High-Flow Nasal Cannula Application After Extubation in Acute Respiratory Failure Patients
Abstract
1. Introduction
2. Materials and Methods
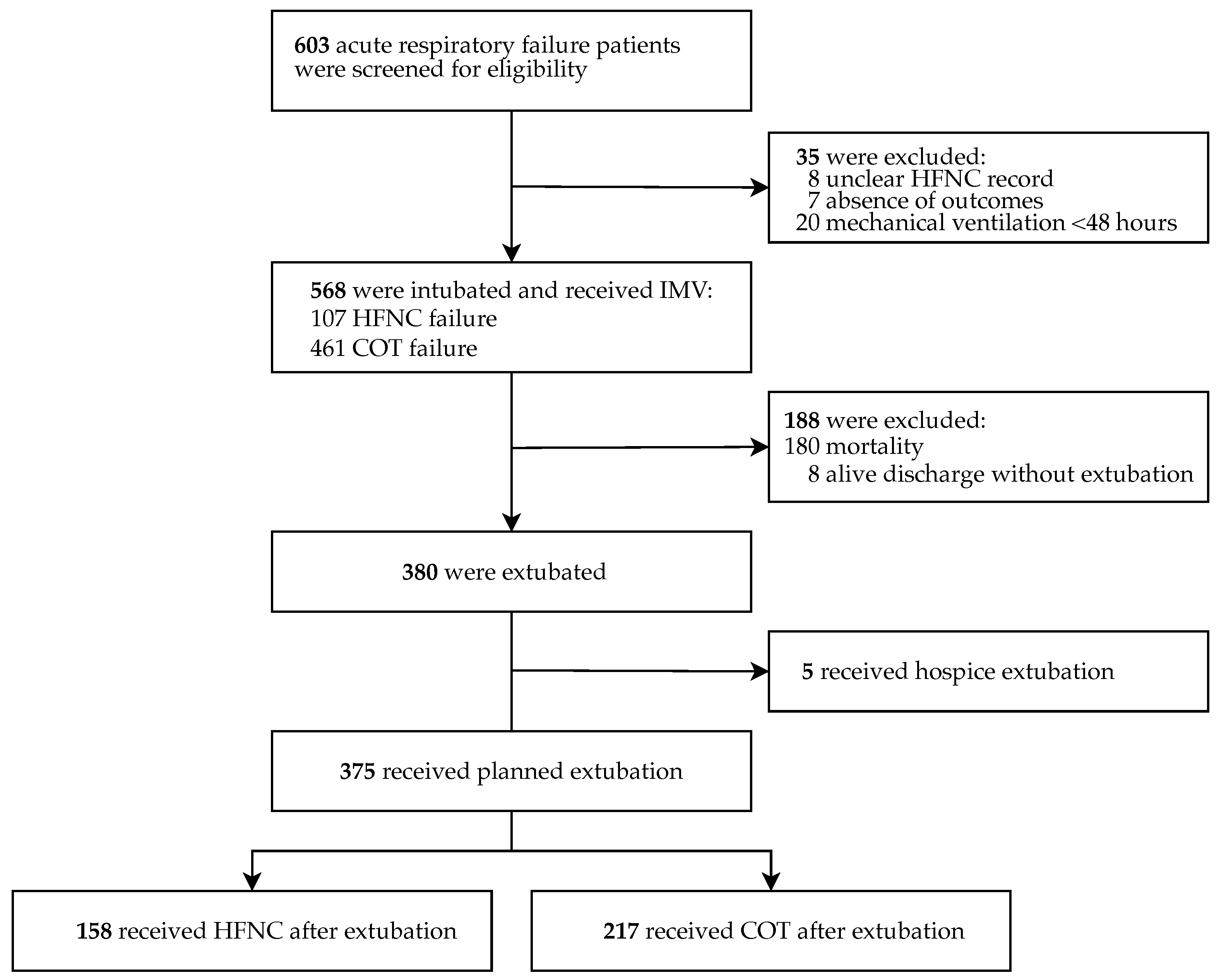
2.1. Study Design and Patient Selection
2.2. Statistical Analysis
3. Results
HFNC Application After Extubation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cuquemelle, E.; Pham, T.; Papon, J.F.; Louis, B.; Danin, P.E.; Brochard, L. Heated and humidified high-flow oxygen therapy reduces discomfort during hypoxemic respiratory failure. Respir. Care 2012, 57, 1571–1577. [Google Scholar] [CrossRef] [PubMed]
- Frat, J.P.; Thille, A.W.; Mercat, A.; Girault, C.; Ragot, S.; Perbet, S.; Prat, G.; Boulain, T.; Morawiec, E.; Cottereau, A.; et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N. Engl. J. Med. 2015, 372, 2185–2196. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, H.; Okano, H.; Mayumi, T.; Narita, C.; Onodera, Y.; Nakane, M.; Shime, N. Post-extubation oxygenation strategies in acute respiratory failure: A systematic review and network meta-analysis. Crit. Care 2021, 25, 135. [Google Scholar] [CrossRef] [PubMed]
- Renda, T.; Corrado, A.; Iskandar, G.; Pelaia, G.; Abdalla, K.; Navalesi, P. High-flow nasal oxygen therapy in intensive care and anaesthesia. Br. J. Anaesth. 2018, 120, 18–27. [Google Scholar] [CrossRef]
- Glossop, A.J.; Shephard, N.; Bryden, D.C.; Mills, G.H. Non-invasive ventilation for weaning, avoiding reintubation after extubation and in the postoperative period: A meta-analysis. Br. J. Anaesth. 2012, 109, 305–314. [Google Scholar] [CrossRef]
- Grieco, D.L.; Maggiore, S.M.; Roca, O.; Spinelli, E.; Patel, B.K.; Thille, A.W.; Barbas, C.S.V.; de Acilu, M.G.; Cutuli, S.L.; Bongiovanni, F.; et al. Non-invasive ventilatory support and high-flow nasal oxygen as first-line treatment of acute hypoxemic respiratory failure and ARDS. Intensive Care Med. 2021, 47, 851–866. [Google Scholar] [CrossRef]
- Papazian, L.; Corley, A.; Hess, D.; Fraser, J.F.; Frat, J.P.; Guitton, C.; Jaber, S.; Maggiore, S.M.; Nava, S.; Rello, J.; et al. Use of high-flow nasal cannula oxygenation in ICU adults: A narrative review. Intensive Care Med. 2016, 42, 1336–1349. [Google Scholar] [CrossRef]
- Dysart, K.; Miller, T.L.; Wolfson, M.R.; Shaffer, T.H. Research in high flow therapy: Mechanisms of action. Respir. Med. 2009, 103, 1400–1405. [Google Scholar] [CrossRef]
- Oczkowski, S.; Ergan, B.; Bos, L.; Chatwin, M.; Ferrer, M.; Gregoretti, C.; Heunks, L.; Frat, J.P.; Longhini, F.; Nava, S.; et al. ERS clinical practice guidelines: High-flow nasal cannula in acute respiratory failure. Eur. Respir. J. 2022, 59, 2101574. [Google Scholar] [CrossRef]
