, S.; D’Amico, J. Preservation of functional descending input to paralyzed upper extremity muscles in motor complete cervical spinal cord injury. Clin. Neurophysiol. 2023, 150, 56–68. [Google Scholar] [CrossRef]
- Awad, A.; Levi, R.; Waller, M.; Westling, G.; Lindgren, L.; Eriksson, J. Preserved somatosensory conduction in complete spinal cord injury: Discomplete SCI. Clin. Neurophysiol. 2020, 131, 1059–1067. [Google Scholar] [CrossRef]
- Wrigley, P.J.; Siddall, P.J.; Gustin, S.M. New evidence for preserved somatosensory pathways in complete spinal cord injury: A fMRI study. Hum. Brain Mapp. 2018, 39, 588–598. [Google Scholar] [CrossRef]
- Wahlgren, C.; Levi, R.; Amezcua, S.; Thorell, O.; Thordstein, M. Prevalence of discomplete sensorimotor spinal cord injury as evidenced by neurophysiological methods: A cross-sectional study. J. Rehabil. Med. 2021, 53, jrm00156. [Google Scholar] [CrossRef]
- Militskova, A.; Mukhametova, E.; Fatykhova, E.; Sharifullin, S.; Cuellar, C.A.; Calvert, J.S.; Grahn, P.J.; Baltina, T.; Lavrov, I. Supraspinal and Afferent Signaling Facilitate Spinal Sensorimotor Network Excitability After Discomplete Spinal Cord Injury: A Case Report. Front. Neurosci. 2020, 14, 552. [Google Scholar] [CrossRef]
- Ditunno, J.F.; Little, J.W.; Tessler, A.; Burns, A.S. Spinal shock revisited: A four-phase model. Spinal Cord. 2004, 42, 383–395. [Google Scholar] [CrossRef]
- Chio, J.C.T.; Xu, K.J.; Popovich, P.; David, S.; Fehlings, M.G. Neuroimmunological therapies for treating spinal cord injury: Evidence and future perspectives. Exp. Neurol. 2021, 341, 113704. [Google Scholar] [CrossRef]
- Lambert, C.; Cisternas, P.; Inestrosa, N.C. Role of Wnt signaling in central nervous system injury. Mol. Neurobiol. 2016, 53, 2297–2311. [Google Scholar] [CrossRef] [PubMed]
- Yates, A.G.; Anthony, D.C.; Ruitenberg, M.J.; Couch, Y. Systemic immune response to traumatic CNS injuries—Are extracellular vesicles the missing link? Front. Immunol. 2019, 10, 2723. [Google Scholar] [CrossRef]
- Eckert, M.J.; Martin, M.J. Trauma: Spinal cord injury. Surg. Clin. 2017, 97, 1031–1045. [Google Scholar] [CrossRef]
- Divi, S.N.; Schroeder, G.D.; Oner, F.C.; Kandziora, F.; Schnake, K.J.; Dvorak, M.F.; Benneker, L.M.; Chapman, J.R.; Vaccaro, A.R. AOSpine—Spine trauma classification system: The value of modifiers: A narrative review with commentary on evolving descriptive principles. Glob. Spine J. 2019, 9 (Suppl. S1), 77S–88S. [Google Scholar] [CrossRef] [PubMed]
- Weisbrod, L.J.; Nilles-Melchert, T.T.; Bergjord, J.R.; Surdell, D.L. Granulocyte-Colony Stimulating Factor Improves Neurological and Functional Outcomes in Patients With Traumatic Incomplete Spinal Cord Injuries: A Systematic Review With Meta-Analyses. Neurotrauma Rep. 2024, 5, 467–482. [Google Scholar] [CrossRef]
- Sterner, R.C.; Sterner, R.M. Immune response following traumatic spinal cord injury: Pathophysiology and therapies. Front. Immunol. 2022, 13, 1084101. [Google Scholar] [CrossRef]
- Bracken, M.B.; Collins, W.F.; Freeman, D.F.; Shepard, M.J.; Wagner, F.W.; Silten, R.M.; Hellenbrand, K.G.; Ransohoff, J.; Hunt, W.E.; Perot, P.L.; et al. Efficacy of methylprednisolone in acute spinal cord injury. JAMA 1984, 251, 45–52. [Google Scholar] [CrossRef]
- Bracken, M.B.; Shepard, M.J.; Collins, W.F.; Holford, T.R.; Young, W.; Baskin, D.S.; Eisenberg, H.M.; Flamm, E.; Leo-Summers, L.; Maroon, J.; et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury: Results of the Second National Acute Spinal Cord Injury Study. N. Engl. J. Med. 1990, 322, 1405–1411. [Google Scholar] [CrossRef]
- Bracken, M.B.; Shepard, M.J.; Holford, T.R.; Leo-Summers, L.; Aldrich, E.F.; Fazl, M.; Fehlings, M.; Herr, D.L.; Hitchon, P.W.; Marshall, L.F.; et al. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury. Results of the Third National Acute Spinal Cord Injury Randomized Controlled Trial. National Acute Spinal Cord Injury Study. JAMA 1997, 277, 1597–1604. [Google Scholar] [CrossRef]
- Geisler, F.H.; Moghaddamjou, A.; Wilson, J.R.F.; Fehlings, M.G. Methylprednisolone in acute traumatic spinal cord injury: Case-matched outcomes from the NASCIS2 and Sygen historical spinal cord injury studies with contemporary statistical analysis. J. Neurosurg. Spine 2023, 38, 595–606. [Google Scholar] [CrossRef]
- Hurlbert, R.J.; Hadley, M.N.; Walters, B.C.; Aarabi, B.; Dhall, S.S.; Gelb, D.E.; Rozzelle, C.J.; Ryken, T.C.; Theodore, N. Pharmacological therapy for acute spinal cord injury. Neurosurgery 2013, 72 (Suppl. S2), 93–105. [Google Scholar] [CrossRef] [PubMed]
- Fehlings, M.G.; Wilson, J.R.; Tetreault, L.A.; Aarabi, B.; Anderson, P.; Arnold, P.M.; Brodke, D.S.; Burns, A.S.; Chiba, K.; Dettori, J.R.; et al. A Clinical Practice Guideline for the Management of Patients With Acute Spinal Cord Injury: Recommendations on the Use of Methylprednisolone Sodium Succinate. Glob. Spine J. 2017, 7 (Suppl. S7), 203S–211S. [Google Scholar] [CrossRef] [PubMed]
