High Risk of Chronic Endometritis in Isthmocele—A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Registration of Protocols
2.2. Search Strategy
2.3. Inclusion and Exclusion Criteria
2.4. Data Extraction
2.5. Quality Assessment
2.6. Data Synthesis
3. Results
3.1. Results of the Systematic Review
3.2. Study Characteristics
| First Author, Year of Publication | Study Design | Total Study Population | Number of Participants of Interest (Total) | Number of Participants in Control Group (Total) | Age (y) Mean SD or Median | Age (y) Mean SD or Median (Control Group) | Parameters | Chronic Pain/Pelvic Pain Associated | Infertility Associated (Number/Total) | Endometriosis Associated (Number/Total) | Antibiotic Therapy |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Glukhov E. Yu. et al., 2019 [31] | prospective observational incomparable study | 50 (CSD) | 26/50 (CE) | 24/50 (non CE) | 33.5 | 32.0 | CE group: 14/26 Non CE group: 6/24 | NM | CE group: 15/26 Non CE group: 9/24 | CE group: 10/26 Non CE group: 8/24 | Yes Most patients received intraoperative intravenous Amoxiclav (92%) or ceftriaxone (8%); in cases of severe CE, antibiotics were continued for up to 5 days. |
| Higuchi et al., 2022 [32] | retrospective case-control study | 84 | 63 (CSS) Secondary infertility, LSK repair of CSD | 21 (non-CSS) Underwent HE, with a history of CS | 35.9 ± 3.3 | 43.6 ± 3.3 | CD138, CD3, CD20, CD56, CD 68, CD138, MPO, Tryptase CD138 significantly higher in the CSS group CD138+ plasma cells in 5 HPFs within 2500 μm from cavity side of CSD CD3, CD20, CD68, tryptase was significantly lower in the CSS group | NM | 63/63 (Secondary infertility was inclusion criteria) | NM | no |
| Yang et al., 2021 [33] | case-control study | 16 | 9/16 (CS with CSD) | 7/16 (vaginal delivery) | 32.2 ± 3.5 | 33.8 ± 3.1 | the MIP-1alpha, SDF-1alpha, IL-27, IL-1beta, IL-2, IL-4, IL-5, IP-10, IL-6, IL-7, IL-8, IL-10, Eotaxin, IL-12p70, IL-13, IL-17A, IL-31, IL-1RA, RANTES, IFN-γ, GM-CSF, TNF-α, MIP-1β, IFN- α, MCP-1, IL-9, TNF-β, GRO-α, IL-1α, IL-23, IL-15, IL-18, IL- 21, and IL-22 | NM | 16/16 | NM | NM |
| Nobuta et al., 2022 [34] | retrospective case-control study | 201 | 38 (CSS) | 163 (Non-CSS) | 38 | 37 | CD138, TNF-α, IL-1β ≥1 CD138+ plasma cell per 10 HPFs (random fields) only in endometrial stroma TNF-α (pg/mL): 0.88 (CSS), 0.15 (non-CSS) IL-1β (pg/mL): 17 (CSS), 10 (non-CSS) | NM | 201/201 | 51/73 (only from the CSS group) | No |