- Winck, J.C.; Ambrosino, N. COVID-19 pandemic and non invasive respiratory management: Every Goliath needs a David. An evidence based evaluation of problems. Pulmonology 2020, 26, 213–220. [Google Scholar] [CrossRef]
- Li, X.; Ma, X. Acute respiratory failure in COVID-19: Is it “typical" ARDS? Crit. Care 2020, 24, 198. [Google Scholar] [CrossRef]
- Agarwal, A.; Basmaji, J.; Muttalib, F.; Granton, D.; Chaudhuri, D.; Chetan, D.; Hu, M.; Fernando, S.M.; Honarmand, K.; Bakaa, L.; et al. High-flow nasal cannula for acute hypoxemic respiratory failure in patients with COVID-19: Systematic reviews of effectiveness and its risks of aerosolization, dispersion, and infection transmission. Can. J. Anesth. 2020, 67, 1217–1248. [Google Scholar] [CrossRef] [PubMed]
- Alhazzani, W.; Moller, M.H.; Arabi, Y.M.; Loeb, M.; Gong, M.N.; Fan, E.; Oczkowski, S.; Levy, M.M.; Derde, L.; Dzierba, A.; et al. Surviving Sepsis Campaign: Guidelines on the Management of Critically Ill Adults with Coronavirus Disease 2019 (COVID-19). Crit. Care Med. 2020, 48, e440–e469. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.J.; Koh, Y.; Lim, C.M.; Huh, J.W.; Baek, S.; Han, M.; Seo, H.S.; Suh, H.J.; Seo, G.J.; Kim, E.Y.; et al. Failure of high-flow nasal cannula therapy may delay intubation and increase mortality. Intensive Care Med. 2015, 41, 623–632. [Google Scholar] [CrossRef]
- Lai, C.C.; Lee, P.I.; Hsueh, P.R. How Taiwan has responded to COVID-19 and how COVID-19 has affected Taiwan, 2020–2022. J. Microbiol. Immunol. Infect. 2023, 56, 433–441. [Google Scholar] [CrossRef]
- Mauri, T.; Turrini, C.; Eronia, N.; Grasselli, G.; Volta, C.A.; Bellani, G.; Pesenti, A. Physiologic Effects of High-Flow Nasal Cannula in Acute Hypoxemic Respiratory Failure. Am. J. Respir. Crit. Care Med. 2017, 195, 1207–1215. [Google Scholar] [CrossRef]
- Force, A.D.T.; Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute respiratory distress syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar] [CrossRef]
- Kin, K.C.; Zhou, H.; Gysi, M.; Huang, C.W.; Selevan, D.C.; Lu, D.D.; Sim, J.J. Outcomes Among Hospitalized Patients with COVID-19 and Acute Kidney Injury Requiring Renal Replacement Therapy. Perm. J. 2022, 26, 39–45. [Google Scholar] [CrossRef]
- Samaan, F.; Carneiro de Paula, E.; de Lima Souza, F.B.G.; Mendes, L.F.C.; Rossi, P.R.G.; Freitas, R.A.P.; Nakagawa, F.T.; Maciel, A.T.; Aranha, S.; Osawa, E.; et al. COVID-19-associated acute kidney injury patients treated with renal replacement therapy in the intensive care unit: A multicenter study in Sao Paulo, Brazil. PLoS ONE 2022, 17, e0261958. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, J.; Pan, J.; Xu, Z.; Xu, J. The ROX index as a predictor of high-flow nasal cannula outcome in pneumonia patients with acute hypoxemic respiratory failure: A systematic review and meta-analysis. BMC Pulm. Med. 2022, 22, 121. [Google Scholar] [CrossRef]
