Inflammatory Indices in Patients with Myocardial Infarction Complicated by Cardiogenic Shock, and Their Interconnections with SCAI Stages and Patients’ Survival: A Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients Characteristics
2.2. Evaluation of Immune Response Reactions
2.3. Calculation of Indices of Systemic Inflammation
- NEU—absolute neutrophil count;
- MON—absolute monocyte count;
- PL—absolute platelet count;
- LYMPH—absolute lymphocyte count.
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Patients
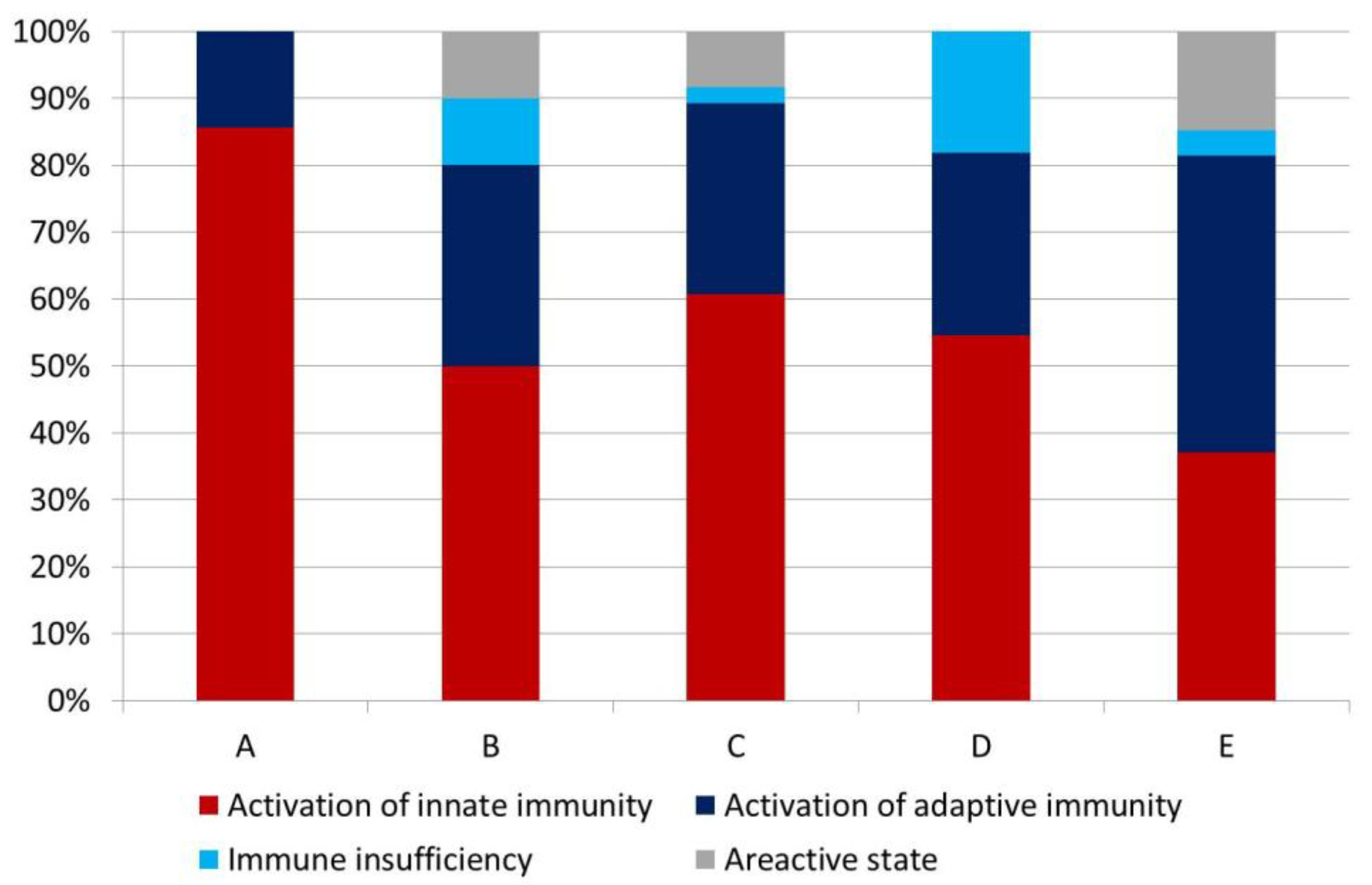
3.2. Complete Blood Count Test and Types of Immune Reactions
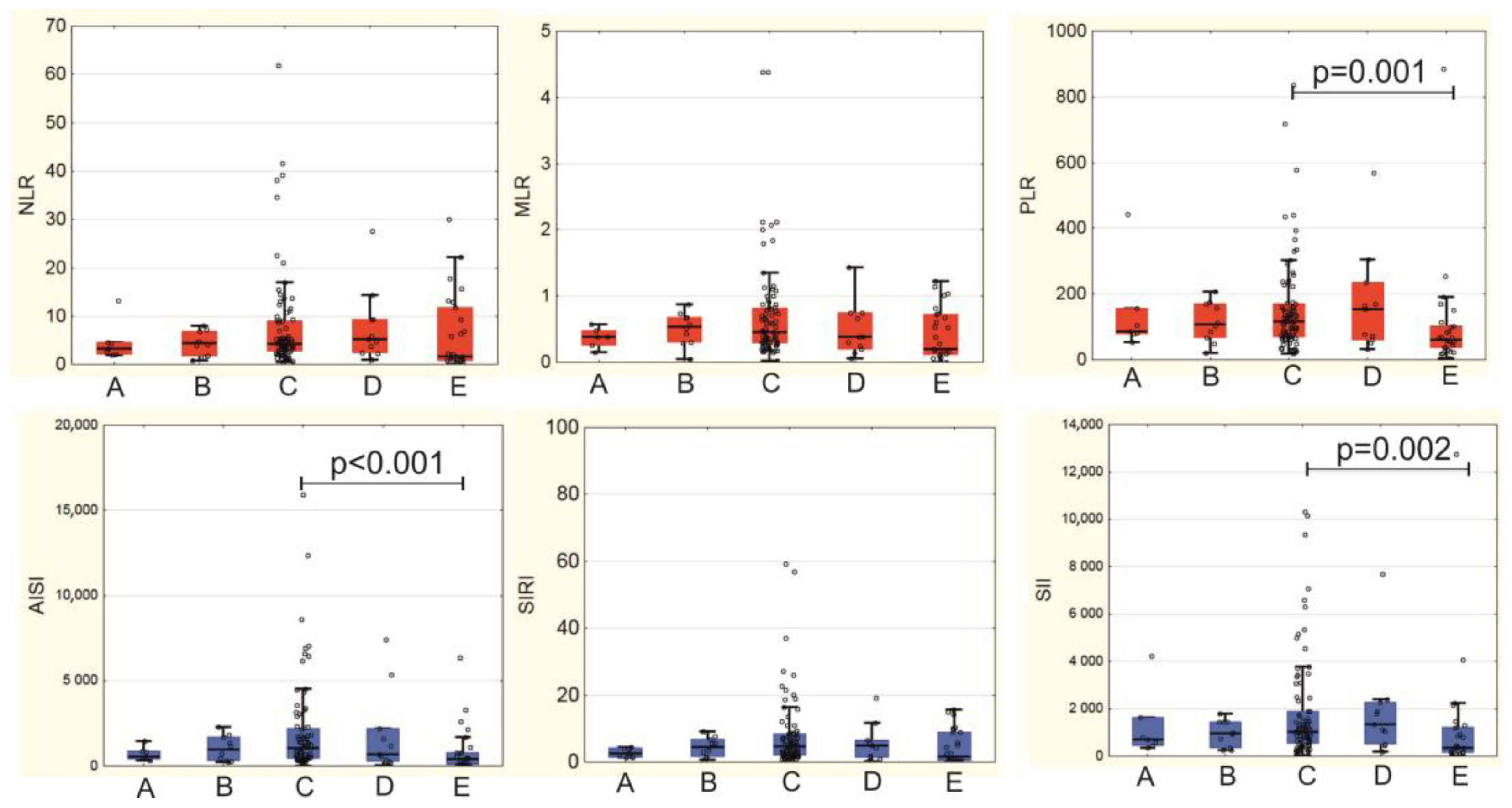
3.3. Systemic Inflammation Indices in MI-CS Patients
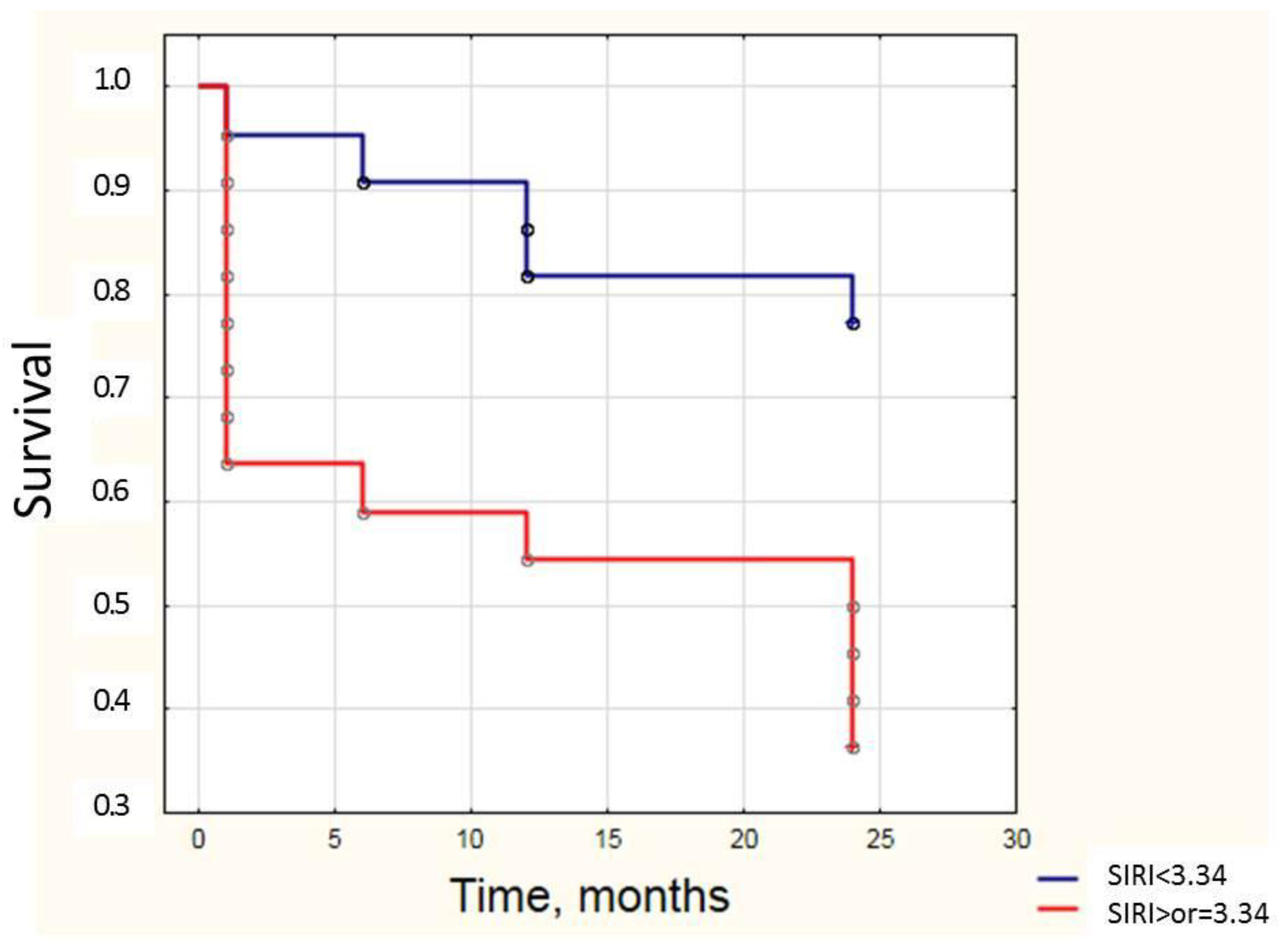
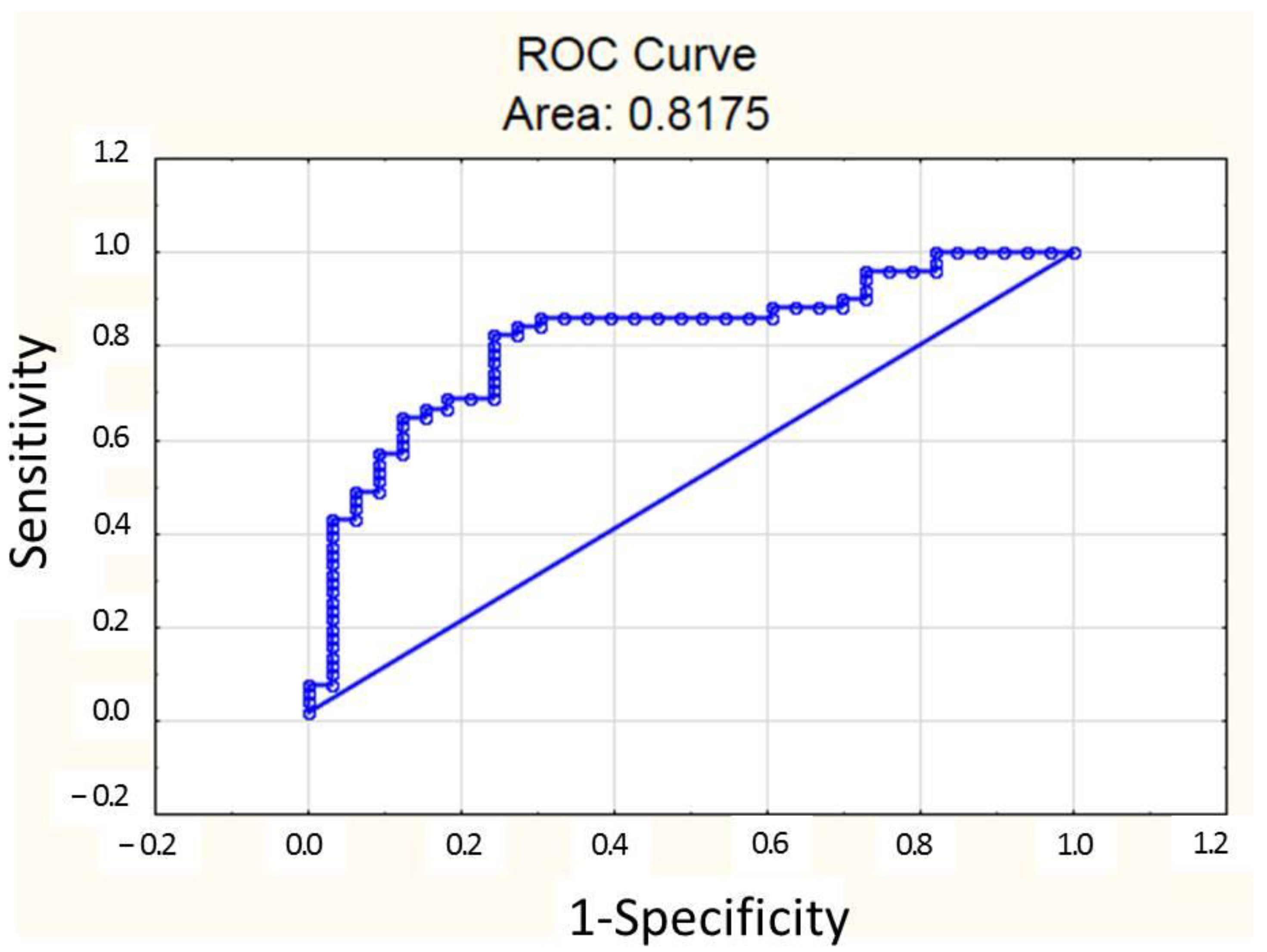
3.4. Systemic Inflammation Indices May Be Used for Prognosis During MI-CS
3.5. Predictors of SIRI Values in MI-CS Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MI-CS | Myocardial infarction complicated by cardiogenic shock |
| ACS | Acute coronary syndrome |
| AISI | Aggregate Index of Systemic Inflammation |
| AUC | Area under curve |
| CBC | Complete blood count test |
| CK-MB | MB fraction of creatine kinase |
| CTLA-4 | Cytotoxic T-lymphocyte associated protein 4 |
| ECG | Electrocardiography |
| Hb | Hemoglobin |
| HRV | Heart rate variability |
| hsCRP | High-sensitivity C-reactive protein |
| ICU | Intensive care unit |
| IFN | Interferon |
| IL | Interleukin |
| LAG-3 | Lymphocyte-activation gene 3 |
| MDSC | Myeloid-derived suppressor cells |
| MLR | Monocytes-lymphocytes ratio |
| NLR | Neutrophils-lymphocytes ratio |
| NSTEMI | non-ST-elevation myocardial infarction |
| PCI | Percutaneous coronary intervention |
| PD-1 | Programmed cell death 1 |
| PIV | Pan-immune inflammation value |
| PLR | Platelet-lymphocytes ratio |
| SAPSII | Simplified Acute Physiology Score II |
| SCAI | American Society of Cardiovascular Angiography and Intervention |
| SII | Systemic Immune Inflammation Index |
| SIRI | Systemic Inflammation Response Index |
| SOFA | Sequential Organ Failure Assessment |
| STEMI | ST-elevation myocardial infarction |
| TIM-3 | T-cell immunoglobulin and mucin domain-containing protein-3 |
| TIMI | Thrombolysis in myocardial infarction |
| Treg | T regulatory lymphocytes |
| WMSI | Wall motion score index |
References
- Samsky, M.D.; Morrow, D.A.; Proudfoot, A.G.; Hochman, J.S.; Thiele, H.; Rao, S.V. Cardiogenic Shock After Acute Myocardial Infarction: A Review. JAMA 2021, 326, 1840–1850. [Google Scholar] [CrossRef] [PubMed]
- Pepe, M.; Bortone, A.S.; Giordano, A.; Cecere, A.; Burattini, O.; Nestola, P.L.; Patti, G.; Di Cillo, O.; Signore, N.; Forleo, C.; et al. Cardiogenic Shock Following Acute Myocardial Infarction: What’s New? Shock 2020, 53, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Dil, S.; Kercheva, M.; Panteleev, O.; Demianov, S.; Kanev, A.; Belich, N.; Kornienko, B.; Ryabov, V. Myocardial Infarction-Associated Shock: A Comprehensive Analysis of Phenotypes, SCAI Classification, and Outcome Assessment. Medicina 2025, 61, 103. [Google Scholar] [CrossRef] [PubMed]
- Sarma, D.; Jentzer, J.C. Cardiogenic Shock: Pathogenesis, Classification, and Management. Crit. Care Clin. 2024, 40, 37–56. [Google Scholar] [CrossRef]
- Thiele, H.; Ohman, E.M.; de Waha-Thiele, S.; Zeymer, U.; Desch, S. Management of cardiogenic shock complicating myocardial infarction: An update 2019. Eur. Heart J. 2019, 40, 2671–2683. [Google Scholar] [CrossRef]