| Wei et al., 2022 [28] | retrospective study using propensitiy score matching | 501 | 170 (CSS) | 331 | 34.9 ± 4.3 | 34.98 ± 4.9 | CD138 CE defined by any of the following: (1) >1 plasma cell per HPF, (2) >5 plasma cells per HPF, (3) >1 plasma cell per section, (4) >1 plasma cell per 10 HPFs, or (5) plasmacyte density index ≥ 0.25. | NM | NM | NM | NM |
| Wang et al., 2024 [29] | retrospective case-control study | 155 with CSD (69 AUB, 86 without AUB) | 30 (AUB after PSM) | 30 (non AUB after PSM) | 37.6 ± 2.6 | 35.9 ± 4.6 | CD138, CD 31 ≥1 CD138+ plasma cell per HPFc | NM | 4/15 (AUB) 2/9 (Non-AUB) | Yes not descriptive | |
| Zhang et al., 2024 [30] | retrospective case-control study | 64 | 44 (CSS) | 20 (CS with TLH) | 30.0 ± 4.3 | 30.25 ± 4.8 | CD138 CE defined as ≥1 CD138+ plasma cell per HPF in 10 HPFs. Mild CE: 1 cell/HPF; Severe CE: ≥2 cells/HPF. | 5/44(CSS) | 7/44 (CSS) | NM | NM |
| Selection | Comparability | Outcome | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| First Author, Year of Publication | Representativeness of Exposed Cohort | Selection of Non-Exposed Cohort | Ascertainment of Exposure | Outcome of Interest Not Present at Study Start | Comparability of Cohorts on the Basis of the Design or Analysis Controlled for Confounders | Assessment of Outcome | Sufficient Length of Follow-Up for Outcomes to Occur | Adequacy of Follow-Up of Cohorts | Total | Quality Assessment |
| Glukhov E.Yu. et al., 2019 [31] | ★ | - | ★ | - | - | - | ★ | ★ | (4/8) | poor |
| Higuchi et al., 2021 [32] | ★ | - | ★ | - | - | - | - | - | (3/8) | poor |
| Yang et al., 2021 [33] | ★ | - | - | - | - | - | - | - | (1/8) | poor |
| Nobuta et al., 2022 [34] | ★ | - | ★ | - | ★ | ★ | ★ | ★ | (6/8) | fair |
| Wei et al., 2022 [28] | ★ | ★ | ★ | - | ★ | ★ | ★ | ★ | (7/8) | good |
| Wang et al., 2024 [29] | ★ | ★ | ★ | ★ | ★ | ★ | - | ★ | (7/8) | good |
| Zhang et al., 2024 [30] | ★ | - | ★ | ★ | ★ | ★ | - | ★ | (7/8) | good |
3.3. Results of the Meta-Analysis
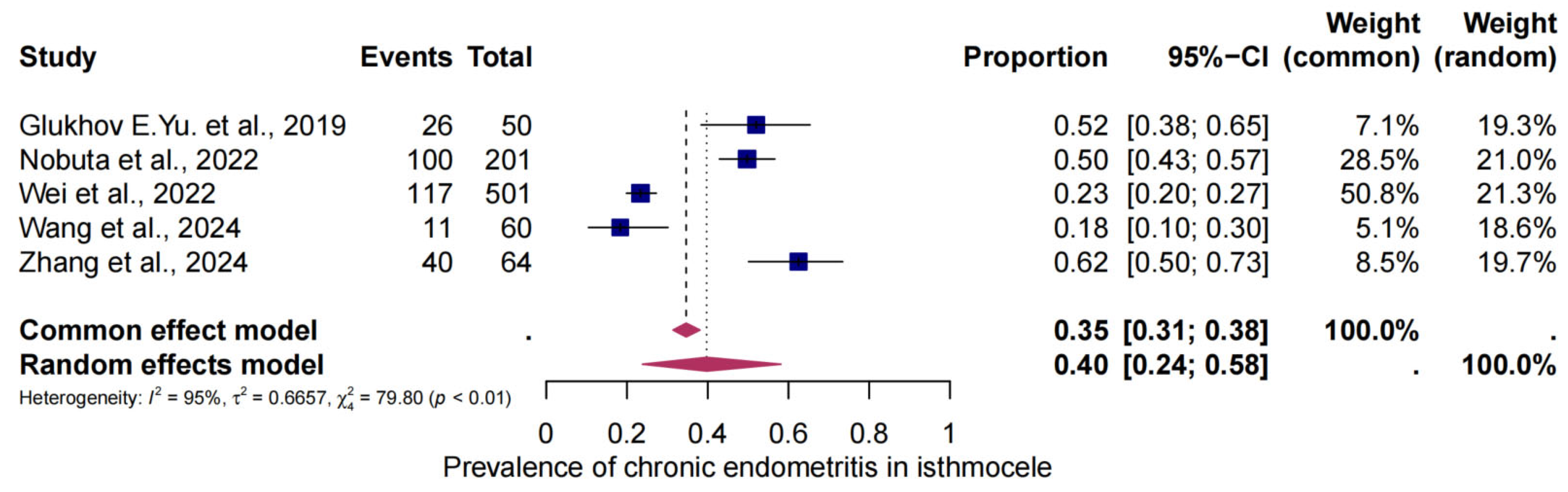
3.4. The Pooled Prevalence of CE in Isthmocele
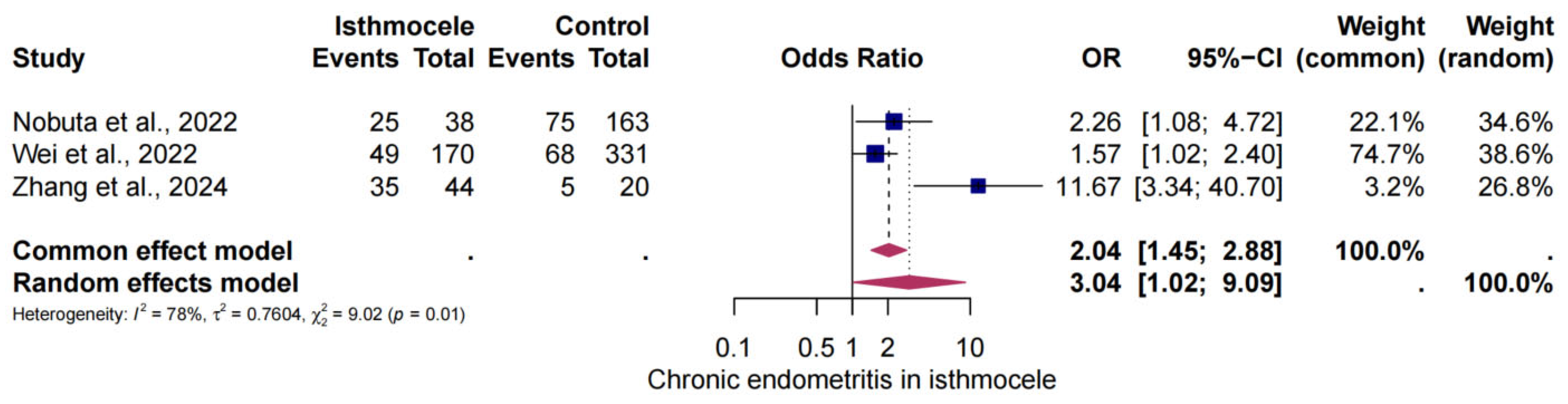
3.5. The Risk of CE in Isthmocele
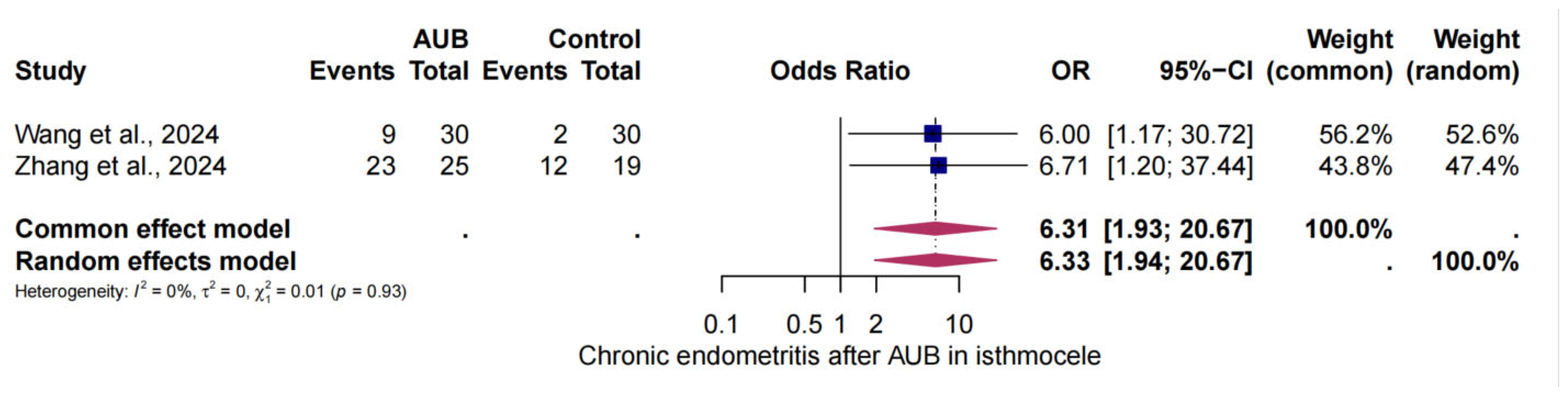
3.6. The Risk of CE in AUB and Isthmocele
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tower, A.M.; Frishman, G.N. Cesarean Scar Defects: An Underrecognized Cause of Abnormal Uterine Bleeding and Other Gynecologic Complications. J. Minim. Invasive Gynecol. 2013, 20, 562–572. [Google Scholar] [CrossRef] [PubMed]
- Bij de Vaate, A.J.M.; Brölmann, H.A.M.; van der Voet, L.F.; van der Slikke, J.W.; Veersema, S.; Huirne, J.A.F. Ultrasound Evaluation of the Cesarean Scar: Relation between a Niche and Postmenstrual Spotting. Ultrasound Obstet. Gynecol. 2011, 37, 93–99. [Google Scholar] [CrossRef] [PubMed]