- Yu, P.T.; Chen, C.H.; Wang, C.J.; Kuo, K.C.; Wu, J.C.; Chung, H.P.; Chen, Y.T.; Tang, Y.H.; Chang, W.K.; Lin, C.Y.; et al. Predicting the successful application of high-flow nasal oxygen cannula in patients with COVID-19 respiratory failure: A retrospective analysis. Expert Rev. Respir. Med. 2023, 17, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, R.; Kaito, D.; Homma, K.; Endo, A.; Tagami, T.; Suzuki, M.; Umetani, N.; Yagi, M.; Nashiki, E.; Suhara, T.; et al. Early intubation and decreased in-hospital mortality in patients with coronavirus disease 2019. Crit. Care 2022, 26, 124. [Google Scholar] [CrossRef] [PubMed]
- Myers, L.C.; Kipnis, P.; Greene, J.D.; Chen, A.; Creekmur, B.; Xu, S.; Sankar, V.; Roubinian, N.H.; Langer-Gould, A.; Gould, M.K.; et al. The impact of timing of initiating invasive mechanical ventilation in COVID-19-related respiratory failure. J. Crit. Care 2023, 77, 154322. [Google Scholar] [CrossRef]
- Frat, J.P.; Quenot, J.P.; Badie, J.; Coudroy, R.; Guitton, C.; Ehrmann, S.; Gacouin, A.; Merdji, H.; Auchabie, J.; Daubin, C.; et al. Effect of High-Flow Nasal Cannula Oxygen, vs. Standard Oxygen Therapy on Mortality in Patients with Respiratory Failure Due to COVID-19: The SOHO-COVID Randomized Clinical Trial. JAMA 2022, 328, 1212–1222. [Google Scholar] [CrossRef]
- Azoulay, E.; Pickkers, P.; Soares, M.; Perner, A.; Rello, J.; Bauer, P.R.; van de Louw, A.; Hemelaar, P.; Lemiale, V.; Taccone, F.S.; et al. Acute hypoxemic respiratory failure in immunocompromised patients: The Efraim multinational prospective cohort study. Intensive Care Med. 2017, 43, 1808–1819. [Google Scholar] [CrossRef]
- Huang, H.B.; Peng, J.M.; Weng, L.; Liu, G.Y.; Du, B. High-flow oxygen therapy in immunocompromised patients with acute respiratory failure: A review and meta-analysis. J. Crit. Care 2018, 43, 300–305. [Google Scholar] [CrossRef]
- Lopez-Ramirez, V.Y.; Sanabria-Rodriguez, O.O.; Bottia-Cordoba, S.; Munoz-Velandia, O.M. Delayed mechanical ventilation with prolonged high-flow nasal cannula exposure time as a risk factor for mortality in acute respiratory distress syndrome due to SARS-CoV-2. Intern. Emerg. Med. 2023, 18, 429–437. [Google Scholar] [CrossRef]
- Ionescu, F.; Zimmer, M.S.; Petrescu, I.; Castillo, E.; Bozyk, P.; Abbas, A.; Abplanalp, L.; Dogra, S.; Nair, G.B. Extubation Failure in Critically Ill COVID-19 Patients: Risk Factors and Impact on In-Hospital Mortality. J. Intensive Care Med. 2021, 36, 1018–1024. [Google Scholar] [CrossRef]
- Thille, A.W.; Richard, J.C.; Brochard, L. The decision to extubate in the intensive care unit. Am. J. Respir. Crit. Care Med. 2013, 187, 1294–1302. [Google Scholar] [CrossRef]