- Hayta, E.; Elden, H. Acute spinal cord injury: A review of pathophysiology and potential of non-steroidal anti-inflammatory drugs for pharmacological intervention. J. Chem. Neuroanat. 2018, 87, 25–31. [Google Scholar] [CrossRef]
- Lambrechts, M.J.; Cook, J.L. Nonsteroidal Anti-Inflammatory Drugs and Their Neuroprotective Role After an Acute Spinal Cord Injury: A Systematic Review of Animal Models. Glob. Spine J. 2021, 11, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Al Mamun, A.; Yuan, Y.; Lu, Q.; Xiong, J.; Yang, S.; Wu, C.; Wu, Y.; Wang, J. Acute spinal cord injury: Pathophysiology and pharmacological intervention. Mol. Med. Rep. 2021, 23, 1–18. [Google Scholar] [CrossRef]
- Geisler, F.; Dorsey, F.; Coleman, W. Recovery of motor function after spinal-cord injury—A randomized, placebo-controlled trial with GM-1 ganglioside. N. Engl. J. Med. 1991, 325, 1659–1660. [Google Scholar] [CrossRef]
- Geisler, F.H.; Coleman, W.P.; Grieco, G.; Poonian, D.; Group, S.S. The Sygen® multicenter acute spinal cord injury study. Spine 2001, 26, S87–S98. [Google Scholar] [CrossRef] [PubMed]
- Chinnock, P.; Roberts, I. Gangliosides for acute spinal cord injury. Cochrane Database Syst. Rev. 2005, 2010, CD004444. [Google Scholar] [CrossRef]
- Chu, H.; Gao, J. Treatment effects of monosialotetrahexosylganglioside on severe traumatic brain injury in adults. Am. J. Transl. Res. 2022, 14, 6638–6646. [Google Scholar]
- Nagoshi, N.; Tsuji, O.; Kitamura, K.; Suda, K.; Maeda, T.; Yato, Y.; Abe, T.; Hayata, D.; Matsumoto, M.; Okano, H.; et al. Phase I/II Study of Intrathecal Administration of Recombinant Human Hepatocyte Growth Factor in Patients with Acute Spinal Cord Injury: A Double-Blind, Randomized Clinical Trial of Safety and Efficacy. J. Neurotrauma 2020, 37, 1752–1758. [Google Scholar] [CrossRef]
- Knoller, N.; Auerbach, G.; Fulga, V.; Zelig, G.; Attias, J.; Bakimer, R.; Marder, J.B.; Yoles, E.; Belkin, M.; Schwartz, M.; et al. Clinical experience using incubated autologous macrophages as a treatment for complete spinal cord injury: Phase I study results. J. Neurosurg. Spine 2005, 3, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Lammertse, D.P.; Jones, L.A.T.; Charlifue, S.B.; Kirshblum, S.C.; Apple, D.F.; Ragnarsson, K.T.; Falci, S.P.; Heary, R.F.; Choudhri, T.F.; Jenkins, A.L.; et al. Autologous incubated macrophage therapy in acute, complete spinal cord injury: Results of the phase 2 randomized controlled multicenter trial. Spinal Cord 2012, 50, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liang, Z.; Lin, Y.; Rao, J.; Lin, F.; Yang, Z.; Wang, R.; Chen, C. Comparing the efficacy and safety of cell transplantation for spinal cord injury: A systematic review and Bayesian network meta-analysis. Front. Cell. Neurosci. 2022, 16, 860131. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, E.; Dickman, R. Growth factors and their peptide mimetics for treatment of traumatic brain injury. Bioorganic Med. Chem. 2023, 90, 117368. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, L.; James, L.; Hubert, C.; Dennis, M. SUN13837 in Treatment of Acute Spinal Cord Injury, the ASCENT-ASCI Study. Clin. Neurol. Neurosci. 2017, 2, 1. [Google Scholar] [CrossRef]
- Shultz, R.B.; Zhong, Y. Minocycline targets multiple secondary injury mechanisms in traumatic spinal cord injury. Neural Regen. Res. 2017, 12, 702–713. [Google Scholar] [CrossRef]
- Yune, T.Y.; Lee, J.Y.; Jung, G.Y.; Kim, S.J.; Jiang, M.H.; Kim, Y.C.; Oh, Y.J.; Markelonis, G.J.; Oh, T.H. Minocycline alleviates death of oligodendrocytes by inhibiting pro-nerve growth factor production in microglia after spinal cord injury. J. Neurosci. 2007, 27, 7751–7761. [Google Scholar] [CrossRef]
- An, J.; Jiang, X.; Wang, Z.; Li, Y.; Zou, Z.; Wu, Q.; Tong, L.; Mei, X.; Tian, H.; Wu, C. Codelivery of minocycline hydrochloride and dextran sulfate via bionic liposomes for the treatment of spinal cord injury. Int. J. Pharm. 2022, 628, 122285. [Google Scholar] [CrossRef]
- Aras, M.; Altas, M.; Motor, S.; Dokuyucu, R.; Yilmaz, A.; Ozgiray, E.; Seraslan, Y.; Yilmaz, N. Protective effects of minocycline on experimental spinal cord injury in rats. Injury 2015, 46, 1471–1474. [Google Scholar] [CrossRef]
- Pourkhodadad, S.; Oryan, S.; Hadipour, M.M.; Kaka, G.; Sadraie, S.H. Minocycline Enhance the Restorative Ability of Olfactory Ensheathing Cells by the Upregulation of BDNF and GDNF Expression After Spinal Cord Injury. Basic Clin. Neurosci. 2021, 12, 777–788. [Google Scholar] [CrossRef]
- Wei, Y.; Andrews, M.R. Advances in chondroitinase delivery for spinal cord repair. J. Integr. Neurosci. 2022, 21, 118. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, E.J.; Carter, L.M. Manipulating the glial scar: Chondroitinase ABC as a therapy for spinal cord injury. Brain Res. Bull. 2011, 84, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.Z.; Granger, N.; Pai, S.B.; Bellamkonda, R.V.; Jeffery, N.D. Therapeutic efficacy of microtube-embedded chondroitinase ABC in a canine clinical model of spinal cord injury. Brain 2018, 141, 1017–1027. [Google Scholar] [CrossRef]