- van der Voet, L.F.; Bij de Vaate, A.M.; Veersema, S.; Brölmann, H.A.M.; Huirne, J.A.F. Long-Term Complications of Caesarean Section. The Niche in the Scar: A Prospective Cohort Study on Niche Prevalence and Its Relation to Abnormal Uterine Bleeding. BJOG 2014, 121, 236–244. [Google Scholar] [CrossRef]
- Tulandi, T.; Cohen, A. Emerging Manifestations of Cesarean Scar Defect in Reproductive-Aged Women. J. Minim. Invasive Gynecol. 2016, 23, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Betran, A.P.; Ye, J.; Moller, A.-B.; Souza, J.P.; Zhang, J. Trends and Projections of Caesarean Section Rates: Global and Regional Estimates. BMJ Glob. Health 2021, 6, e005671. [Google Scholar] [CrossRef] [PubMed]
- Florio, P.; Filippeschi, M.; Moncini, I.; Marra, E.; Franchini, M.; Gubbini, G. Hysteroscopic Treatment of the Cesarean-Induced Isthmocele in Restoring Infertility. Curr. Opin. Obstet. Gynecol. 2012, 24, 180–186. [Google Scholar] [CrossRef]
- Vachon-Marceau, C.; Demers, S.; Bujold, E.; Roberge, S.; Gauthier, R.J.; Pasquier, J.-C.; Girard, M.; Chaillet, N.; Boulvain, M.; Jastrow, N. Single versus Double-Layer Uterine Closure at Cesarean: Impact on Lower Uterine Segment Thickness at next Pregnancy. Am. J. Obstet. Gynecol. 2017, 217, 65.e1–65.e5. [Google Scholar] [CrossRef]
- Di Spiezio Sardo, A.; Saccone, G.; McCurdy, R.; Bujold, E.; Bifulco, G.; Berghella, V. Risk of Cesarean Scar Defect Following Single- vs. Double-layer Uterine Closure: Systematic Review and Meta-analysis of Randomized Controlled Trials. Ultrasound Obstet. Gynecol. 2017, 50, 578–583. [Google Scholar] [CrossRef]
- Alper, E.; Aksakal, E.; Usta, I.; Urman, B. The Novel Parallel Closure Technique Compared to Single-Layer Closure of the Uterus After Primary Cesarean Section Decreases the Incidence of Isthmocele Formation and Increases Residual Myometrial Thickness. Cureus 2024, 16, e60932. [Google Scholar] [CrossRef]
- Asoglu, M.R.; Celik, C.; Ozturk, E.; Cavkaytar, S.; Bahceci, M. Impact of Isthmocele on Assisted Reproductive Treatment Outcomes: An Age-Matched Retrospective Study. J. Minim. Invasive Gynecol. 2021, 28, 1113–1120. [Google Scholar] [CrossRef]
- Vitagliano, A.; Cicinelli, E.; Viganò, P.; Sorgente, G.; Nicolì, P.; Busnelli, A.; Dellino, M.; Damiani, G.R.; Gerli, S.; Favilli, A. Isthmocele, Not Cesarean Section per Se, Reduces in Vitro Fertilization Success: A Systematic Review and Meta-Analysis of over 10,000 Embryo Transfer Cycles. Fertil. Steril. 2024, 121, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Vidal, A.; Geiger, J.; Vinayahalingam, V.; Pape, J.; Gulz, M.; Karrer, T.; Mueller, M.D.; Von Wolff, M. High Live Birth Rates after Laparoscopic Isthmocele Repair in Infertility: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2025, 16, 1507482. [Google Scholar] [CrossRef]