- Panteleev, O.O.; Ryabov, V.V. Cardiogenic shock: What’s new? Sib. J. Clin. Exp. Med. 2021, 36, 45–51. [Google Scholar] [CrossRef]
- Baran, D.A.; Grines, C.L.; Bailey, S.; Burkhoff, D.; Hall, S.A.; Henry, T.D.; Hollenberg, S.M.; Kapur, N.K.; O’Neill, W.; Ornato, J.P.; et al. SCAI clinical expert consensus statement on the classification of cardiogenic shock: This document was endorsed by the American College of Cardiology (ACC), the American Heart Association (AHA), the Society of Critical Care Medicine (SCCM), and the Society of Thoracic Surgeons (STS) in April 2019. Catheter. Cardiovasc. Interv. 2019, 94, 29–37. [Google Scholar] [CrossRef]
- Kercheva, M.; Ryabov, V.; Gombozhapova, A.; Rebenkova, M.; Kzhyshkowska, J. Macrophages of the "Heart-Kidney" Axis: Their Dynamics and Correlations with Clinical Data and Outcomes in Patients with Myocardial Infarction. J. Pers. Med. 2022, 12, 127. [Google Scholar] [CrossRef]
- Dettling, A.; Weimann, J.; Sundermeyer, J.; Beer, B.N.; Besch, L.; Becher, P.M.; Brunner, F.J.; Kluge, S.; Kirchhof, P.; Blankenberg, S.; et al. Association of systemic inflammation with shock severity, 30-day mortality, and therapy response in patients with cardiogenic shock. Clin. Res. Cardiol. 2024, 113, 324–335. [Google Scholar] [CrossRef]
- Ceglarek, U.; Schellong, P.; Rosolowski, M.; Scholz, M.; Willenberg, A.; Kratzsch, J.; Zeymer, U.; Fuernau, G.; de Waha-Thiele, S.; Büttner, P.; et al. The novel cystatin C, lactate, interleukin-6, and N-terminal pro-B-type natriuretic peptide (CLIP)-based mortality risk score in cardiogenic shock after acute myocardial infarction. Eur. Heart J. 2021, 42, 2344–2352. [Google Scholar] [CrossRef]
- Rueda, F.; Borràs, E.; García-García, C.; Iborra-Egea, O.; Revuelta-López, E.; Harjola, V.P.; Cediel, G.; Lassus, J.; Tarvasmäki, T.; Mebazaa, A.; et al. Protein-based cardiogenic shock patient classifier. Eur. Heart J. 2019, 40, 2684–2694. [Google Scholar] [CrossRef] [PubMed]
- Galusko, V.; Wenzl, F.A.; Vandenbriele, C.; Panoulas, V.; Lüscher, T.F.; Gorog, D.A. Current and novel biomarkers in cardiogenic shock. Eur. J. Heart Fail. 2025. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, Y.; Liu, S.; Lu, P.; Zhou, H.; Yang, M. The systemic inflammation indexes predict all-cause mortality in peritoneal dialysis patients. Ren. Fail. 2023, 45, 2160348. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Geng, X. Prognostic value of the systemic inflammation response index on 3-year outcomes of elderly patients with acute coronary syndrome after stent implantation. Adv. Clin. Exp. Med. 2024. [Google Scholar] [CrossRef] [PubMed]
- Weiß, E.; Ramos, G.C.; Delgobo, M. Myocardial-Treg Crosstalk: How to Tame a Wolf. Front. Immunol. 2022, 13, 914033. [Google Scholar] [CrossRef]
- Jentzer, J.C.; Szekely, Y.; Burstein, B.; Ballal, Y.; Kim, E.Y.; van Diepen, S.; Tabi, M.; Wiley, B.; Kashani, K.B.; Lawler, P.R. Peripheral blood neutrophil-to-lymphocyte ratio is associated with mortality across the spectrum of cardiogenic shock severity. J. Crit. Care 2022, 68, 50–58. [Google Scholar] [CrossRef]