- Wang, D.; Ichiyama, R.M.; Zhao, R.; Andrews, M.R.; Fawcett, J.W. Chondroitinase combined with rehabilitation promotes recovery of forelimb function in rats with chronic spinal cord injury. J. Neurosci. 2011, 31, 9332–9344. [Google Scholar] [CrossRef]
- Gold, B.G. Neuroimmunophilin ligands: Evaluation of their therapeutic potential for the treatment of neurological disorders. Expert Opin. Investig. Drugs 2000, 9, 2331–2342. [Google Scholar] [CrossRef]
- Al Mamun, A.; Monalisa, I.; Kubra, K.T.; Akter, A.; Akter, J.; Sarker, T.; Munir, F.; Wu, Y.; Jia, C.; Taniya, M.A.; et al. Advances in immunotherapy for the treatment of spinal cord injury. Immunobiology 2021, 226, 152033. [Google Scholar] [CrossRef] [PubMed]
- Hausch, F. FKBPs and their role in neuronal signaling. Biochim. Biophys. Acta 2015, 1850, 2035–2040. [Google Scholar] [CrossRef]
- Gao, S.-J.; Liu, Y.; Wang, H.-J.; Ban, D.-X.; Cheng, S.-Z.; Ning, G.-Z.; Wang, L.-L.; Chang, J.; Feng, S.-Q. New approach to treating spinal cord injury using PEG-TAT-modified, cyclosporine-A-loaded PLGA/polymeric liposomes. J. Drug Target. 2017, 25, 75–82. [Google Scholar] [CrossRef]
- Ineichen, B.V.; Kapitza, S.; Bleul, C.; Good, N.; Plattner, P.S.; Seyedsadr, M.S.; Kaiser, J.; Schneider, M.P.; Zörner, B.; Martin, R.; et al. Nogo-A antibodies enhance axonal repair and remyelination in neuro-inflammatory and demyelinating pathology. Acta Neuropathol. 2017, 134, 423–440. [Google Scholar] [CrossRef]
- Schneider, M.P.; Sartori, A.M.; Ineichen, B.V.; Moors, S.; Engmann, A.K.; Hofer, A.-S.; Weinmann, O.; Kessler, T.M.; Schwab, M.E. Anti-Nogo-A Antibodies As a Potential Causal Therapy for Lower Urinary Tract Dysfunction after Spinal Cord Injury. J. Neurosci. 2019, 39, 4066–4076. [Google Scholar] [CrossRef]
- Freund, P.; Wannier, T.; Schmidlin, E.; Bloch, J.; Mir, A.; Schwab, M.E.; Rouiller, E.M. Anti-Nogo-A antibody treatment enhances sprouting of corticospinal axons rostral to a unilateral cervical spinal cord lesion in adult macaque monkey. J. Comp. Neurol. 2007, 502, 644–659. [Google Scholar] [CrossRef] [PubMed]
- Kucher, K.; Johns, D.; Maier, D.; Abel, R.; Badke, A.; Baron, H.; Thietje, R.; Casha, S.; Meindl, R.; Gomez-Mancilla, B.; et al. First-in-Man Intrathecal Application of Neurite Growth-Promoting Anti-Nogo-A Antibodies in Acute Spinal Cord Injury. Neurorehabil. Neural Repair. 2018, 32, 578–589. [Google Scholar] [CrossRef] [PubMed]
- Maynard, G.; Kannan, R.; Liu, J.; Wang, W.; Lam, T.K.T.; Wang, X.; Adamson, C.; Hackett, C.; Schwab, J.M.; Liu, C.; et al. Soluble Nogo-Receptor-Fc decoy (AXER-204) in patients with chronic cervical spinal cord injury in the USA: A first-in-human and randomised clinical trial. Lancet Neurol. 2023, 22, 672–684. [Google Scholar] [CrossRef]
- Lee, J.K.; Geoffroy, C.G.; Chan, A.F.; Tolentino, K.E.; Crawford, M.J.; Leal, M.A.; Kang, B.; Zheng, B. Assessing spinal axon regeneration and sprouting in Nogo-, MAG-, and OMgp-deficient mice. Neuron 2010, 66, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Cafferty, W.B.; Duffy, P.; Huebner, E.; Strittmatter, S.M. MAG and OMgp synergize with Nogo-A to restrict axonal growth and neurological recovery after spinal cord trauma. J. Neurosci. 2010, 30, 6825–6837. [Google Scholar] [CrossRef]
- Forgione, N.; Fehlings, M.G. Rho-ROCK inhibition in the treatment of spinal cord injury. World Neurosurg. 2014, 82, e535–e539. [Google Scholar] [CrossRef]
- Giraldo, E.; Bonilla, P.; Mellado, M.; Garcia-Manau, P.; Rodo, C.; Alastrue, A.; Lopez, E.; Moratonas, E.C.; Pellise, F.; Đorđević, S.; et al. Transplantation of Human-Fetal-Spinal-Cord-Derived NPCs Primed with a Polyglutamate-Conjugated Rho/Rock Inhibitor in Acute Spinal Cord Injury. Cells 2022, 11, 3304. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Jiang, C.; Chen, Z.; Ni, S.; Fan, H.; Wang, Z.; Tian, F.; An, J.; Yang, H.; et al. Rho Kinase Inhibitor Y27632 Improves Recovery After Spinal Cord Injury by Shifting Astrocyte Phenotype and Morphology via the ROCK/NF-κB/C3 Pathway. Neurochem. Res. 2022, 47, 3733–3744. [Google Scholar] [CrossRef]
- Fehlings, M.G.; Theodore, N.; Harrop, J.; Maurais, G.; Kuntz, C.; Shaffrey, C.I.; Kwon, B.K.; Chapman, J.; Yee, A.; Tighe, A.; et al. A phase I/IIa clinical trial of a recombinant Rho protein antagonist in acute spinal cord injury. J. Neurotrauma 2011, 28, 787–796. [Google Scholar] [CrossRef]
- Fehlings, M.G.; Chen, Y.; Aarabi, B.; Ahmad, F.; Anderson, K.D.; Dumont, T.; Fourney, D.R.; Harrop, J.S.; Kim, K.D.; Kwon, B.K.; et al. A Randomized Controlled Trial of Local Delivery of a Rho Inhibitor (VX-210) in Patients with Acute Traumatic Cervical Spinal Cord Injury. J. Neurotrauma 2021, 38, 2065–2072. [Google Scholar] [CrossRef]