- Vidal, A.; Bora, C.; Von Holzen, J.; Gulz, M.; Obmann, V.C.; Pape, J.; Karrer, T.; Yilmaz, G.; Von Wolff, M. Cine-MRI for Quantifying Uterine Peristalsis: A Systematic Review and Meta-Analysis. J. Clin. Med. 2025, 14, 1021. [Google Scholar] [CrossRef]
- Vidal, A.; Trejos, V.; Pape, J.; Karrer, T.; Yilmaz, G.; Von Wolff, M. Lower Pregnancy Rate in Women with High Uterine Peristalsis before Embryo Transfer: A Systematic Review and Meta-Analysis. Reprod. Biol. Endocrinol. 2025, 23, 49. [Google Scholar] [CrossRef]
- Vissers, J.; Hehenkamp, W.; Lambalk, C.B.; Huirne, J.A. Post-Caesarean Section Niche-Related Impaired Fertility: Hypothetical Mechanisms. Hum. Reprod. 2020, 35, 1484–1494. [Google Scholar] [CrossRef]
- Baldini, G.M.; Lot, D.; Malvasi, A.; Di Nanni, D.; Laganà, A.S.; Angelucci, C.; Tinelli, A.; Baldini, D.; Trojano, G. Isthmocele and Infertility. J. Clin. Med. 2024, 13, 2192. [Google Scholar] [CrossRef]
- Greenwood, S.M.; Moran, J.J. Chronic Endometritis: Morphologic and Clinical Observations. Obstet. Gynecol. 1981, 58, 176–184. [Google Scholar]
- Bouet, P.-E.; El Hachem, H.; Monceau, E.; Gariépy, G.; Kadoch, I.-J.; Sylvestre, C. Chronic Endometritis in Women with Recurrent Pregnancy Loss and Recurrent Implantation Failure: Prevalence and Role of Office Hysteroscopy and Immunohistochemistry in Diagnosis. Fertil. Steril. 2016, 105, 106–110. [Google Scholar] [CrossRef]
- Kimura, F.; Takebayashi, A.; Ishida, M.; Nakamura, A.; Kitazawa, J.; Morimune, A.; Hirata, K.; Takahashi, A.; Tsuji, S.; Takashima, A.; et al. Review: Chronic Endometritis and Its Effect on Reproduction. J. Obstet. Gynaecol. 2019, 45, 951–960. [Google Scholar] [CrossRef]
- Moreno, O.M.; Paredes, A.C.; Suarez-Obando, F.; Rojas, A. An Update on Fanconi Anemia: Clinical, Cytogenetic and Molecular Approaches (Review). Biomed. Rep. 2021, 15, 74. [Google Scholar] [CrossRef]
- Kitaya, K.; Takeuchi, T.; Mizuta, S.; Matsubayashi, H.; Ishikawa, T. Endometritis: New Time, New Concepts. Fertil. Steril. 2018, 110, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Cicinelli, E.; Matteo, M.; Trojano, G.; Mitola, P.C.; Tinelli, R.; Vitagliano, A.; Crupano, F.M.; Lepera, A.; Miragliotta, G.; Resta, L. Chronic Endometritis in Patients with Unexplained Infertility: Prevalence and Effects of Antibiotic Treatment on Spontaneous Conception. Am. J. Rep. Immunol. 2018, 79, e12782. [Google Scholar] [CrossRef]