- Zhu, Y.; Yin, H.; Zhang, R.; Ye, X.; Wei, J. High-flow nasal cannula oxygen therapy versus conventional oxygen therapy in patients after planned extubation: A systematic review and meta-analysis. Crit. Care 2019, 23, 180. [Google Scholar] [CrossRef]
- Simioli, F.; Annunziata, A.; Langella, G.; Polistina, G.E.; Martino, M.; Fiorentino, G. Clinical outcomes of high-flow nasal cannula in COVID-19 associated postextubation respiratory failure. A single-centre case series. Anaesthesiol. Intensive Ther. 2020, 52, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Flinspach, A.N.; Booke, H.; Zacharowski, K.; Balaban, U.; Herrmann, E.; Adam, E.H. High sedation needs of critically ill COVID-19 ARDS patients-A monocentric observational study. PLoS ONE 2021, 16, e0253778. [Google Scholar] [CrossRef] [PubMed]
- Tsolaki, V.; Zakynthinos, G.E.; Papadonta, M.E.; Bardaka, F.; Fotakopoulos, G.; Pantazopoulos, I.; Makris, D.; Zakynthinos, E. Neuromuscular Blockade in the Pre- and COVID-19 ARDS Patients. J. Pers. Med. 2022, 12, 1538. [Google Scholar] [CrossRef]
- Schmidt, D.; Piva, T.C.; Glaeser, S.S.; Piekala, D.M.; Berto, P.P.; Friedman, G.; Sbruzzi, G. Intensive Care Unit-Acquired Weakness in Patients with COVID-19: Occurrence and Associated Factors. Phys. Ther. 2022, 102, pzac028. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Murthy, S.; Diaz, J.V.; Slutsky, A.S.; Villar, J.; Angus, D.C.; Annane, D.; Azevedo, L.C.P.; Berwanger, O.; Cavalcanti, A.B.; et al. Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients with COVID-19: A Meta-analysis. JAMA 2020, 324, 1330–1341. [Google Scholar] [CrossRef]
- Group, R.C.; Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; et al. Dexamethasone in Hospitalized Patients with Covid-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef]
- Hermans, G.; Van den Berghe, G. Clinical review: Intensive care unit acquired weakness. Crit. Care 2015, 19, 274. [Google Scholar] [CrossRef]
- Boscolo, A.; Pettenuzzo, T.; Sella, N.; Zatta, M.; Salvagno, M.; Tassone, M.; Pretto, C.; Peralta, A.; Muraro, L.; Zarantonello, F.; et al. Noninvasive respiratory support after extubation: A systematic review and network meta-analysis. Eur. Respir. Rev. 2023, 32, 220196. [Google Scholar] [CrossRef]
- Hu, T.Y.; Lee, C.H.; Cheng, K.H.; Tan, M.C.; Hua, H.F.; Kuo, L.K. Effect of High-Flow Nasal Oxygen vs. Conventional Oxygen Therapy on Extubation Outcomes and Physiologic Changes for Patients with High Risk of Extubation Failure in the Medical ICU: A Tertiary Center, Randomized, Controlled Trial. Int. J. Gerontol. 2020, 14, 36–41. [Google Scholar] [CrossRef]