- Wu, B.; Matic, D.; Djogo, N.; Szpotowicz, E.; Schachner, M.; Jakovcevski, I. Improved regeneration after spinal cord injury in mice lacking functional T- and B-lymphocytes. Exp. Neurol. 2012, 237, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Pellkofer, H.; Krumbholz, M.; Berthele, A.; Hemmer, B.; Gerdes, L.; Havla, J.; Bittner, R.; Canis, M.; Meinl, E.; Hohlfeld, R.; et al. Long-term follow-up of patients with neuromyelitis optica after repeated therapy with rituximab. Neurology 2011, 76, 1310–1315. [Google Scholar] [CrossRef] [PubMed]
- Kap, Y.S.; Bauer, J.; van Driel, N.; Bleeker, W.K.; Parren, P.W.; Kooi, E.-J.; Geurts, J.J.; Laman, J.D.; Craigen, J.L.; Blezer, E.; et al. B-cell depletion attenuates white and gray matter pathology in marmoset experimental autoimmune encephalomyelitis. J. Neuropathol. Exp. Neurol. 2011, 70, 992–1005. [Google Scholar] [CrossRef]
- Carnasciali, A.; Amoriello, R.; Bonechi, E.; Mazzoni, A.; Ravagli, C.; Doumett, S.; Cappiello, L.; D’elios, M.M.; Baldi, G.; Ballerini, C. T Cell Delivery of Nanoparticles-Bound Anti-CD20 Monoclonal Antibody: Successful B Cell Depletion in the Spinal Cord during Experimental Autoimmune Encephalomyelitis. J. Neuroimmune Pharmacol. 2021, 16, 376–389. [Google Scholar] [CrossRef]
- Wilson, J.R.; Fehlings, M.G. Riluzole for acute traumatic spinal cord injury: A promising neuroprotective treatment strategy. World Neurosurg. 2014, 81, 825–829. [Google Scholar] [CrossRef]
- Xu, S.; Wu, Q.; Zhang, W.; Liu, T.; Zhang, Y.; Zhang, W.; Zhang, Y.; Chen, X. Riluzole Promotes Neurite Growth in Rats after Spinal Cord Injury through the GSK-3β/CRMP-2 Pathway. Biol. Pharm. Bull. 2022, 45, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Caglar, Y.S.; Demirel, A.; Dogan, I.; Huseynov, R.; Eroglu, U.; Ozgural, O.; Cansiz, C.; Bahadir, B.; Kilinc, M.C.; Al-Beyati, E.S. Effect of Riluzole on Spinal Cord Regeneration with Hemisection Method Before Injury. World Neurosurg. 2018, 114, e247–e253. [Google Scholar] [CrossRef]
- Tetreault, L.A.; Zhu, M.P.; Wilson, J.R.; Karadimas, S.K.; Fehlings, M.G. The Impact of Riluzole on Neurobehavioral Outcomes in Preclinical Models of Traumatic and Nontraumatic Spinal Cord Injury: Results From a Systematic Review of the Literature. Glob. Spine J. 2020, 10, 216–229. [Google Scholar] [CrossRef]
- Grossman, R.G.; Fehlings, M.G.; Frankowski, R.F.; Burau, K.D.; Chow, D.S.; Tator, C.; Teng, A.; Toups, E.G.; Harrop, J.S.; Aarabi, B.; et al. A prospective, multicenter, phase I matched-comparison group trial of safety, pharmacokinetics, and preliminary efficacy of riluzole in patients with traumatic spinal cord injury. J. Neurotrauma 2014, 31, 239–255. [Google Scholar] [CrossRef]
- Fehlings, M.G.; Moghaddamjou, A.; Harrop, J.S.; Stanford, R.; Ball, J.R.; Aarabi, B.; Freeman, B.J.C.; Arnold, P.M.; Guest, J.D.; Kurpad, S.N.; et al. Safety and Efficacy of Riluzole in Acute Spinal Cord Injury Study (RISCIS): A Multi-Center, Randomized, Placebo-Controlled, Double-Blinded Trial. J. Neurotrauma 2023, 40, 1878–1888. [Google Scholar] [CrossRef]
- Hu, X.; Xu, W.; Ren, Y.; Wang, Z.; He, X.; Huang, R.; Ma, B.; Zhao, J.; Zhu, R.; Cheng, L. Spinal cord injury: Molecular mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2023, 8, 245. [Google Scholar] [CrossRef] [PubMed]
- Shao, A.; Tu, S.; Lu, J.; Zhang, J. Crosstalk between stem cell and spinal cord injury: Pathophysiology and treatment strategies. Stem Cell Res. Ther. 2019, 10, 238. [Google Scholar] [CrossRef]
- Curtis, E.; Martin, J.R.; Gabel, B.; Sidhu, N.; Rzesiewicz, T.K.; Mandeville, R.; Van Gorp, S.; Leerink, M.; Tadokoro, T.; Marsala, S.; et al. A First-in-Human, Phase I Study of Neural Stem Cell Transplantation for Chronic Spinal Cord Injury. Cell Stem Cell. 2018, 22, 941–950.e6. [Google Scholar] [CrossRef] [PubMed]
- Levi, A.D.; Anderson, K.D.; Okonkwo, D.O.; Park, P.; Bryce, T.N.; Kurpad, S.N.; Aarabi, B.; Hsieh, J.; Gant, K. Clinical Outcomes from a Multi-Center Study of Human Neural Stem Cell Transplantation in Chronic Cervical Spinal Cord Injury. J. Neurotrauma 2019, 36, 891–902. [Google Scholar] [CrossRef]
- Curt, A.; Hsieh, J.; Schubert, M.; Hupp, M.; Friedl, S.; Freund, P.; Huber, E.; Pfyffer, D.; Sutter, R.; Jutzeler, C.; et al. The Damaged Spinal Cord Is a Suitable Target for Stem Cell Transplantation. Neurorehabil. Neural Repair 2020, 34, 758–768. [Google Scholar] [CrossRef] [PubMed]
- All, A.H.; Gharibani, P.; Gupta, S.; Bazley, F.A.; Pashai, N.; Chou, B.-K.; Shah, S.; Resar, L.M.; Cheng, L.; Gearhart, J.D.; et al. Early intervention for spinal cord injury with human induced pluripotent stem cells oligodendrocyte progenitors. PLoS ONE 2015, 10, e0116933. [Google Scholar] [CrossRef]
- Fessler, R.G.; Ehsanian, R.; Liu, C.Y.; Steinberg, G.K.; Jones, L.; Lebkowski, J.S.; Wirth, E.D.; McKenna, S.L. A phase 1/2a dose-escalation study of oligodendrocyte progenitor cells in individuals with subacute cervical spinal cord injury. J. Neurosurg. Spine 2022, 37, 812–820. [Google Scholar] [CrossRef]