- Cicinelli, E.; Matteo, M.; Tinelli, R.; Lepera, A.; Alfonso, R.; Indraccolo, U.; Marrocchella, S.; Greco, P.; Resta, L. Prevalence of Chronic Endometritis in Repeated Unexplained Implantation Failure and the IVF Success Rate after Antibiotic Therapy. Hum. Reprod. 2015, 30, 323–330. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Borissov, N.; Haas, Q.; Minder, B.; Kopp-Heim, D.; von Gernler, M.; Janka, H.; Teodoro, D.; Amini, P. Reducing Systematic Review Burden Using Deduklick: A Novel, Automated, Reliable, and Explainable Deduplication Algorithm to Foster Medical Research. Syst. Rev. 2022, 11, 172. [Google Scholar] [CrossRef]
- Van der Mierden, S.; Tsaioun, K.; Bleich, A.; Leenaars, C.H.C. Software Tools for Literature Screening in Systematic Reviews in Biomedical Research. ALTEX 2019, 36, 508–517. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses; Ottawa Hospital Research Institute: Ottawa, ON, Canada, 2009. [Google Scholar]
- Wei, L.; Xu, C.; Zhao, Y.; Zhang, C. Higher Prevalence of Chronic Endometritis in Women with Cesarean Scar Defect: A Retrospective Study Using Propensity Score Matching. J. Pers. Med. 2022, 13, 39. [Google Scholar] [CrossRef]
- Wang, Y.; Han, Y.; Guo, X.; Wei, Q.; Xia, Y.; Gao, L.; Wang, H.; Lu, X.; Shu, J. Caesarean Scar Endometrial Defects Contribute to Post-Caesarean Abnormal Uterine Bleeding and Chronic Endometritis: A Retrospective Case–Control Study. BJOG 2025, 132, 132–139. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, J.; Xu, Z.; Yang, Q.; Zeng, L.; Zhou, L.; Deng, K. The Correlation between Chronic Endometritis and Caesarean Scar Diverticulum. J. Reprod. Immunol. 2024, 166, 104324. [Google Scholar] [CrossRef]
- Glukhov, E.Y.; Dikke, G.B.; Neff, E.I.; Glukhova, V.E.; Svyazhina, A.V. Chronic Endometritis and an Incompetent Uterine Scar after Cesarean Section: Long-Term Outcomes of Metroplasty. Obstet. Gynecol. 2019, 2019, 126–134. [Google Scholar] [CrossRef]
- Higuchi, A.; Tsuji, S.; Nobuta, Y.; Nakamura, A.; Katsura, D.; Amano, T.; Kimura, F.; Tanimura, S.; Murakami, T. Histopathological Evaluation of Cesarean Scar Defect in Women with Cesarean Scar Syndrome. Reprod. Med. Biol. 2022, 21, e12431. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Pan, X.; Cai, M.; Zhang, B.; Liang, X.; Liu, G. Microbial Flora Changes in Cesarean Section Uterus and Its Possible Correlation with Inflammation. Front. Med. 2021, 8, 651938. [Google Scholar] [CrossRef] [PubMed]
