Consensus-Based Recommendations for Assessing Post-Intensive Care Syndrome: A Systematic Review
Abstract
1. Introduction
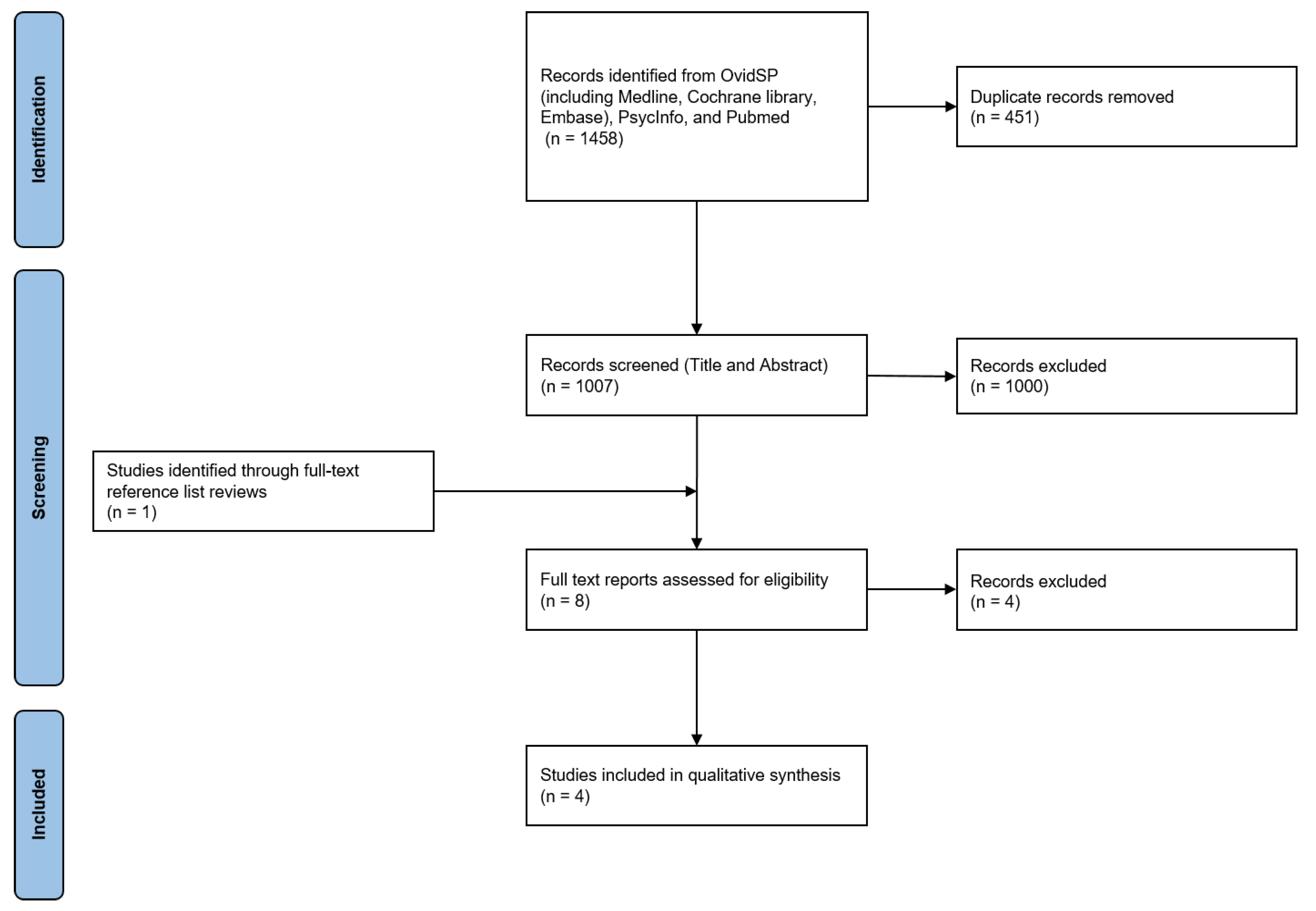
2. Methods
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Needham, D.M.; Davidson, J.; Cohen, H.; Hopkins, R.O.; Weinert, C.; Wunsch, H.; Zawistowski, C.; Bemis-Dougherty, A.; Berney, S.C.; Bienvenu, O.J.; et al. Improving long-term outcomes after discharge from intensive care unit: Report from a stakeholders’ conference. Crit. Care Med. 2012, 40, 502–509. [Google Scholar] [CrossRef]
- Ayenew, T.; Gete, M.; Gedfew, M.; Getie, A.; Afenigus, A.D.; Edmealem, A.; Amha, H.; Alem, G.; Tiruneh, B.G.; Messelu, M.A. Prevalence of Post-intensive care syndrome among intensive care unit-survivors and its association with intensive care unit length of stay: Systematic review and meta-analysis. PLoS ONE 2025, 20, e0323311. [Google Scholar] [CrossRef]
- Gao, S.; Liang, X.; Pan, Z.; Zhang, X.; Zhang, L. Effect size estimates of risk factors for post-intensive care syndrome: A systematic review and meta-analysis. Intensive Crit. Care Nurs. 2025, 87, 103888. [Google Scholar] [CrossRef] [PubMed]
- Inoue, S.; Nakanishi, N.; Amaya, F.; Fujinami, Y.; Hatakeyama, J.; Hifumi, T.; Iida, Y.; Kawakami, D.; Kawai, Y.; Kondo, Y.; et al. Post-intensive care syndrome: Recent advances and future directions. Acute Med. Surg. 2024, 11, e929. [Google Scholar] [CrossRef]
- Paul, N.; Weiss, B. Post-Intensive Care Syndrome: Functional impairments of critical illness survivors. Anaesthesiologie 2025, 74, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Geense, W.W.; Zegers, M.; Peters, M.A.A.; Ewalds, E.; Simons, K.S.; Vermeulen, H.; van der Hoeven, J.G.; van den Boogaard, M. New Physical, Mental, and Cognitive Problems 1 Year after ICU Admission: A Prospective Multicenter Study. Am. J. Respir. Crit. Care Med. 2021, 203, 1512–1521. [Google Scholar] [CrossRef] [PubMed]
- Yoo, K.H.; Lee, J.; Oh, J.; Choi, N.; Lim, T.H.; Kang, H.; Ko, B.S.; Cho, Y. Depression or anxiety and long-term mortality among adult survivors of intensive care unit: A population-based cohort study. Crit. Care 2025, 29, 179. [Google Scholar] [CrossRef]
- Probert, J.M.; Lin, S.; Yan, H.; Leoutsakos, J.-M.S.; Dinglas, V.D.; Hosey, M.M.; Parker, A.M.; Hopkins, R.O.; Needham, D.M.; Neufeld, K.J. Bodily pain in survivors of acute respiratory distress syndrome: A 1-year longitudinal follow-up study. J. Psychosom. Res. 2021, 144, 110418. [Google Scholar] [CrossRef]
- Hayhurst, C.J.; Jackson, J.C.; Archer, K.R.; Thompson, J.L.; Chandrasekhar, R.; Hughes, C.G. Pain and Its Long-term Interference of Daily Life After Critical Illness. Anesth. Analg. 2018, 127, 690–697. [Google Scholar] [CrossRef]
- Schittek, G.A.; Simonis, H.; Bornemann-Cimenti, H. Pain, nausea, vomiting, thirst, cold, … the challenge of well-being in post-operative patients. Intensive Crit. Care Nurs. 2021, 66, 103090. [Google Scholar] [CrossRef]