- Cofano, F.; Boido, M.; Monticelli, M.; Zenga, F.; Ducati, A.; Vercelli, A.; Garbossa, D. Mesenchymal Stem Cells for Spinal Cord Injury: Current Options, Limitations, and Future of Cell Therapy. Int. J. Mol. Sci. 2019, 20, 2698. [Google Scholar] [CrossRef]
- Dasari, V.R.; Veeravalli, K.K.; Dinh, D.H. Mesenchymal stem cells in the treatment of spinal cord injuries: A review. World J. Stem Cells. 2014, 6, 120–133. [Google Scholar] [CrossRef]
- Bydon, M.; Qu, W.; Moinuddin, F.M.; Hunt, C.L.; Garlanger, K.L.; Reeves, R.K.; Windebank, A.J.; Zhao, K.D.; Jarrah, R.; Trammell, B.C.; et al. Intrathecal delivery of adipose-derived mesenchymal stem cells in traumatic spinal cord injury: Phase I trial. Nat. Commun. 2024, 15, 2201. [Google Scholar] [CrossRef]
- El-Kheir, W.A.; Gabr, H.; Awad, M.R.; Ghannam, O.; Barakat, Y.; Farghali, H.A.M.A.; El Maadawi, Z.M.; Ewes, I.; Sabaawy, H.E. Autologous bone marrow-derived cell therapy combined with physical therapy induces functional improvement in chronic spinal cord injury patients. Cell Transplant. 2014, 23, 729–745. [Google Scholar] [CrossRef] [PubMed]
- Dai, G.; Liu, X.; Zhang, Z.; Yang, Z.; Dai, Y.; Xu, R. Transplantation of autologous bone marrow mesenchymal stem cells in the treatment of complete and chronic cervical spinal cord injury. Brain Res. 2013, 1533, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Albu, S.; Kumru, H.; Coll, R.; Vives, J.; Vallés, M.; Benito-Penalva, J.; Rodríguez, L.; Codinach, M.; Hernández, J.; Navarro, X.; et al. Clinical effects of intrathecal administration of expanded Wharton jelly mesenchymal stromal cells in patients with chronic complete spinal cord injury: A randomized controlled study. Cytotherapy 2021, 23, 146–156. [Google Scholar] [CrossRef]
- Cheng, H.; Liu, X.; Hua, R.; Dai, G.; Wang, X.; Gao, J.; An, Y. Clinical observation of umbilical cord mesenchymal stem cell transplantation in treatment for sequelae of thoracolumbar spinal cord injury. J. Transl. Med. 2014, 12, 253. [Google Scholar] [CrossRef]
- Montoto-Meijide, R.; Meijide-Faílde, R.; Díaz-Prado, S.M.; Montoto-Marqués, A. Mesenchymal Stem Cell Therapy in Traumatic Spinal Cord Injury: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 11719. [Google Scholar] [CrossRef]
- Wang, T.; Huang, G.; Yi, Z.; Dai, S.; Zhuang, W.; Guo, S. Advances in extracellular vesicle-based combination therapies for spinal cord injury. Neural Regen. Res. 2024, 19, 369–374. [Google Scholar] [CrossRef]
- Guo, S.; Perets, N.; Betzer, O.; Ben-Shaul, S.; Sheinin, A.; Michaelevski, I.; Popovtzer, R.; Offen, D.; Levenberg, S. Intranasal Delivery of Mesenchymal Stem Cell Derived Exosomes Loaded with Phosphatase and Tensin Homolog siRNA Repairs Complete Spinal Cord Injury. ACS Nano 2019, 13, 10015–10028. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Li, C.; Kong, G.; Zeng, Q.; Wang, S.; Yin, G.; Gu, J.; Fan, J. Treg cell-derived exosomes miR-709 attenuates microglia pyroptosis and promotes motor function recovery after spinal cord injury. J. Nanobiotechnol. 2022, 20, 529. [Google Scholar] [CrossRef]
- Liu, W.Z.; Ma, Z.J.; Li, J.R.; Kang, X.W. Mesenchymal stem cell-derived exosomes: Therapeutic opportunities and challenges for spinal cord injury. Stem Cell Res. Ther. 2021, 12, 102. [Google Scholar] [CrossRef]
- Xie, Y.; Sun, Y.; Liu, Y.; Zhao, J.; Liu, Q.; Xu, J.; Qin, Y.; He, R.; Yuan, F.; Wu, T.; et al. Targeted Delivery of RGD-CD146(+)CD271(+) Human Umbilical Cord Mesenchymal Stem Cell-Derived Exosomes Promotes Blood-Spinal Cord Barrier Repair after Spinal Cord Injury. ACS Nano 2023, 17, 18008–18024. [Google Scholar] [CrossRef]
- Ren, J.; Zhu, B.; Gu, G.; Zhang, W.; Li, J.; Wang, H.; Wang, M.; Song, X.; Wei, Z.; Feng, S. Schwann cell-derived exosomes containing MFG-E8 modify macrophage/microglial polarization for attenuating inflammation via the SOCS3/STAT3 pathway after spinal cord injury. Cell Death Dis. 2023, 14, 70. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Liu, C.; Chen, X.; Zheng, L.; Zou, Y.; Wen, H.; Guan, P.; Lu, F.; Luo, Y.; Tan, G.; et al. Exosomes-Loaded Electroconductive Hydrogel Synergistically Promotes Tissue Repair after Spinal Cord Injury via Immunoregulation and Enhancement of Myelinated Axon Growth. Adv. Sci. 2022, 9, e2105586. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Gu, G.; Ren, J.; Song, X.; Li, J.; Wang, C.; Zhang, W.; Huo, Y.; Wang, H.; Jin, L.; et al. Schwann Cell-Derived Exosomes and Methylprednisolone Composite Patch for Spinal Cord Injury Repair. ACS Nano 2023, 17, 22928–22943. [Google Scholar] [CrossRef] [PubMed]
- Fang, A.; Wang, Y.; Guan, N.; Zuo, Y.; Lin, L.; Guo, B.; Mo, A.; Wu, Y.; Lin, X.; Cai, W.; et al. Porous microneedle patch with sustained delivery of extracellular vesicles mitigates severe spinal cord injury. Nat. Commun. 2023, 14, 4011. [Google Scholar] [CrossRef]
- Wilson, J.R.; Fehlings, M.G. Emerging Approaches to the Surgical Management of Acute Traumatic Spinal Cord Injury. Neurotherapeutics 2011, 8, 187–194. [Google Scholar] [CrossRef]