- Djordjevic, D.; Rondovic, G.; Surbatovic, M.; Stanojevic, I.; Udovicic, I.; Andjelic, T.; Zeba, S.; Milosavljevic, S.; Stankovic, N.; Abazovic, D.; et al. Neutrophil-to-Lymphocyte Ratio, Monocyte-to-Lymphocyte Ratio, Platelet-to-Lymphocyte Ratio, and Mean Platelet Volume-to-Platelet Count Ratio as Biomarkers in Critically Ill and Injured Patients: Which Ratio to Choose to Predict Outcome and Nature of Bacteremia? Mediat. Inflamm. 2018, 2018, 3758068. [Google Scholar] [CrossRef]
- Wei, X.; Zhang, Z.; Wei, J.; Luo, C. Association of systemic immune inflammation index and system inflammation response index with clinical risk of acute myocardial infarction. Front. Cardiovasc. Med. 2023, 10, 1248655. [Google Scholar] [CrossRef]
- Zhou, H.; Li, X.; Wang, W.; Zha, Y.; Gao, G.; Li, S.; Liu, B.; Guo, R. Immune-inflammatory biomarkers for the occurrence of MACE in patients with myocardial infarction with non-obstructive coronary arteries. Front. Cardiovasc. Med. 2024, 11, 1367919. [Google Scholar] [CrossRef]
- Yao, L.; Wang, X.; Wang, Z.; Wang, X. A Comprehensive Analysis Exploring the Vital Role of the Systemic Immune-Inflammatory Index Upon Admission in Severe Hemorrhagic Fever with Renal Syndrome. Int. J. Gen. Med. 2024, 17, 4857–4866. [Google Scholar] [CrossRef]
- Ru, S.; Luo, Y. The association and prognostic value of systemic inflammatory response index with short and long-term mortality in patients with sepsis. Medicine 2023, 102, e33967. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yang, L.; Yuan, Z.; Wu, Q.; Lyu, X. The Combination of Systemic Immune-Inflammation Index and Serum Procalcitonin has High Auxiliary Predictive Value for Short-Term Adverse Prognosis in Septic Shock Patients. J. Emerg. Med. 2024, 67, e357–e367. [Google Scholar] [CrossRef] [PubMed]
- Kislitsina, O.N.; Rich, J.D.; Wilcox, J.E.; Pham, D.T.; Churyla, A.; Vorovich, E.B.; Ghafourian, K.; Yancy, C.W. Shock—Classification and Pathophysiological Principles of Therapeutics. Curr. Cardiol. Rev. 2019, 15, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Marchi, F.; Pylypiv, N.; Parlanti, A.; Storti, S.; Gaggini, M.; Paradossi, U.; Berti, S.; Vassalle, C. Systemic Immune-Inflammation Index and Systemic Inflammatory Response Index as Predictors of Mortality in ST-Elevation Myocardial Infarction. J. Clin. Med. 2024, 13, 1256. [Google Scholar] [CrossRef]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.A.; Dweck, M.R.; Galbraith, M.; et al. ESC Scientific Document Group. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef]
- Naidu, S.S.; Baran, D.A.; Jentzer, J.C.; Hollenberg, S.M.; van Diepen, S.; Basir, M.B.; Grines, C.L.; Diercks, D.B.; Hall, S.; Kapur, N.K.; et al. SCAI SHOCK Stage Classification Expert Consensus Update: A Review and Incorporation of Validation Studies: This statement was endorsed by the American College of Cardiology (ACC), American College of Emergency Physicians (ACEP), American Heart Association (AHA), European Society of Cardiology (ESC) Association for Acute Cardiovascular Care (ACVC), International Society for Heart and Lung Transplantation (ISHLT), Society of Critical Care Medicine (SCCM), and Society of Thoracic Surgeons (STS) in December 2021. J. Am. Coll. Cardiol. 2022, 79, 933–946. [Google Scholar] [CrossRef]
- Borisov, R.N.; Zdzitovetskii, D.E.; Kasparov, E.V.; Savchenko, A.A.; Borisov, S.A.; Berdnikov, D.S.; Govorukha, E.S.; Boldyrev, P.N. Types of immune system reactions and their characteristic in patients with generalized purulent peritonitis. Sib. Med. Rev. 2019, 80–87. [Google Scholar] [CrossRef]