- Schittek, G.; Schwantzer, G.; Papamargaritis, V.; Gebauer, D.; Bornemann-Cimenti, H. Influence of intraoperative administration of glycopyrronium on early post-operative thirst, dry mouth and wellbeing. A post hoc analysis of an interventional study. Intensive Crit. Care Nurs. 2021, 66, 103078. [Google Scholar] [CrossRef] [PubMed]
- Voiriot, G.; Oualha, M.; Pierre, A.; Salmon-Gandonniere, C.; Gaudet, A.; Jouan, Y.; Kallel, H.; Radermacher, P.; Vodovar, D.; Sarton, B.; et al. Chronic critical illness and post-intensive care syndrome: From pathophysiology to clinical challenges. Ann. Intensive Care 2022, 12, 58. [Google Scholar] [CrossRef] [PubMed]
- Schittek, G.A.; Schwantzer, G.; Zoidl, P.; Orlob, S.; Holger, S.; Eichinger, M.; Sampl, L.; Bornemann-Cimenti, H.; Sandner-Kiesling, A. Adult patients’ wellbeing and disturbances during early recovery in the post anaesthesia care unit. A cross-sectional study. Intensive Crit. Care Nurs. 2020, 61, 102912. [Google Scholar] [CrossRef]
- Ribet Buse, E.; Grunow, J.J.; Spies, C.D.; Weiss, B.; Paul, N. Health-related quality of life correlates with patient-reported and proxy-reported disability in critical illness survivors: A secondary analysis of the ERIC trial. Crit. Care 2025, 29, 158. [Google Scholar] [CrossRef] [PubMed]
- Herridge, M.S.; Cheung, A.M.; Tansey, C.M.; Matte-Martyn, A.; Diaz-Granados, N.; Al-Saidi, F.; Cooper, A.B.; Guest, C.B.; Mazer, C.D.; Mehta, S.; et al. One-year outcomes in survivors of the acute respiratory distress syndrome. N. Engl. J. Med. 2003, 348, 683–693. [Google Scholar] [CrossRef]
- Kamdar, B.B.; Suri, R.; Suchyta, M.R.; Digrande, K.F.; Sherwood, K.D.; Colantuoni, E.; Dinglas, V.D.; Needham, D.M.; Hopkins, R.O. Return to work after critical illness: A systematic review and meta-analysis. Thorax 2020, 75, 17–27. [Google Scholar] [CrossRef]
- Davidson, J.E.; Jones, C.; Bienvenu, O.J. Family response to critical illness: Postintensive care syndrome-family. Crit. Care Med. 2012, 40, 618–624. [Google Scholar] [CrossRef]
- Miyamoto, Y.; Ohbe, H.; Goto, T.; Yasunaga, H. Association between intensive care unit admission of a patient and mental disorders in the spouse: A retrospective matched-pair cohort study. J. Intensive Care 2021, 9, 69. [Google Scholar] [CrossRef]
- Paul, N.; Albrecht, V.; Denke, C.; Spies, C.D.; Krampe, H.; Weiss, B. A Decade of Post-Intensive Care Syndrome: A Bibliometric Network Analysis. Medicina 2022, 58, 170. [Google Scholar] [CrossRef]
- Djulbegovic, B.; Guyatt, G. Evidence vs. Consensus in Clinical Practice Guidelines. JAMA 2019, 322, 725–726. [Google Scholar] [CrossRef]
- Pant, U.; Vyas, K.; Meghani, S.; Park, T.; Norris, C.M.; Papathanassoglou, E. Screening tools for post-intensive care syndrome and post-traumatic symptoms in intensive care unit survivors: A scoping review. Aust. Crit. Care 2023, 36, 863–871. [Google Scholar] [CrossRef]