- Basoalto, R.; Damiani, L.F.; Jalil, Y.; Bachmann, M.C.; Oviedo, V.; Alegría, L.; Valenzuela, E.D.; Rovegno, M.; Ruiz-Rudolph, P.; Cornejo, R.; et al. Physiological effects of high-flow nasal cannula oxygen therapy after extubation: A randomized crossover study. Ann. Intensive Care 2023, 13, 104. [Google Scholar] [CrossRef] [PubMed]
- Rosas, I.O.; Brau, N.; Waters, M.; Go, R.C.; Hunter, B.D.; Bhagani, S.; Skiest, D.; Aziz, M.S.; Cooper, N.; Douglas, I.S.; et al. Tocilizumab in Hospitalized Patients with Severe Covid-19 Pneumonia. N. Engl. J. Med. 2021, 384, 1503–1516. [Google Scholar] [CrossRef] [PubMed]
| Variable | Pre-Intubation HFNC Failure (n = 107) | COT (n = 461) | p-Value |
|---|---|---|---|
| Patient profile | |||
| Age, years | 69 (62–74.5) | 68 (60–73) | 0.177 |
| Male | 67 (62.6) | 304 (65.9) | 0.590 |
| BMI ≥ 30 | 21 (20.2) | 69 (15.8) | 0.344 |
| Smoking | 27 (25.5) | 116 (25.5) | 1.000 |
| Comorbidities | |||
| COPD | 8 (7.5) | 24 (5.2) | 0.493 |
| Hypertension | 69 (64.5) | 240 (52) | 0.027 |
| CHF | 10 (9.3) | 43 (9.3) | 1.000 |
| DM | 42 (39.3) | 157 (34.1) | 0.367 |
| Cirrhosis | 8 (7.5) | 26 (5.6) | 0.620 |
| CCI ≥ 3 | 80 (74.8) | 284 (61.6) | 0.015 |
| Laboratory profiles * | |||
| Lymphocyte count, ×106/L | 763 (480–1091) | 740 (474–1026) | 0.995 |
| Procalcitonin ≥ 0.5 ng/mL | 14 (12.8) | 122 (31.4) | 0.002 |
| LDH ≥ 300 U/L | 73 (75.3) | 310 (82.2) | 0.159 |
| Ferritin ≥ 800 ng/mL | 62 (64.6) | 245 (66.9) | 0.754 |
| Intensive care-related profiles | |||
| APACHE II ≥ 25 | 12 (11.2) | 116 (23.5) | 0.008 |
| PaO2/FiO2 ratio * | 109.2 (70–148) | 138.6 (83–218) | <0.001 |
| ROX index * | 5.5 (4.5–7.5) | 6.1 (4.7–8.1) | 0.128 |
| ARDS | 84 (78.5) | 359 (77.9) | 0.990 |
| AKI-RRT | 15 (14.0) | 56 (12.3) | 0.745 |
| IMV duration, days | 15 (8–27) | 11 (7–21) | 0.031 |
| ICU length of stay, days | 24.5 (13–34) | 14 (8–27) | 0.002 |
| DNR order | 38 (35.5) | 161 (34.9) | 0.148 |
| Hospital mortality | 41 (38.3) | 164 (35.6) | 0.674 |
| Variable | Post-Extubation HFNC (n = 158) | COT (n = 217) | p-Value |
|---|---|---|---|
| Patient profile | |||
| Age, years | 66 (59–72) | 65 (59–70) | 0.547 |
| Male | 102 (64.6) | 133 (61.3) | 0.591 |
| BMI ≥ 30 | 28 (18.3) | 33 (15.9) | 0.654 |
| Smoking | 42 (26.9) | 44 (20.7) | 0.200 |
| Comorbidities | |||
| COPD | 5 (3.2) | 10 (4.6) | 0.661 |
| Hypertension | 87 (55.1) | 108 (49.8) | 0.367 |
| CHF | 12 (7.6) | 16 (7.4) | 1.000 |
| DM | 53 (33.5) | 68 (31.3) | 0.734 |
| Cirrhosis | 11 (7.0) | 12 (5.5) | 0.724 |
| CCI ≥ 3 | 90 (57.0) | 118 (54.4) | 0.695 |
| Intensive care-related profiles | |||
| APACHE II ≥ 25 | 24 (15.2) | 38 (17.5) | 0.648 |
| Tocilizumab | 123 (77.8) | 151 (69.6) | 0.096 |
| Sedation | 142 (90.4) | 201 (93.1) | 0.470 |
| NMB | 102 (64.6) | 141 (65) | 1.000 |
| ARDS | 120 (75.9) | 151 (69.6) | 0.293 |
| AKI-RRT | 11 (7.1) | 9 (4.2) | 0.336 |
