The Role of Cardiovascular Magnetic Resonance Imaging in Athletic Individuals—A Narrative Review
Abstract
1. Introduction
2. Clinical Indications for Cardiac MRI in Athletes
3. Cardiac Magnetic Resonance Imaging in Athletes—A Practical Perspective
3.1. Preparation
3.2. Expected or Anticipated? Think Twice
3.3. Technical Challenges—Practical Tips
3.4. Contrast Agents
3.5. Cardiac MRI in Athletes with Cardiac Implantable Electronic Devices
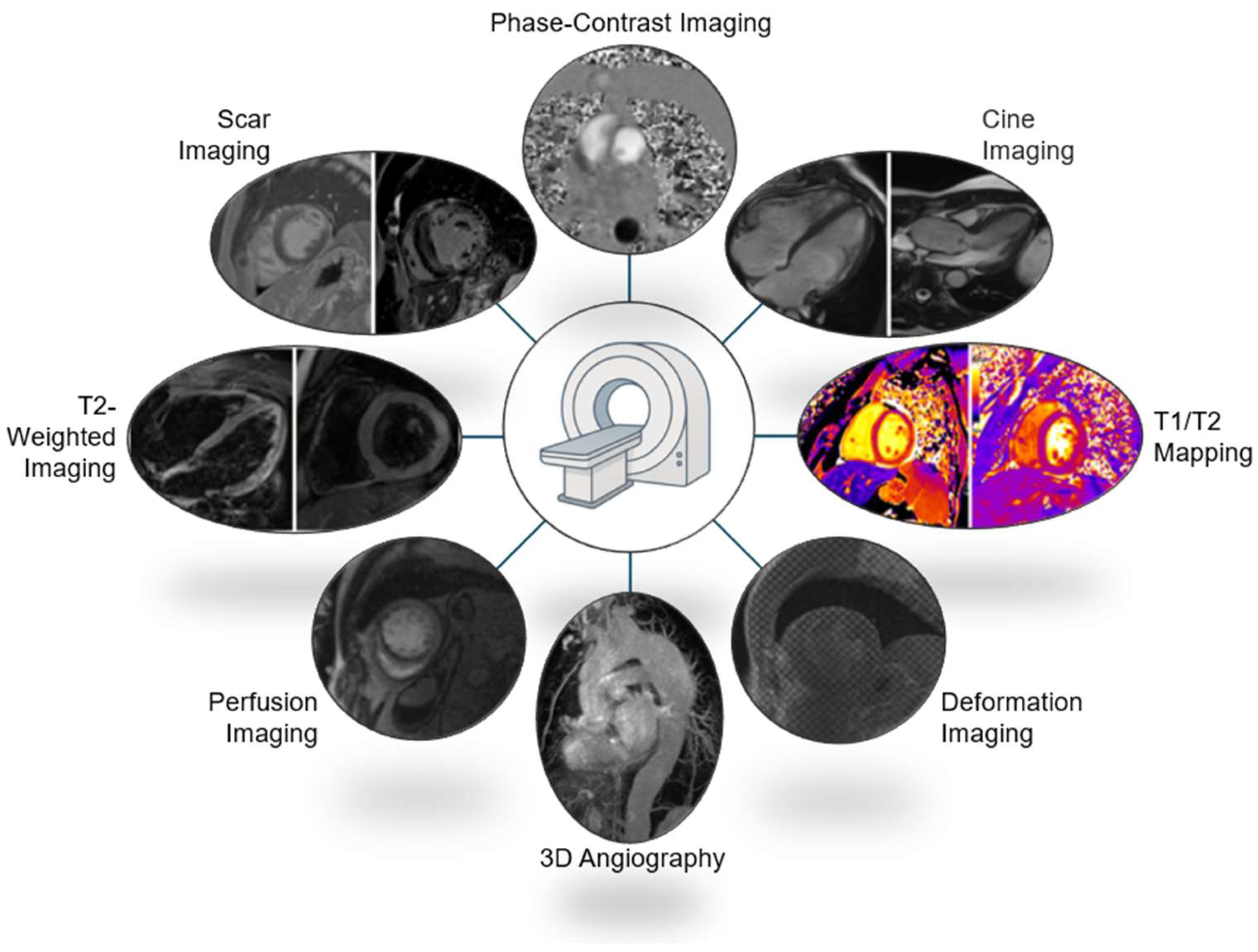
3.6. Cardiac MRI Protocols
3.7. Gaps in Evidence
4. Athlete’s Heart
5. Family History of Cardiomyopathy
6. Pre-Clinical Cardiomyopathy
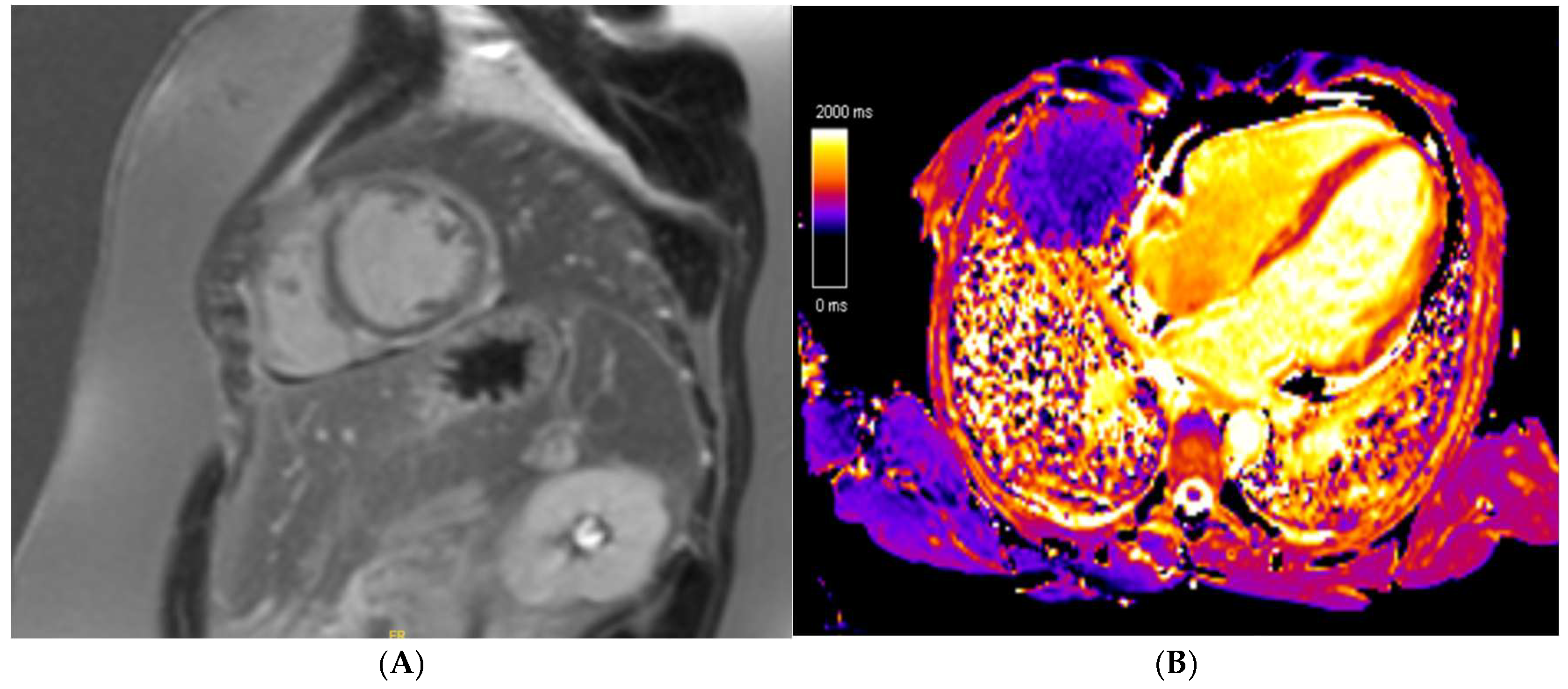
7. Hypertrophic Cardiomyopathy
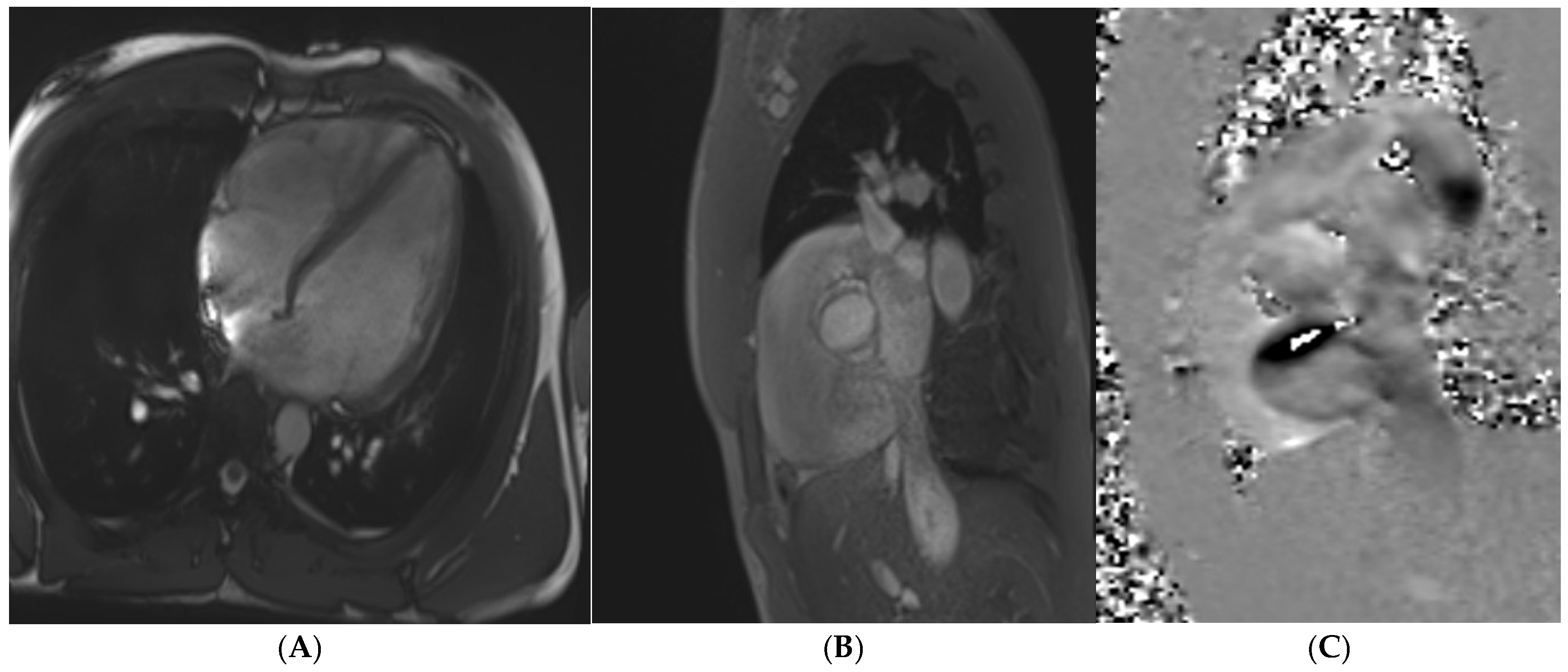
8. Dilated Cardiomyopathy
9. Non-Dilated Left Ventricular Cardiomyopathy