- Kea, B.; Sun, B.C. Consensus development for healthcare professionals. Intern. Emerg. Med. 2015, 10, 373–383. [Google Scholar] [CrossRef]
- Nair, R.; Aggarwal, R.; Khanna, D. Methods of formal consensus in classification/diagnostic criteria and guideline development. Semin. Arthritis Rheum. 2011, 41, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Brouwers, M.C.; Kho, M.E.; Browman, G.P.; Burgers, J.S.; Cluzeau, F.; Feder, G.; Fervers, B.; Graham, I.D.; Hanna, S.E.; Makarski, J.; et al. Development of the AGREE II, part 1: Performance, usefulness and areas for improvement. CMAJ 2010, 182, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Amer, Y.S.; Sabr, Y.; ElGohary, G.M.; Altaki, A.M.; Khojah, O.T.; El-Malky, A.; Alzahrani, M.F. Quality assessment of evidence-based clinical practice guidelines for the management of pregnant women with sickle cell disease using the AGREE II instrument: A systematic review. BMC Pregnancy Childbirth 2020, 20, 595. [Google Scholar] [CrossRef]
- Santero, M.; de Mas, J.; Rifà, B.; Clavero, I.; Rexach, I.; Bonfill Cosp, X. Assessing the methodological strengths and limitations of the Spanish Society of Medical Oncology (SEOM) guidelines: A critical appraisal using AGREE II and AGREE-REX tool. Clin. Transl. Oncol. 2024, 26, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Central Intelligence Agency. CIA’s World Factbook. Available online: https://www.cia.gov/the-world-factbook/countries/world/#people-and-society (accessed on 20 March 2025).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Major, M.E.; Kwakman, R.; Kho, M.E.; Connolly, B.; McWilliams, D.; Denehy, L.; Hanekom, S.; Patman, S.; Gosselink, R.; Jones, C.; et al. Surviving critical illness: What is next? An expert consensus statement on physical rehabilitation after hospital discharge. Crit. Care 2016, 20, 354. [Google Scholar] [CrossRef]
- Mikkelsen, M.E.; Still, M.; Anderson, B.J.; Bienvenu, O.J.; Brodsky, M.B.; Brummel, N.; Butcher, B.; Clay, A.S.; Felt, H.; Ferrante, L.E.; et al. Society of Critical Care Medicine’s International Consensus Conference on Prediction and Identification of Long-Term Impairments After Critical Illness. Crit. Care Med. 2020, 48, 1670–1679. [Google Scholar] [CrossRef]
- Spies, C.D.; Krampe, H.; Paul, N.; Denke, C.; Kiselev, J.; Piper, S.K.; Kruppa, J.; Grunow, J.J.; Steinecke, K.; Gulmez, T.; et al. Instruments to measure outcomes of post-intensive care syndrome in outpatient care settings—Results of an expert consensus and feasibility field test. J. Intensive Care Soc. 2021, 22, 159–174. [Google Scholar] [CrossRef]