- Jentzer, J.C.; Lawler, P.R.; van Diepen, S.; Henry, T.D.; Menon, V.; Baran, D.A.; Džavík, V.; Barsness, G.W.; Holmes, D.R., Jr.; Kashani, K.B. Systemic Inflammatory Response Syndrome Is Associated With Increased Mortality Across the Spectrum of Shock Severity in Cardiac Intensive Care Patients. Circ. Cardiovasc. Qual. Outcomes 2020, 13, e006956. [Google Scholar] [CrossRef]
- Cuinet, J.; Garbagnati, A.; Rusca, M.; Yerly, P.; Schneider, A.G.; Kirsch, M.; Liaudet, L. Cardiogenic shock elicits acute inflammation, delayed eosinophilia, and depletion of immune cells in most severe cases. Sci. Rep. 2020, 10, 7639. [Google Scholar] [CrossRef]
- Kunkel, J.B.; Josiassen, J.; Helgestad, O.K.L.; Schmidt, H.; Holmvang, L.; Jensen, L.O.; Thøgersen, M.; Fosbøl, E.; Ravn, H.B.; Møller, J.E.; et al. Inflammatory response by 48 h after admission and mortality in patients with acute myocardial infarction complicated by cardiogenic shock. Eur. Heart J. Acute Cardiovasc. Care 2023, 12, 306–314. [Google Scholar] [CrossRef]
- Dessap, A.M.; Bagate, F.; Delmas, C.; Morichau-Beauchant, T.; Cholley, B.; Cariou, A.; Lattuca, B.; Moussa, M.; Mongardon, N.; Fard, D.; et al. Low-dose corticosteroid therapy for cardiogenic shock in adults (COCCA): Study protocol for a randomized controlled trial. Trials 2022, 23, 4. [Google Scholar] [CrossRef] [PubMed]
- Parenica, J.; Jarkovsky, J.; Malaska, J.; Mebazaa, A.; Gottwaldova, J.; Helanova, K.; Litzman, J.; Dastych, M.; Tomandl, J.; Spinar, J.; et al. GREAT Network. Infectious Complications and Immune/Inflammatory Response in Cardiogenic Shock Patients: A Prospective Observational Study. Shock 2017, 47, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Soussi, S.; Tarvasmäki, T.; Kimmoun, A.; Ahmadiankalati, M.; Azibani, F.; Dos Santos, C.C.; Duarte, K.; Gayat, E.; Jentzer, J.C.; Harjola, V.P.; et al. Identifying biomarker-driven subphenotypes of cardiogenic shock: Analysis of prospective cohorts and randomized controlled trials. EClinicalMedicine 2024, 79, 103013. [Google Scholar] [CrossRef] [PubMed]
- Nunez, J.I.; Uehara, M.; Patel, S.R.; Forest, S.J.; Rochlani, Y.; Madan, S.; Sims, D.B.; Mellas, N.; Ashley, J.E.; Rahmanian, M.; et al. Changes in Neutrophil-to-Lymphocyte Ratio During Venoarterial Extracorporeal Membrane Oxygenation. ASAIO J. 2025. [Google Scholar] [CrossRef]
- Kologrivova, I.; Kercheva, M.; Panteleev, O.; Ryabov, V. The Role of Inflammation in the Pathogenesis of Cardiogenic Shock Secondary to Acute Myocardial Infarction: A Narrative Review. Biomedicines 2024, 12, 2073. [Google Scholar] [CrossRef]
- Jung, C.; Bruno, R.R.; Jumean, M.; Price, S.; Krychtiuk, K.A.; Ramanathan, K.; Dankiewicz, J.; French, J.; Delmas, C.; Mendoza, A.A.; et al. Management of cardiogenic shock: State-of-the-art. Intensive Care Med. 2024, 50, 1814–1829. [Google Scholar] [CrossRef]
- Lee, H.H.; Hong, S.J.; Ahn, C.M.; Yang, J.H.; Gwon, H.C.; Kim, J.S.; Kim, B.K.; Ko, Y.G.; Choi, D.; Hong, M.K.; et al. Clinical Implications of Thrombocytopenia at Cardiogenic Shock Presentation: Data from a Multicenter Registry. Yonsei Med. J. 2020, 61, 851–859. [Google Scholar] [CrossRef]
- Wang, L.; Shao, J.; Shao, C.; Wang, H.; Jia, M.; Hou, X. The Relative Early Decrease in Platelet Count Is Associated with Mortality in Post-cardiotomy Patients Undergoing Venoarterial Extracorporeal Membrane Oxygenation. Front. Med. 2021, 8, 733946. [Google Scholar] [CrossRef]