10. Arrhythmogenic Right Ventricular Cardiomyopathy
11. Left Ventricular Hypertrabeculation
12. Mitral Valve Prolapse
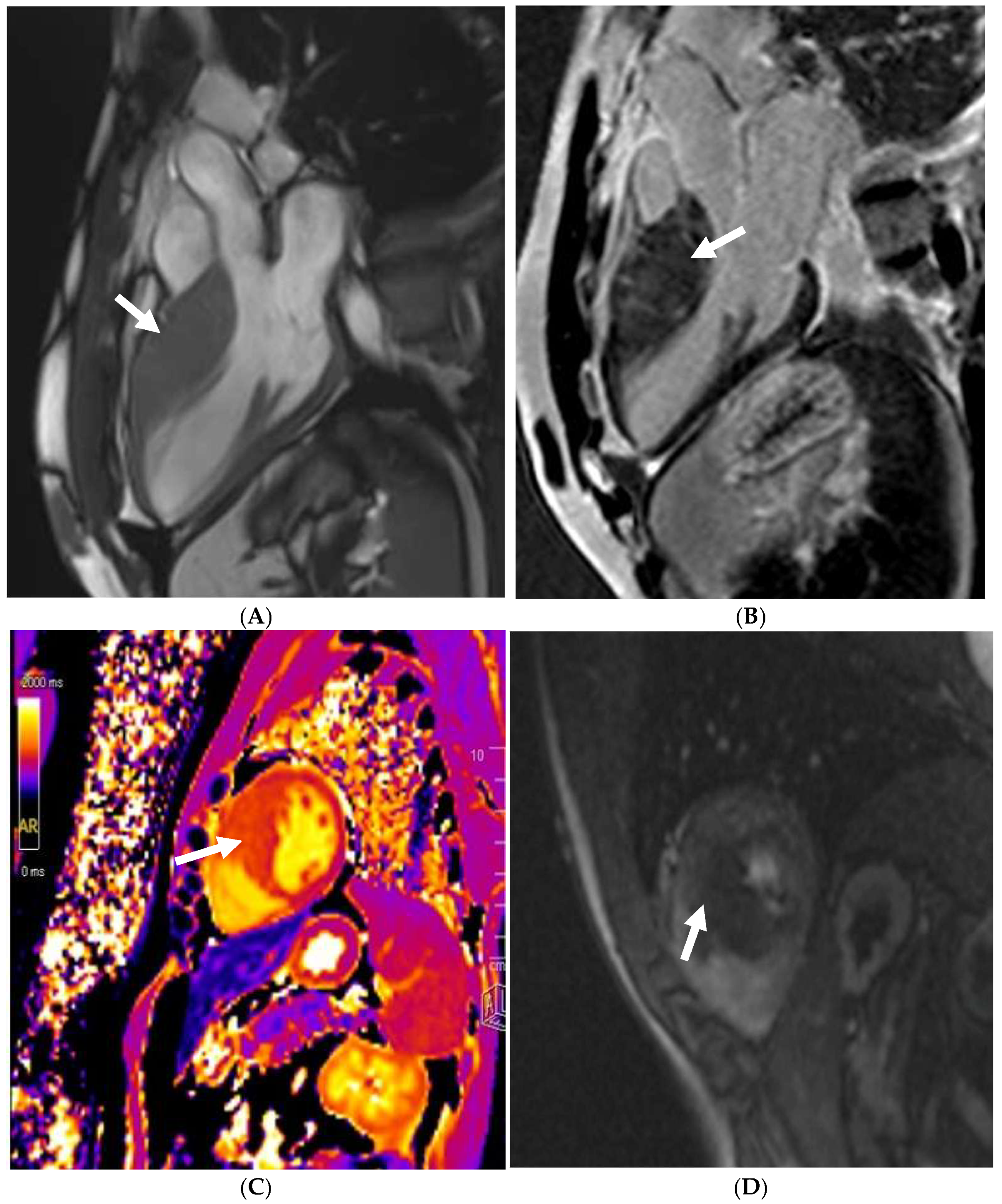
13. Bicuspid Aortic Valve and Aortopathy
14. Other Valvular Heart Disease
15. Congenital Heart Disease
16. Ischaemic Heart Disease
17. Myocardial Infarction with Non-Obstructive Coronaries
18. Coronary Artery Anomalies
19. Myocarditis
20. Pericarditis
21. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharma, S.; Merghani, A.; Mont, L. Exercise and the heart: The good, the bad, and the ugly. Eur. Heart J. 2015, 36, 1445–1453. [Google Scholar] [CrossRef]
- Kokkinos, P.; Myers, J.; Faselis, C.; Panagiotakos, D.B.; Doumas, M.; Pittaras, A.; Manolis, A.; Kokkinos, J.P.; Karasik, P.; Greenberg, M.; et al. Exercise Capacity and Mortality in Older Men. Circulation 2010, 122, 790–797. [Google Scholar] [CrossRef]