- Nakanishi, N.; Liu, K.; Kawauchi, A.; Okamura, M.; Tanaka, K.; Katayama, S.; Mitani, Y.; Ota, K.; Taito, S.; Fudeyasu, K.; et al. Instruments to assess post-intensive care syndrome assessment: A scoping review and modified Delphi method study. Crit. Care 2023, 27, 430. [Google Scholar] [CrossRef] [PubMed]
- Van Schaaf, M.D.; Bakhshi-Raiez, F.; Van Der Steen, M.; Dongelmans, D.A.; De Keizer, N.F. Recommendations for intensive care follow-up clinics; Report from a survey and conference of Dutch intensive cares. Minerva Anestesiol. 2015, 81, 135–144. [Google Scholar]
- Appleton, R.T.; Kinsella, J.; Quasim, T. The incidence of intensive care unit-acquired weakness syndromes: A systematic review. J. Intensive Care Soc. 2015, 16, 126–136. [Google Scholar] [CrossRef]
- Mgbemena, N.; Jones, A.; Leicht, A.S. Relationship between handgrip strength and lung function in adults: A systematic review. Physiother. Theory Pract. 2022, 38, 1908–1927. [Google Scholar] [CrossRef]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [PubMed]
- D’Andria Ursoleo, J.; Bottussi, A.; Epstein, A.S.; Agosta, V.T.; Monaco, F.; Rosa, W.E. Communicating about the end of life: The path of prognostic awareness. Palliat. Support Care 2025, 23, e23. [Google Scholar] [CrossRef] [PubMed]
- Hayes, K.; Harding, S.; Blackwood, B.; Latour, J.M. How and when post intensive care syndrome-family is measured: A scoping review. Intensive Crit. Care Nurs. 2024, 84, 103768. [Google Scholar] [CrossRef]
- Eaton, T.L.; Lewis, A.; Donovan, H.S.; Davis, B.C.; Butcher, B.W.; Alexander, S.A.; Iwashyna, T.J.; Scheunemann, L.P.; Seaman, J. Examining the needs of survivors of critical illness through the lens of palliative care: A qualitative study of survivor experiences. Intensive Crit. Care Nurs. 2023, 75, 103362. [Google Scholar] [CrossRef]
- Eaton, T.L.; Scheunemann, L.P.; Butcher, B.W.; Donovan, H.S.; Alexander, S.; Iwashyna, T.J. The Prevalence of Spiritual and Social Support Needs and Their Association with Postintensive Care Syndrome Symptoms Among Critical Illness Survivors Seen in a Post-ICU Follow-Up Clinic. Crit. Care Explor. 2022, 4, e0676. [Google Scholar] [CrossRef]
- Sollgruber, A.; Bornemann-Cimenti, H.; Szilagyi, I.S.; Sandner-Kiesling, A. Spirituality in pain medicine: A randomized experiment of pain perception, heart rate and religious spiritual well-being by using a single session meditation methodology. PLoS ONE 2018, 13, e0203336. [Google Scholar] [CrossRef]
- Jeong, Y.J.; Kang, J. Development and validation of a questionnaire to measure post-intensive care syndrome. Intensive Crit. Care Nurs. 2019, 55, 102756. [Google Scholar] [CrossRef]