- Yoshihara, H. Indirect decompression in spinal surgery. J. Clin. Neurosci. 2017, 44, 63–68. [Google Scholar] [CrossRef]
- Hersh, A.M.; Weber-Levine, C.; Jiang, K.; Theodore, N. Spinal cord injury: Emerging technologies. Neurosurg. Clin. 2024, 35, 243–251. [Google Scholar] [CrossRef]
- Torregrossa, F.; Sallì, M.; Grasso, G. Emerging therapeutic strategies for traumatic spinal cord injury. World Neurosurg. 2020, 140, 591–601. [Google Scholar] [CrossRef]
- Shin, H.K.; Park, J.H.; Roh, S.W.; Jeon, S.R. Meta-Analysis on the Effect of Hypothermia in Acute Spinal Cord Injury. Neurospine 2022, 19, 748–756. [Google Scholar] [CrossRef]
- Badjatia, N. Hyperthermia and fever control in brain injury. Crit. Care Med. 2009, 37, S250–S257. [Google Scholar] [CrossRef]
- Hamid, S.; Hayek, R. Role of electrical stimulation for rehabilitation and regeneration after spinal cord injury: An overview. Eur. Spine J. 2008, 17, 1256–1269. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Xu, H.; Zuo, Y.; Liu, X.; All, A.H. A review of functional electrical stimulation treatment in spinal cord injury. Neuromolecular Med. 2020, 22, 447–463. [Google Scholar] [CrossRef]
- Lee, H.U.; Blasiak, A.; Agrawal, D.R.; Loong, D.T.B.; Thakor, N.V.; All, A.H.; Ho, J.S.; Yang, I.H. Subcellular electrical stimulation of neurons enhances the myelination of axons by oligodendrocytes. PLoS ONE 2017, 12, e0179642. [Google Scholar] [CrossRef]
- Ojha, R.; George, J.; Chandy, B.R.; Tharion, G.; Devasahayam, S.R. Neuromodulation by surface electrical stimulation of peripheral nerves for reduction of detrusor overactivity in patients with spinal cord injury: A pilot study. J. Spinal Cord Med. 2015, 38, 207–213. [Google Scholar] [CrossRef]
- Sun, L.; Peng, C.; Joosten, E.; Cheung, C.W.; Tan, F.; Jiang, W.; Shen, X. Spinal Cord Stimulation and Treatment of Peripheral or Central Neuropathic Pain: Mechanisms and Clinical Application. Neural Plast. 2021, 2021, 5607898. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.H. Neuroplasticity of spinal cord injury and repair. Handb. Clin. Neurol. 2022, 184, 317–330. [Google Scholar] [CrossRef]
- Hofer, A.S.; Schwab, M.E. Enhancing rehabilitation and functional recovery after brain and spinal cord trauma with electrical neuromodulation. Curr. Opin. Neurol. 2019, 32, 828–835. [Google Scholar] [CrossRef]
- Kathe, C.; Skinnider, M.A.; Hutson, T.H.; Regazzi, N.; Gautier, M.; Demesmaeker, R.; Komi, S.; Ceto, S.; James, N.D.; Cho, N.; et al. The neurons that restore walking after paralysis. Nature 2022, 611, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Wagner, F.B.; Mignardot, J.B.; Le Goff-Mignardot, C.G.; Demesmaeker, R.; Komi, S.; Capogrosso, M.; Rowald, A.; Seáñez, I.; Caban, M.; Pirondini, E.; et al. Targeted neurotechnology restores walking in humans with spinal cord injury. Nature 2018, 563, 65–71. [Google Scholar] [CrossRef]
- Inanici, F.; Brighton, L.N.; Samejima, S.; Hofstetter, C.P.; Moritz, C.T. Transcutaneous Spinal Cord Stimulation Restores Hand and Arm Function After Spinal Cord Injury. IEEE Trans. Neural. Syst. Rehabil. Eng. 2021, 29, 310–319. [Google Scholar] [CrossRef]
- Epstein, N.E. Cerebrospinal fluid drains reduce risk of spinal cord injury for thoracic/thoracoabdominal aneurysm surgery: A review. Surg. Neurol. Int. 2018, 9, 48. [Google Scholar] [CrossRef]
- Theodore, N.; Martirosyan, N.; Hersh, A.M.; Ehresman, J.; Ahmed, A.K.; Danielson, J.; Sullivan, C.; Shank, C.D.; Almefty, K.; Lemole, G.M.; et al. Cerebrospinal fluid drainage in patients with acute spinal cord injury: A multi-center randomized controlled trial. World Neurosurg. 2023, 177, e472–e479. [Google Scholar] [CrossRef] [PubMed]
- Martirosyan, N.L.; Kalani, M.Y.S.; Bichard, W.D.; Baaj, A.A.; Gonzalez, L.F.; Preul, M.C.; Theodore, N. Cerebrospinal fluid drainage and induced hypertension improve spinal cord perfusion after acute spinal cord injury in pigs. Neurosurgery 2015, 76, 461–469. [Google Scholar] [CrossRef]
- Kwon, B.K.; Curt, A.; Belanger, L.M.; Bernardo, A.; Chan, D.; Markez, J.A.; Gorelik, S.; Slobogean, G.P.; Umedaly, H.; Giffin, M.; et al. Intrathecal pressure monitoring and cerebrospinal fluid drainage in acute spinal cord injury: A prospective randomized trial. J. Neurosurg. Spine 2009, 10, 181–193. [Google Scholar] [CrossRef]
- Hogg, F.R.; Gallagher, M.J.; Kearney, S.; Zoumprouli, A.; Papadopoulos, M.C.; Saadoun, S. Acute spinal cord injury: Monitoring lumbar cerebrospinal fluid provides limited information about the injury site. J. Neurotrauma 2020, 37, 1156–1164. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tan, J.; Xiao, Z.; Zhao, Y.; Han, S.; Liu, D.; Yin, W.; Li, J.; Li, J.; Wanggou, S.; et al. Transplantation of hUC-MSCs seeded collagen scaffolds reduces scar formation and promotes functional recovery in canines with chronic spinal cord injury. Sci. Rep. 2017, 7, 43559. [Google Scholar] [CrossRef]