- Nobuta, Y.; Tsuji, S.; Kitazawa, J.; Hanada, T.; Nakamura, A.; Zen, R.; Amano, T.; Murakami, T. Decreased Fertility in Women with Cesarean Scar Syndrome Is Associated with Chronic Inflammation in the Uterine Cavity. Tohoku J. Exp. Med. 2022, 258, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Puente, E.; Alonso, L.; Laganà, A.S.; Ghezzi, F.; Casarin, J.; Carugno, J. Chronic Endometritis: Old Problem, Novel Insights and Future Challenges. Int. J. Fertil. Steril. 2020, 13, 250–256. [Google Scholar] [CrossRef]
- Donnez, O.; Donnez, J.; Orellana, R.; Dolmans, M.-M. Gynecological and Obstetrical Outcomes after Laparoscopic Repair of a Cesarean Scar Defect in a Series of 38 Women. Fertil. Steril. 2017, 107, 289–296.e2. [Google Scholar] [CrossRef]
- Morris, H. Surgical Pathology of the Lower Uterine Segment Caesarean Section Scar: Is the Scar a Source of Clinical Symptoms? Int. J. Gynecol. Pathol. 1995, 14, 16–20. [Google Scholar] [CrossRef]
- Dominguez, J.A.; Pacheco, L.A.; Moratalla, E.; Carugno, J.A.; Carrera, M.; Perez-Milan, F.; Caballero, M.; Alcázar, J.L. Diagnosis and Management of Isthmocele (Cesarean Scar Defect): A SWOT Analysis. Ultrasound Obstet. Gynecol. 2023, 62, 336–344. [Google Scholar] [CrossRef]
- Becker, C.M.; Bokor, A.; Heikinheimo, O.; Horne, A.; Jansen, F.; Kiesel, L.; King, K.; Kvaskoff, M.; Nap, A.; Petersen, K.; et al. ESHRE Guideline: Endometriosis. Hum. Reprod. Open 2022, 2022, hoac009. [Google Scholar] [CrossRef]
- Gulz, M.; Imboden, S.; Nirgianakis, K.; Siegenthaler, F.; Rau, T.T.; Mueller, M.D. Endometriosis and Isthmocele: Common or Rare? J. Clin. Med. 2022, 11, 1158. [Google Scholar] [CrossRef]
- Cicinelli, E.; Matteo, M.; Tinelli, R.; Pinto, V.; Marinaccio, M.; Indraccolo, U.; De Ziegler, D.; Resta, L. Chronic Endometritis Due to Common Bacteria Is Prevalent in Women with Recurrent Miscarriage as Confirmed by Improved Pregnancy Outcome After Antibiotic Treatment. Reprod. Sci. 2014, 21, 640–647. [Google Scholar] [CrossRef]
- Lozano, F.M.; Bernabeu, A.; Lledo, B.; Morales, R.; Diaz, M.; Aranda, F.I.; Llacer, J.; Bernabeu, R. Characterization of the Vaginal and Endometrial Microbiome in Patients with Chronic Endometritis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 263, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Hsu, I.; Hsu, L.; Dorjee, S.; Hsu, C.-C. Bacterial Colonization at Caesarean Section Defects in Women of Secondary Infertility: An Observational Study. BMC Pregnancy Childbirth 2022, 22, 135. [Google Scholar] [CrossRef]