- Wang, S.; Allen, D.; Perkins, A.; Monahan, P.; Khan, S.; Lasiter, S.; Boustani, M.; Khan, B. Validation of a New Clinical Tool for Post-Intensive Care Syndrome. Am. J. Crit. Care 2019, 28, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Needham, D.M.; Sepulveda, K.A.; Dinglas, V.D.; Chessare, C.M.; Friedman, L.A.; Bingham, C.O., 3rd; Turnbull, A.E. Core Outcome Measures for Clinical Research in Acute Respiratory Failure Survivors. An International Modified Delphi Consensus Study. Am. J. Respir. Crit. Care Med. 2017, 196, 1122–1130. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, C.L.; Burrell, A.J.C.; Engeler, D.M.; Pellegrino, V.A.; Brodie, D.; Fan, E.; International, E.N. Core Outcome Measures for Research in Critically Ill Patients Receiving Extracorporeal Membrane Oxygenation for Acute Respiratory or Cardiac Failure: An International, Multidisciplinary, Modified Delphi Consensus Study. Crit. Care Med. 2019, 47, 1557–1563. [Google Scholar] [CrossRef]
- Haywood, K.; Whitehead, L.; Nadkarni, V.M.; Achana, F.; Beesems, S.; Böttiger, B.W.; Brooks, A.; Castrén, M.; Ong, M.E.; Hazinski, M.F. COSCA (Core Outcome Set for Cardiac Arrest) in adults: An advisory statement from the International Liaison Committee on Resuscitation. Circulation 2018, 137, e783–e801. [Google Scholar] [CrossRef] [PubMed]
| PubMed | (“post intensive care syndrome”[All Fields] OR “postintensive care syndrome”[All Fields] OR “PICS”[All Fields] OR “post ICU syndrome”[All Fields]) AND (“guideline”[Publication Type] OR “guidelines as topic”[MeSH Terms] OR “guideline”[All Fields] OR “consens*”[All Fields] OR “consensus”[MeSH Terms] OR “consensus”[All Fields] OR “statement”[All Fields] OR “statements”[All Fields] OR “delphi”[All Fields] OR “societies”[All Fields] OR “society”[All Fields] OR “meeting”[All Fields] OR “conference”[All Fields] OR “experts”[All Fields]) |
| Ovid | ((“post intensive care syndrome” or “postintensive care syndrome” or “PICS” or “post ICU syndrome”) and (“guideline” or “consens*” or “consensus” or “statement” or “statements” or “delphi” or “societies” or “society” or “meeting” or “conference” or “experts”)).mp. [mp=ti, ot, ab, tx, kw, ct, sh, fx, hw, tn, dm, mf, dv, kf, dq, bt, nm, ox, px, rx, an, ui, sy, ux, mx] |
| Author | Major, 2016 [29] | Mikkelsen, 2020 [30] | Spies, 2021 [31] | Nakanishi, 2023 [32] |
|---|---|---|---|---|
| Methodology | Delphi consensus process | Consensus development conference | Semi-structured consensus meeting | Delphi consensus process |
| Type of attendance | Online | Online and in person | In person | Online |
| Systematic review of the literature | Yes | Yes | Yes | Yes |
| Number of rounds | 3 | 4 | 3 | 3 |
| Number of participants | 10 | 31 | 14 | 23 |
| Nationalities | International | International | Germany | Japan |
| Intensive care physician/anesthesiologist | Yes | Yes | Yes | Yes |
| Nurse | Yes | Yes | Yes | Yes |
| Physio-, ergo-, occupational therapist | Yes | Yes | Yes | Yes |
| Psychologist, psychiatrist | Yes | Yes | Yes | No |
| Patients’ representative | No | Yes | No | No |