- Ralph, P.C.; Choi, S.-W.; Back, M.J.; Lee, S.J. Regenerative Medicine Approaches for the Treatment of Spinal Cord Injuries: Progress and Challenges. Acta Biomater. 2024, 189, 57–72. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Ge, R.; Zhang, C.; Zhao, Z.; Han, L.; Zhang, W.; Yue, W.; Zhang, J.; Zhao, Y.; Hou, S.; et al. Promotion of nerve regeneration and motor function recovery in SCI rats using LOCAS-iPSCs-NSCs. Stem Cell Res. Ther. 2024, 15, 376. [Google Scholar] [CrossRef]
- Xiao, Z.; Tang, F.; Zhao, Y.; Han, G.; Yin, N.; Li, X.; Chen, B.; Han, S.; Jiang, X.; Yun, C.; et al. Significant improvement of acute complete spinal cord injury patients diagnosed by a combined criteria implanted with NeuroRegen scaffolds and mesenchymal stem cells. Cell Transplant. 2018, 27, 907–915. [Google Scholar] [CrossRef]
- Theodore, N.; Hlubek, R.; Danielson, J.; Neff, K.; Vaickus, L.; Ulich, T.R.; Ropper, A.E. First Human Implantation of a Bioresorbable Polymer Scaffold for Acute Traumatic Spinal Cord Injury. Neurosurgery 2016, 79, E305–E312. [Google Scholar] [CrossRef]
- Liu, C.-W.; Huang, C.-C.; Yang, Y.-H.; Chen, S.-C.; Weng, M.-C.; Huang, M.-H. Relationship between neurogenic bowel dysfunction and health-related quality of life in persons with spinal cord injury. J. Rehabil. Med. 2009, 41, 35–40. [Google Scholar] [CrossRef]
- Lynch, A.; Antony, A.; Dobbs, B.; Frizelle, F. Bowel dysfunction following spinal cord injury. Spinal Cord 2001, 39, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Coggrave, M.; Norton, C.; Wilson-Barnett, J. Management of neurogenic bowel dysfunction in the community after spinal cord injury: A postal survey in the United Kingdom. Spinal Cord 2009, 47, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Krassioukov, A.; Eng, J.J.; Claxton, G.; Sakakibara, B.M.; Shum, S. Neurogenic bowel management after spinal cord injury: A systematic review of the evidence. Spinal Cord 2010, 48, 718–733. [Google Scholar] [CrossRef]
- Frisbie, J.H.; Tun, C.G.; Nguyen, C.H. Effect of enterostomy on quality of life in spinal cord injury patients. J. Am. Paraplegia Soc. 1986, 9, 3–5. [Google Scholar] [CrossRef]
- Furlan, J.C.; Urbach, D.R.; Fehlings, M.G. Optimal treatment for severe neurogenic bowel dysfunction after chronic spinal cord injury: A decision analysis. Br. J. Surg. 2007, 94, 1139–1150. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Ou, Y.-C.; Hu, J.-C.; Yang, M.-H.; Lin, W.-Y.; Huang, S.-W.; Lin, C.-C.; Lin, V.C.; Chuang, Y.-C.; Kuo, H.-C. Bladder management strategies for urological complications in patients with chronic spinal cord injury. J. Clin. Med. 2022, 11, 6850. [Google Scholar] [CrossRef] [PubMed]
- Perez, N.E.; Godbole, N.P.; Amin, K.; Syan, R.; Gater, D.R., Jr. Neurogenic bladder physiology, pathogenesis, and management after spinal cord injury. J. Pers. Med. 2022, 12, 968. [Google Scholar] [CrossRef]
- Gould, M.K.; Garcia, D.A.; Wren, S.M.; Karanicolas, P.J.; Arcelus, J.I.; Heit, J.A.; Samama, C.M. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic therapy and prevention of thrombosis: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012, 141, e227S. [Google Scholar] [CrossRef]
- Christie, S.; Thibault-Halman, G.; Casha, S. Acute pharmacological DVT prophylaxis after spinal cord injury. J. Neurotrauma 2011, 28, 1509–1514. [Google Scholar] [CrossRef]
- Donadini, M.P.; Dentali, F.; Ageno, W.; Marazzi, M.; Bocchi, R.; Imberti, D.; Pierfranceschi, M.G. The short-and long-term risk of venous thromboembolism in patients with acute spinal cord injury. Thromb. Haemost. 2013, 109, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Statements PC-o-I. Prevention of venous thromboembolism in individuals with spinal cord injury: Clinical practice guidelines for health care providers. Top. Spinal Cord Inj. Rehabil. 2016, 22, 209–240. [Google Scholar] [CrossRef] [PubMed]
- Ryce, A.L.; Lee, S.J.; Ahmed, O.; Majdalany, B.S.; Kokabi, N. Contemporary use of prophylactic inferior vena cava filters in patients with severe traumatic injuries and high thromboembolic event risk. J. Am. Coll. Radiol. 2024, 21, 712–720. [Google Scholar] [CrossRef]
- Filipcic, T.; Sember, V.; Pajek, M.; Jerman, J. Quality of life and physical activity of persons with spinal cord injury. Int. J. Environ. Res. Public Health 2021, 18, 9148. [Google Scholar] [CrossRef]
- Post, M.; van Leeuwen, C.M. Psychosocial issues in spinal cord injury: A review. Spinal Cord 2012, 50, 382–389. [Google Scholar] [CrossRef]
- Migliorini, C.; Tonge, B.; Taleporos, G. Spinal cord injury and mental health. Aust. N. Z. J. Psychiatry 2008, 42, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Ginis, K.A.M.; Hicks, A.L.; Latimer, A.E.; Warburton, D.E.R.; Bourne, C.; Ditor, D.S.; Goodwin, D.L.; Hayes, K.C.; McCartney, N.; McIlraith, A.; et al. The development of evidence-informed physical activity guidelines for adults with spinal cord injury. Spinal Cord 2011, 49, 1088–1096. [Google Scholar] [CrossRef]
- Pelletier, C. Exercise prescription for persons with spinal cord injury: A review of physiological considerations and evidence-based guidelines. Appl. Physiol. Nutr. Metab. 2023, 48, 882–895. [Google Scholar] [CrossRef]