- Tortorella, C.; Piazzolla, G.; Matteo, M.; Pinto, V.; Tinelli, R.; Sabbà, C.; Fanelli, M.; Cicinelli, E. Interleukin-6, Interleukin-1β, and Tumor Necrosis Factor α in Menstrual Effluents as Biomarkers of Chronic Endometritis. Fertil. Steril. 2014, 101, 242–247. [Google Scholar] [CrossRef]
- Song, D.; Feng, X.; Zhang, Q.; Xia, E.; Xiao, Y.; Xie, W.; Li, T.C. Prevalence and Confounders of Chronic Endometritis in Premenopausal Women with Abnormal Bleeding or Reproductive Failure. Reprod. Biomed. Online 2018, 36, 78–83. [Google Scholar] [CrossRef]
- Cheng, X.; Huang, Z.; Xiao, Z.; Bai, Y. Does Antibiotic Therapy for Chronic Endometritis Improve Clinical Outcomes of Patients with Recurrent Implantation Failure in Subsequent IVF Cycles? A Systematic Review and Meta-Analysis. J. Assist. Reprod. Genet. 2022, 39, 1797–1813. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Z.A.; Liu, Y.; Cheng, L.; Yan, L. Impact of Antibiotic Treatment for Chronic Endometritis on Pregnancy Outcomes in Women with Reproductive Failures (RIF and RPL): A Systematic Review and Meta-Analysis. Front. Med. 2022, 9, 980511. [Google Scholar] [CrossRef]
- Di Gennaro, F.; Guido, G.; Frallonardo, L.; Pennazzi, L.; Bevilacqua, M.; Locantore, P.; Vitagliano, A.; Saracino, A.; Cicinelli, E. Chronic Endometritis and Antimicrobial Resistance: Towards a Multidrug-Resistant Endometritis? An Expert Opinion. Microorganisms 2025, 13, 197. [Google Scholar] [CrossRef]
- Strug, M.R.; Hartup, L.A.; Ryan, E.; Lathi, R.B. Unveiling the Silver Lining: A Narrative Review of Clinical Evaluation and Management of Chronic Endometritis and Its Impact on Fertility. FS Rev. 2024, 5, 100073. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vidal, A.; Pape, J.; Vinayahalingam, V.; Gulz, M.; Karrer, T.; von Wolff, M. High Risk of Chronic Endometritis in Isthmocele—A Systematic Review and Meta-Analysis. J. Clin. Med. 2025, 14, 3628. https://doi.org/10.3390/jcm14113628
Vidal A, Pape J, Vinayahalingam V, Gulz M, Karrer T, von Wolff M. High Risk of Chronic Endometritis in Isthmocele—A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2025; 14(11):3628. https://doi.org/10.3390/jcm14113628
Chicago/Turabian StyleVidal, Angela, Janna Pape, Vithusha Vinayahalingam, Marietta Gulz, Tanya Karrer, and Michael von Wolff. 2025. "High Risk of Chronic Endometritis in Isthmocele—A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 14, no. 11: 3628. https://doi.org/10.3390/jcm14113628
APA StyleVidal, A., Pape, J., Vinayahalingam, V., Gulz, M., Karrer, T., & von Wolff, M. (2025). High Risk of Chronic Endometritis in Isthmocele—A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 14(11), 3628. https://doi.org/10.3390/jcm14113628