- del Rosario Espinoza Mora, M.; Böhm, M.; Link, A. The Th17/Treg imbalance in patients with cardiogenic shock. Clin. Res. Cardiol. 2014, 103, 301–313. [Google Scholar] [CrossRef]
- Kleinschnitz, C.; Kraft, P.; Dreykluft, A.; Hagedorn, I.; Göbel, K.; Schuhmann, M.K.; Langhauser, F.; Helluy, X.; Schwarz, T.; Bittner, S.; et al. Regulatory T cells are strong promoters of acute ischemic stroke in mice by inducing dysfunction of the cerebral microvasculature. Blood 2013, 121, 679–691. [Google Scholar] [CrossRef]
- Liu, D.; Huang, S.Y.; Sun, J.H.; Zhang, H.C.; Cai, Q.L.; Gao, C.; Li, L.; Cao, J.; Xu, F.; Zhou, Y.; et al. Sepsis-induced immunosuppression: Mechanisms, diagnosis and current treatment options. Mil. Med. Res. 2022, 9, 56. [Google Scholar] [CrossRef] [PubMed]
- Yilu, Z.; Zhanglong, W.; Fanke, H.; Jing, G.; Yue, W.; Yuwen, C.; Bingqing, L.; Jianfeng, L. The progression of non-culprit coronary lesion is related to higher SII, SIRI, and PIV in patients with ACS. Medicine 2024, 103, e41094. [Google Scholar] [CrossRef] [PubMed]
- Qu, C.; Li, X.; Gao, H. The Impact of Systemic Inflammation Response Index on the Prognosis of Patients with ST-Segment Elevation Myocardial Infarction Undergoing Percutaneous Coronary Intervention. Rev. Cardiovasc. Med. 2023, 24, 153. [Google Scholar] [CrossRef] [PubMed]
- Han, K.; Shi, D.; Yang, L.; Wang, Z.; Li, Y.; Gao, F.; Liu, Y.; Ma, X.; Zhou, Y. Prognostic value of systemic inflammatory response index in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Ann. Med. 2022, 54, 1667–1677. [Google Scholar] [CrossRef]
- Fan, W.; Wei, C.; Liu, Y.; Sun, Q.; Tian, Y.; Wang, X.; Liu, J.; Zhang, Y.; Sun, L. The Prognostic Value of Hematologic Inflammatory Markers in Patients with Acute Coronary Syndrome Undergoing Percutaneous Coronary Intervention. Clin. Appl. Thromb. Hemost. 2022, 28, 10760296221146183. [Google Scholar] [CrossRef]
- Rajakumar, H.K.; Coimbatore Sathyabal, V.; Vasanthan, M.; Dasarathan, R. The predictive role of Systemic Inflammation Response Index (SIRI), Neutrophil-Lymphocyte Ratio (NLR), and Platelet-Lymphocyte Ratio (PLR) in the prognosis of acute coronary syndrome in the prognosis of acute coronary syndrome in a tertiary care hospital. Heliyon 2024, 10, e39029. [Google Scholar] [CrossRef]
- Orrem, H.L.; Nilsson, P.H.; Pischke, S.E.; Grindheim, G.; Garred, P.; Seljeflot, I.; Husebye, T.; Aukrust, P.; Yndestad, A.; Andersen, G.Ø.; et al. Acute heart failure following myocardial infarction: Complement activation correlates with the severity of heart failure in patients developing cardiogenic shock. ESC Heart Fail. 2018, 5, 292–301. [Google Scholar] [CrossRef]
- Okun, E.; Griffioen, K.J.; Rothman, S.; Wan, R.; Cong, W.N.; De Cabo, R.; Martin-Montalvo, A.; Levette, A.; Maudsley, S.; Martin, B.; et al. Toll-like receptors 2 and 4 modulate autonomic control of heart rate and energy metabolism. Brain Behav. Immun. 2014, 36, 90–100. [Google Scholar] [CrossRef]
- Al-Rashed, F.; Sindhu, S.; Al Madhoun, A.; Ahmad, Z.; AlMekhled, D.; Azim, R.; Al-Kandari, S.; Wahid, M.A.; Al-Mulla, F.; Ahmad, R. Elevated resting heart rate as a predictor of inflammation and cardiovascular risk in healthy obese individuals. Sci. Rep. 2021, 11, 13883. [Google Scholar] [CrossRef]
- Frasch, M.G. Heart Rate as a Non-Invasive Biomarker of Inflammation: Implications for Digital Health. Front. Immunol. 2022, 13, 930445. [Google Scholar] [CrossRef]