- Tordi, N.; Dugue, B.; Klupzinski, D.; Rasseneur, L.; Rouillon, J.; Lonsdorfer, J. Interval training program on a wheelchair ergometer for paraplegic subjects. Spinal Cord 2001, 39, 532–537. [Google Scholar] [CrossRef]
- Eng, J.; Teasell, R.; Miller, W.; Wolfe, D.; Townson, A.; Aubut, J.-A.; Abramson, C.; Hsieh, J.; Connolly, S.; Konnyu, K. Spinal cord injury rehabilitation evidence: Method of the SCIRE systematic review. Top. Spinal Cord Inj. Rehabil. 2007, 13, 1–10. [Google Scholar] [CrossRef]
| A | Complete | No motor or sensory function remains in the sacral segments S4–S5. |
| B | Incomplete | Sensory function is retained below the neurological level, but motor function is absent, including the sacral segments S4–S5. |
| C | Incomplete | Motor function is present below the neurological level, but more than half of the key muscles have a grade lower than 3 |
| D | Incomplete | Motor function is preserved below the neurological level, and at least half of the key muscles below the neurological level have a muscle grade of 3 or higher. |
| E | Normal | Motor and sensory function are normal. |
| Intervention | Research Stage | Findings and Outcomes |
|---|---|---|
| Steroids (methylprednisolone) | Clinical | There is no difference in neurological recovery of motor function or pinprick and light touch sensation. |
| Non-Steroidal Anti-inflammatory Drugs (NSAIDs) | Animal | Benefits only be observed in animal models. No clinical significance in patients. |
| Monosialotetrahexosylganglioside (GM-1) | Clinical | Conflicting results: some studies show improvement in tSCI signs and symptoms, while others fail to provide evidence of effectiveness. |
| Anti-CD11d Antibodies | Animal | Only studied in rat models |
| Fibroblast Growth Factors (FGFs) | Clinical | No significant clinical benefit |
| Macrophage Transplantation | Clinical | No significant difference between macrophage transplantation and placebo group. |
| Granulocyte Colony-Stimulating Factor (G-CSF) | Clinical | G-CSF for the treatment of incomplete tSCI may result in improved neurological outcomes compared to controls [25]. |
| Minocycline | Clinical | No evidence of neurological improvement |
| Chondroitinase ABC (ChABC) Enzyme | Clinical | A randomized controlled study in canine models with chronic tSCI showed a 23% improvement in coordination in the ChABC group, with a subgroup of recipients regaining the ability to walk unassisted. |
| Neuroimmunophilin Ligands | Animal | In vivo rodent model study showed significant improvement in motor function at both four weeks and three months following spinal cord injury. |
| Anti-Nogo-A Antibodies (ATI-355) | Clinical | There is a lack of consensus on whether ATI-355 administration results in any significant motor improvements. |
| Rho/ROCK Inhibitors (VX-210/Cethrin/BA-210, C3 transferase, fasudil, Y27632) | Clinical | Studies did not show remarkable success, but preclinical evidence of the regulatory actions of Rho/ROCK inhibitors may warrant future revisitation of these drugs as adjunct therapies. |
| B-Cell Depletion Therapies | Animal | The use of B-cell depletion therapies to treat tSCI still requires further investigation. |
| Riluzole | Clinical | Studies did not show statistical significance in the primary outcome of upper extremity motor scores. |
| Stem Cell Therapy for tSCI | Clinical | There is no strong evidence to support functional improvement using neural stem cells (NSCs). Administering increasing doses of oligodendrocyte progenitor cells (OPCs) might help with one level of neurological function after one year. Mesenchymal stem cell (MSC) administration results in improved neurological recovery of function compared to rehabilitation alone. |
| Extracellular Vesicle Therapy for tSCI | Clinical | MSC therapy might increase the ASIA sensory score, 14 showed increases in the ASIA motor score, and improve neurophysiological assessment |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zarmer, L.; Khan, M.; Islat, G.; Alameddin, H.; Massey, M.; Chaudhry, R. Traumatic Spinal Cord Injury: Review of the Literature. J. Clin. Med. 2025, 14, 3649. https://doi.org/10.3390/jcm14113649
Zarmer L, Khan M, Islat G, Alameddin H, Massey M, Chaudhry R. Traumatic Spinal Cord Injury: Review of the Literature. Journal of Clinical Medicine. 2025; 14(11):3649. https://doi.org/10.3390/jcm14113649
Chicago/Turabian StyleZarmer, Lori, Maaz Khan, Glenn Islat, Hanan Alameddin, Maria Massey, and Rabail Chaudhry. 2025. "Traumatic Spinal Cord Injury: Review of the Literature" Journal of Clinical Medicine 14, no. 11: 3649. https://doi.org/10.3390/jcm14113649
APA StyleZarmer, L., Khan, M., Islat, G., Alameddin, H., Massey, M., & Chaudhry, R. (2025). Traumatic Spinal Cord Injury: Review of the Literature. Journal of Clinical Medicine, 14(11), 3649. https://doi.org/10.3390/jcm14113649