| IMV duration, days | 13 (8–22) | 11 (7–20) | 0.834 |
| ICU length of stay, days | 19 (11–32) | 14 (9–27) | 0.118 |
| DNR order | 23 (14.6) | 35 (16.1) | 0.786 |
| Post-extubation prognosis | |||
| 14-day mortality | 0 (0.0) | 12 (5.5) | 0.007 |
| 28-day mortality | 2 (1.3) | 14 (6.5) | 0.028 |
| Variable | Crude HR | 95% CI | p-Value |
|---|---|---|---|
| Age ≥ 65 | 2.375 | 0.764–7.381 | 0.135 |
| Male | 0.575 | 0.216–1.532 | 0.268 |
| BMI ≥ 30 | 0.383 | 0.050–2.927 | 0.355 |
| COPD | 1.610 | 0.213–12.191 | 0.645 |
| Hypertension | 1.419 | 0.515–3.908 | 0.499 |
| CHF | 5.990 | 2.081–17.243 | <0.001 |
| DM | 2.592 | 0.965–6.965 | 0.059 |
| Cirrhosis | 4.352 | 1.238–15.305 | 0.022 |
| CCI ≥ 3 | 5.051 | 1.145–22.274 | 0.032 |
| APACHE II ≥ 25 | 3.620 | 1.346–9.739 | 0.011 |
| Tocilizumab | 1.109 | 0.358–3.440 | 0.857 |
| Sedation | 0.510 | 0.115–2.254 | 0.375 |
| NMB | 1.175 | 0.408–3.382 | 0.765 |
| ARDS | 5.165 | 0.682–39.139 | 0.112 |
| AKI-RRT | 11.482 | 4.082–32.296 | <0.001 |
| Pre-intubation HFNC failure | 1.052 | 0.300–3.692 | 0.937 |
| Post-extubation HFNC | 0.182 | 0.041–0.799 | 0.024 |
| Variable | Adjusted HR | 95% CI | p-Value |
|---|---|---|---|
| CHF | 1.956 | 0.475–8.063 | 0.353 |
| DM | 1.477 | 0.443–4.923 | 0.525 |
| Cirrhosis | 2.407 | 0.495–11.692 | 0.276 |
| CCI ≥ 3 | 2.559 | 0.498–13.144 | 0.260 |
| APACHE II ≥ 25 | 2.251 | 0.719–7.044 | 0.163 |
| AKI-RRT | 11.238 | 3.452–36.589 | <0.001 |
| Post-extubation HFNC | 0.060 | 0.007–0.493 | 0.009 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chao, W.-C.; Wang, S.-Y.; Lin, C.-Y.; Chang, H.-T.; Su, W.-L.; Tseng, C.-H.; Yang, K.-Y.; Ku, S.-C.; Kao, K.-C.; Wang, C.-J. High-Flow Nasal Cannula Application After Extubation in Acute Respiratory Failure Patients. J. Clin. Med. 2025, 14, 3087. https://doi.org/10.3390/jcm14093087
Chao W-C, Wang S-Y, Lin C-Y, Chang H-T, Su W-L, Tseng C-H, Yang K-Y, Ku S-C, Kao K-C, Wang C-J. High-Flow Nasal Cannula Application After Extubation in Acute Respiratory Failure Patients. Journal of Clinical Medicine. 2025; 14(9):3087. https://doi.org/10.3390/jcm14093087
Chicago/Turabian StyleChao, Wen-Chi, Shen-Yung Wang, Chang-Yi Lin, Hou-Tai Chang, Wen-Lin Su, Chien-Hua Tseng, Kuang-Yao Yang, Shih-Chi Ku, Kuo-Chin Kao, and Chieh-Jen Wang. 2025. "High-Flow Nasal Cannula Application After Extubation in Acute Respiratory Failure Patients" Journal of Clinical Medicine 14, no. 9: 3087. https://doi.org/10.3390/jcm14093087
APA StyleChao, W.-C., Wang, S.-Y., Lin, C.-Y., Chang, H.-T., Su, W.-L., Tseng, C.-H., Yang, K.-Y., Ku, S.-C., Kao, K.-C., & Wang, C.-J. (2025). High-Flow Nasal Cannula Application After Extubation in Acute Respiratory Failure Patients. Journal of Clinical Medicine, 14(9), 3087. https://doi.org/10.3390/jcm14093087