- Kunkel, J.B.; Frydland, M.; Holle, S.D.; Kjaergaard, J.; Pecini, R.; Bang, L.E.; Palm, P.; Wiberg, S.; Holmvang, L.; Engstroem, T.; et al. Low-dose dobutamine infusion and single-dose tocilizumab in acute myocardial infarction patients with high risk of cardiogenic shock development -- rationale and design of the DOBERMANN trial. Eur. Heart J. Acute Cardiovasc. Care 2023, 12, zuad036.131. [Google Scholar] [CrossRef]
- Fattouch, K.; Bianco, G.; Speziale, G.; Sampognaro, R.; Lavalle, C.; Guccione, F.; Dioguardi, P.; Ruvolo, G. Beneficial effects of C1 esterase inhibitor in ST-elevation myocardial infarction in patients who underwent surgical reperfusion: A randomised double-blind study. Eur. J. Cardiothorac. Surg. 2007, 32, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Deniau, B.; Rehfeld, L.; Santos, K.; Dienelt, A.; Azibani, F.; Sadoune, M.; Kounde, P.R.; Samuel, J.L.; Tolpannen, H.; Lassus, J.; et al. Circulating dipeptidyl peptidase 3 is a myocardial depressant factor: Dipeptidyl peptidase 3 inhibition rapidly and sustainably improves haemodynamics. Eur. J. Heart Fail. 2020, 22, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Torzewski, J.; Mattecka, S.; Ries, W.; Garlichs, C.D.; Heigl, F.; Fiedler, J.; Sheriff, A. Case report: C-reactive protein apheresis in cardiogenic shock: Case series from the C-reactive protein apheresis in acute myocardial infarction-registry. Front. Drug Discov. 2023, 3, 1286710. [Google Scholar] [CrossRef]
- Cao, M.; Wang, G.; Xie, J. Immune dysregulation in sepsis: Experiences, lessons and perspectives. Cell Death Discov. 2023, 9, 465. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, L.; Li, Y.; Cao, Y.; Wang, M.; Deng, Z.; Kang, H. Immunotherapy in the context of sepsis-induced immunological dysregulation. Front. Immunol. 2024, 15, 1391395. [Google Scholar] [CrossRef]
- Matter, M.A.; Paneni, F.; Libby, P.; Frantz, S.; Stähli, B.E.; Templin, C.; Mengozzi, A.; Wang, Y.J.; Kündig, T.M.; Räber, L.; et al. Inflammation in acute myocardial infarction: The good, the bad and the ugly. Eur. Heart J. 2024, 45, 89–103. [Google Scholar] [CrossRef]
- Montero, S.; Bayes-Genis, A. The overlooked tsunami of systemic inflammation in post-myocardial infarction cardiogenic shock. Eur. J. Prev. Cardiol. 2022, 29, 2052–2054. [Google Scholar] [CrossRef]
- Kunkel, J.B.; Holle, S.L.D.; Hassager, C.; Pecini, R.; Wiberg, S.; Palm, P.; Holmvang, L.; Bang, L.E.; Kjærgaard, J.; Thomsen, J.H.; et al. Interleukin-6 receptor antibodies (tocilizumab) in acute myocardial infarction with intermediate to high risk of cardiogenic shock development (DOBERMANN-T): Study protocol for a double-blinded, placebo-controlled, single-center, randomized clinical trial. Trials 2024, 25, 739. [Google Scholar] [CrossRef]
- Santas, E.; Villar, S.; Palau, P.; Llàcer, P.; de la Espriella, R.; Miñana, G.; Lorenzo, M.; Núñez-Marín, G.; Górriz, J.L.; Carratalá, A.; et al. High-sensitivity C-reactive protein and risk of clinical outcomes in patients with acute heart failure. Sci. Rep. 2024, 14, 21672. [Google Scholar] [CrossRef]
| Parameters | Lymphocytes, Absolute Counts | |||
|---|---|---|---|---|
| Below the Reference Range | Within the Reference Range | Above the Reference Range | ||
| Leucocytes, absolute counts | Above the reference range | Innate immunity activation | Innate immunity activation | Adaptive immunity activation |
| Within the reference range | Immune insufficiency | Areactive state | Adaptive immunity activation | |