| Other specialties | No | Internal medicine, general practitioner, pharmacist, rehabilitation expert, advanced practice provider | Sepsis researchers, telehealth researcher, respiratory specialist, healthcare manager | No |
| Major, 2016 [29] | Mikkelsen, 2020 [30] | Spies, 2021 [31] (Initial Assessment) | Spies, 2021 [31] (Extended Assessment) | Nakanishi, 2023 [32] | |
|---|---|---|---|---|---|
| Cognitive dysfunction | MMSE, Subjective Global Assessment Tool | MoCA | Mini-cog, animal naming | RBANS, TMT | MoCA, MMSE, SMQ |
| Anxiety and depression | HADS | HADS | PHQ-4 | PHQ-8, GAD-7 | HADS |
| Post-traumatic stress disorder | IES-R | IES-R, IES-6 | No recommendation | IES-R | IES-R |
| Physical function | 6MWT, 4 m time walk/gait speed, cycle ergometry testing, DEMMI, TUG, MRC | 6MWT | TUG | 2MWT, SPPB | 6MWT, MRC-score, |
| Physical examination | Handgrip strength and handheld dynamometry, maximum inspiratory pressure, maximum expiratory pressure | No recommendation | Handgrip strength | Handgrip strength | Handgrip strength |
| Activities of daily living | FIM, SPPB, SF-36 (physical function domain), Barthel Index, Katz ADL, Lawton IADL | No recommendation | No recommendation | No recommendation | Barthel Index, IADL, FIM |
| Quality of life | SF-36, EQ-5D | EuroQol-5D-5L | EQ-5D-5L | WHODAS 2.0, EQ-5D-5L | SF-36, EQ-5D-5L, EQ-5D-3L, EQ-VAS, SF-12 |
| New or worsening health problems | No recommendation | No recommendation | No recommendation | Single items and NRS of subjective mental and physical health | No recommendation |
| Fatigue | MFI | No recommendation | No recommendation | No recommendation | No recommendation |
| Nutrition | SNAQ, MUST | No recommendation | No recommendation | No recommendation | No recommendation |
| Sleep | RCSQ | No recommendation | No recommendation | No recommendation | PSQI |
| Pain | VAS for pain | No recommendation | No recommendation | No recommendation | BPI |
| Major, 2016 [29] | Mikkelsen, 2020 [30] | Spies, 2021 [31] | Nakanishi, 2023 [32] | |
|---|---|---|---|---|
| Scope and purpose | 88.9 | 100.0 | 100.0 | 94.4 |
| Stakeholder involvement | 66.7 | 72.2 | 83.3 | 77.8 |
| Rigor of development | 50.0 | 62.5 | 64.6 | 60.4 |
| Clarity of presentation | 50.0 | 55.6 | 50.0 | 50.0 |
| Applicability | 22.2 | 22.2 | 33.3 | 22.2 |
| Editorial independence | 100.0 | 100.0 | 100.0 | 100.0 |
| Overall quality | 66.7 | 83.3 | 66.7 | 66.7 |
| Domain | Tool | License | English | Mandarin Chinese | Hindi | Spanish | French |
|---|---|---|---|---|---|---|---|
| Cognition | Montreal Cognitive Assessment (MOCA) | Free upon request, requires training session | Yes | Yes | Yes | Yes | Yes |
| Mini-Mental State Examination (MMSE) | Commercial | Yes | Yes | Yes | Yes | Yes | |
| Short memory questionnaire (SMQ) | No data | Yes | No | No | No | No | |
| MiniCog | Free upon request | Yes | Yes | Yes | Yes | Yes | |
| Animal naming | Free | Yes | Yes | No | No | No | |