| Below the reference range | Immune insufficiency | Immune insufficiency | Adaptive immunity activation | |
| Parameter | SCAI Stage A (n = 7) | SCAI Stage B (n = 10) | SCAI Stage C (n = 84) | SCAI Stage D (n = 11) | SCAI Stage E (n = 27) | p | |
|---|---|---|---|---|---|---|---|
| Age, years | 68 (58; 71) | 66 (59; 74) | 73 (65; 81) | 80 (69; 82) | 80 (75; 87) | 0.004 | |
| Men, % | 71.4 | 100.0 | 47.6 | 54.5 | 33.3 | 0.006 | |
| Arterial hypertension, % | 85.7 | 100.0 | 94.0 | 90.9 | 92.6 | 0.811 | |
| Diabetes mellitus, % | 42.9 | 10.0 | 32.1 | 45.5 | 14.8 | 0.195 | |
| Obesity, % | 42.9 | 40.0 | 28.6 | 18.2 | 85.2 | 0.655 | |
| STEMI/NSTEMI, % | 71.4/28.6 | 90.0/10.0 | 77.4/22.6 | 81.8/18.2 | 70.4/29.6 | 0.757 | |
| “Pain-to-door” time, minutes | - | 149 (100; 374) | 220 (137; 540) | 324 (139; 1080) | 153 (90; 410) | 0.134 | |
| “Door-to-balloon” time, minutes | - | 84 (50; 120) | 63 (52; 145) | 114 (44; 230) | 51 (43; 115) | 0.130 | |
| PCI, n (%) | 5 (71.4) | 6 (60.0) | 55 (65.5) | 9 (81.8) | 14 (51.9) | 0.710 | |
| TIMI 3 flow in PCI, n (%) | restored | 3 (42.8) | 7 (70) | 38 (45.2) | 8 (72.7) | 9 (33.3) | 0.999 |
| not restored | 1 (14.4) | 1 (10) | 16 (19.1) | 2 (18.2) | 5 (18.5) | ||
| no data | 3 (42.8) | 2 (20) | 30 (35.7) | 1 (9.1) | 13 (48.2) | ||
| Maximum troponin I level, ng/mL | 0.47 (0.15; 1.30) | 0.49 (0.04; 6.4) | 0.50 (0.06; 3.20) | 5.90 (0.61; 14.00) | 0.37 (0.09; 6.32) | 0.336 | |
| Vasocative inotropic score, points | - | 7.0 (5.0; 10.0) | 7.5 (5.0; 26.0) | 30.0 (8.0; 50.0) | 40.0 (5.0; 62.0) | 0.999 | |
| Lactate, mmol/L | 1.9 (1.8; 1.9) | 1.7 (1.3; 1.7) | 3.0 (2.4; 5.1) | 4.6 (2.6; 6.0) | 8.7 (6.9; 11.4) | <0.001 | |
| pH (venous) | 7.41 (7.21; 7.44) | 7.39 (7.33; 7.42) | 7.32 (7.27; 7.35) | 7.30 (7.26; 7.31) | 7.14 (7.06; 7.19) | <0.001 | |
| Hb, g/L | 144 (113; 151) | 148 (119; 156) | 134 (116; 145) | 128 (116; 137) | 120 (103; 138) | 0.125 | |
| Central venous pressure, cm H2O | 5.0 (4.0; 6.0) | 9.0 (6.0; 10.0) | 12.0 (8.0; 15.0) | 11.5 (9.0; 13.0) | 16.0 (8.0; 19.0) | 0.062 | |
| Number of arteries with stenosis, %: | |||||||
| 1 artery | 14.3 | 18.5 | 20.2 | 18.2 | 18.5 | 0.958 | |
| 2 arteries | 42.9 | 14.8 | 27.4 | 36.4 | 14.8 | ||
| 3 arteries | 42.9 | 33.3 | 40.5 | 45.5 | 33.3 | ||
| Mortality, % | 71.4 | 40.0 | 44.0 | 63.6 | 85.2 | 0.003 | |
| Parameters | SCAI Stage A (n = 7) | SCAI Stage B (n = 10) | SCAI Stage C (n = 84) | SCAI Stage D (n = 11) | SCAI Stage E (n = 27) | p |
|---|---|---|---|---|---|---|
| Leucocytes, ×109/L | 10.5 (10.4; 12.8) | 11.6 (9.4; 13.7) | 13.7 (10.3; 15.9) | 11.8 (9.9; 17.1) | 14.1 (8.4; 17.8) | 0.452 |
| Erythrocytes, ×1012/L | 4.5 (3.9; 5.1) | 4.5 (4.2; 5.2) | 4.4 (4.0; 5.0) | 4.5 (4.1; 4.8) | 4.3 (3.6; 5.0) | 0.697 |
| Platelets, ×109/L | 217 (186; 319) | 221 (188; 249) | 244 (175; 297) | 208 (186; 366) | 188 (139; 247) | 0.108 |
| Neutrophils, ×109/L | 7.7 (6.5; 9.5) | 8.5 (6.9; 10.6) | 9.7 (7.1; 13.0) | 8.0 (6.7; 14.8) | 6.8 (3.6; 11.7) | 0.104 |
| Lymphocytes, ×109/L | 2.5 (1.8; 2.6) | 1.9 (1.5; 3.5) | 2.1 (1.5; 3.1) | 1.8 (1.1; 3.2) | 2.7 (1.1; 6.6) | 0.635 |
| Monocytes, ×109/L | 0.98 (0.68; 1.21) | 0.99 (0.72; 1.28) | 1.02 (0.67; 1.31) | 0.55 (0.44; 0.89) | 0.81 (0.49; 0.99) | 0.022 |
| Parameter | SCAI Stage C, Alive Patients (n = 25) | SCAI Stage C, Deceased Patients (n = 19) | p |
|---|---|---|---|
| Age, years | 70 (61; 75) | 75 (68; 81) | 0.028 |
| Men (%) | 14 (56.0) | 11 (57.9) | 0.999 |
| Arterial hypertension (%) | 25 (100) | 18 (94.7) | 0.432 |
| Diabetes mellitus (%) | 8 (32) | 8 (42.1) | 0.999 |
| Obesity (%) | 10 (40) | 7 (36.8) | 0.999 |
| STEMI/NSTEMI (%) | 21/4 (84.0/16.0) | 12/7 (63.2/36.8) | 0.164 |
| “Pain-to-door” time, minutes | 185 (87; 335) | 288 (165; 720) | 0.323 |
| “Door-to-balloon” time, minutes | 68 (54; 118) | 59 (52; 102) | 0.538 |
| Vasocative inotropic score, points | 0.07 (0.04; 0.22) | 0.67 (0.05; 1.50) | 0.309 |
| Lactate, mmol/L | 7 (3; 10) | 6 (2; 10) | 0.917 |
| pH (venous) | 3.0 (2.4; 5.8) | 3.0 (2.4; 5.0) | 0.689 |
| Hb, g/L | 7.31 (7.27; 7.34) | 7.32 (7.23; 7.33) | 0.657 |
| Central venous pressure, cm H2O | 140 (129; 153) | 125 (100; 138) | 0.025 |
| Vasocative inotropic score, points | 10.0 (8.0; 15.0) | 11.5 (7.5; 15.0) | 0.750 |
| Parameter | SCAI Stage C, Alive Patients (n = 25) | SCAI Stage C, Deceased Patients (n = 19) | p |
|---|---|---|---|
| Leucocytes, ×109/L | 12.3 (9.6; 15.8) | 13.4 (10.3; 15.1) | 0.870 |
| Erythrocytes, ×1012/L | 4.7 (4.1; 5.0) | 4.2 (3.9; 4.6) | 0.103 |
| Platelets, ×109/L | 243 (201; 281) | 288 (175; 358) | 0.511 |
| Neutrophils, ×109/L | 9.4 (6.1; 11.5) | 9.5 (7.4; 16.3) | 0.279 |
| Lymphocytes, ×109/L | 2.6 (1.9; 3.2) | 2.2 (1.9; 2.9) | 0.212 |
| Monocytes, ×109/L | 0.91 (0.64; 1.20) | 1.02 (0.68; 1.42) | 0.411 |
| NLR | 3.20 (2.33; 4.92) | 3.96 (3.44; 9.22) | 0.119 |
| MLR | 0.29 (0.24; 0.45) | 0.47 (0.34; 0.70) | 0.046 |
| PLR | 97.4 (71.4; 128.2) | 136.5 (74.9; 225.2) | 0.157 |
| AISI | 600 (363; 1368) | 1430 (546; 2934) | 0.093 |
| SIRI | 2.38 (1.61; 5.23) | 5.47 (2.69; 7.28) | 0.036 |
| SII | 853 (541; 1298) | 1495 (440; 3445.9) | 0.089 |
| Parameters | Estimates | p |
|---|---|---|
| Intercept | −2.74 | 0.008 |
| Heartbeat | 0.02 | 0.020 |
| CK-MB | 0.01 | 0.142 |
| Activation of innate immunity | 1.49 | 0.007 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kologrivova, I.; Kercheva, M.; Panteleev, O.; Dmitriukov, A.; Zenkov, I.; Suslova, T.; Ryabov, V. Inflammatory Indices in Patients with Myocardial Infarction Complicated by Cardiogenic Shock, and Their Interconnections with SCAI Stages and Patients’ Survival: A Retrospective Study. J. Clin. Med. 2025, 14, 4283. https://doi.org/10.3390/jcm14124283
Kologrivova I, Kercheva M, Panteleev O, Dmitriukov A, Zenkov I, Suslova T, Ryabov V. Inflammatory Indices in Patients with Myocardial Infarction Complicated by Cardiogenic Shock, and Their Interconnections with SCAI Stages and Patients’ Survival: A Retrospective Study. Journal of Clinical Medicine. 2025; 14(12):4283. https://doi.org/10.3390/jcm14124283
Chicago/Turabian StyleKologrivova, Irina, Maria Kercheva, Oleg Panteleev, Alexey Dmitriukov, Ivan Zenkov, Tatiana Suslova, and Vyacheslav Ryabov. 2025. "Inflammatory Indices in Patients with Myocardial Infarction Complicated by Cardiogenic Shock, and Their Interconnections with SCAI Stages and Patients’ Survival: A Retrospective Study" Journal of Clinical Medicine 14, no. 12: 4283. https://doi.org/10.3390/jcm14124283
APA StyleKologrivova, I., Kercheva, M., Panteleev, O., Dmitriukov, A., Zenkov, I., Suslova, T., & Ryabov, V. (2025). Inflammatory Indices in Patients with Myocardial Infarction Complicated by Cardiogenic Shock, and Their Interconnections with SCAI Stages and Patients’ Survival: A Retrospective Study. Journal of Clinical Medicine, 14(12), 4283. https://doi.org/10.3390/jcm14124283