| Subjective Global Assessment Tool | Free | Yes | Yes | No | Yes | Yes | |
| Anxiety and Depression | Hospital Anxiety and Depression Scale (HADS) | Free upon request | Yes | Yes | Yes | Yes | Yes |
| Impact of Event Scale—Revised (IES-R) | Free upon request | Yes | Yes | Yes | Yes | Yes | |
| Patient Health Questionnaire-9 (PHQ-9) | Free | Yes | Yes | Yes | Yes | Yes | |
| Patient Health Questionnaire-4 (PHQ-4) | Free | Yes | Yes | No | Yes | Yes | |
| PTSD | Impact of Event Scale—Revised (IES-R) | Free upon request | Yes | Yes | Yes | Yes | Yes |
| Physical function | Medical Research Council score (MRC) | Free with credit line | Yes | No | No | No | No |
| QoL | Short Form-36 Health Survey (SF-36) | Free with credit line | Yes | Yes | Yes | Yes | Yes |
| EuroQol 5-Dimension Questionnaire (EQ-5D-5L) | Free upon request | Yes | Yes | Yes | Yes | Yes | |
| EuroQol 5-Dimension Questionnaire (EQ-5D-3L) | Free upon request | Yes | Yes | Yes | Yes | Yes | |
| EuroQuol-VAS | Free upon request | Yes | Yes | Yes | Yes | Yes | |
| Short Form-12 Health Survey (SF-12) | Commercial | Yes | Yes | Yes | Yes | Yes | |
| World Health Organization Disability Assessment Schedule 2.0 (WHODAS 2.0) | Free for non-commercial use | Yes | Yes | No | Yes | No | |
| Function | Barthel Index | Free for non-funded academic users | Yes | Yes | Yes | Yes | Yes |
| Instrumental Activities of Daily Living (IADL) | Free upon request | Yes | Yes | No | Yes | Yes | |
| Functional Independence Measure (FIM) | Free | Yes | Yes | No | Yes | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bornemann-Cimenti, H.; Lang, J.; Hammer, S.; Lang-Illievich, K.; Labenbacher, S.; Neuwersch-Sommeregger, S.; Klivinyi, C. Consensus-Based Recommendations for Assessing Post-Intensive Care Syndrome: A Systematic Review. J. Clin. Med. 2025, 14, 3595. https://doi.org/10.3390/jcm14103595
Bornemann-Cimenti H, Lang J, Hammer S, Lang-Illievich K, Labenbacher S, Neuwersch-Sommeregger S, Klivinyi C. Consensus-Based Recommendations for Assessing Post-Intensive Care Syndrome: A Systematic Review. Journal of Clinical Medicine. 2025; 14(10):3595. https://doi.org/10.3390/jcm14103595
Chicago/Turabian StyleBornemann-Cimenti, Helmar, Johanna Lang, Sascha Hammer, Kordula Lang-Illievich, Sebastian Labenbacher, Stefan Neuwersch-Sommeregger, and Christoph Klivinyi. 2025. "Consensus-Based Recommendations for Assessing Post-Intensive Care Syndrome: A Systematic Review" Journal of Clinical Medicine 14, no. 10: 3595. https://doi.org/10.3390/jcm14103595
APA StyleBornemann-Cimenti, H., Lang, J., Hammer, S., Lang-Illievich, K., Labenbacher, S., Neuwersch-Sommeregger, S., & Klivinyi, C. (2025). Consensus-Based Recommendations for Assessing Post-Intensive Care Syndrome: A Systematic Review. Journal of Clinical Medicine, 14(10), 3595. https://doi.org/10.3390/jcm14103595