- WHO. Guidelines on Physical Activity and Sedentary Behaviour; World Health Organization: Geneva, Switzerland, 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK566046/ (accessed on 24 January 2025).
- Dvorak, J.; Grimm, K.; Schmied, C.; Junge, A. Development and implementation of a standardized precompetition medical assessment of international elite football players-2006 FIFA world cup Germany. Clin. J. Sport Med. 2009, 19, 316–321. [Google Scholar] [CrossRef]
- Ljungqvist, A.; Jenoure, P.J.; Engebretsen, L.; Alonso, J.M.; Bahr, R.; Clough, A.F.; de Bondt, G.; Dvorak, J.; Maloley, R.; Matheson, G.; et al. The International Olympic Committee (IOC) Consensus Statement on Periodic Health Evaluation of Elite Athletes, March 2009. Clin. J. Sport Med. 2009, 43, 631–643. [Google Scholar] [CrossRef]
- Finocchiaro, G.; Westaby, J.; Sheppard, M.N.; Papadakis, M.; Sharma, S. Sudden Cardiac Death in Young Athletes: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2024, 83, 350–370. [Google Scholar] [CrossRef] [PubMed]
- Finocchiaro, G.; Radaelli, D.; D’errico, S.; Bhatia, R.; Papadakis, M.; Behr, E.R.; Westaby, J.; Sharma, S.; Sheppard, M.N. Ethnicity and sudden cardiac death in athletes: Insights from a large United Kingdom registry. Eur. J. Prev. Cardiol. 2024, 31, 1518–1525. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.W.; Kim, J.H.; Shah, A.B.; Phelan, D.; Emery, M.S.; Wasfy, M.M.; Fernandez, A.B.; Bunch, T.J.; Dean, P.; Danielian, A.; et al. Exercise-Induced Cardiovascular Adaptations and Approach to Exercise and Cardiovascular Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 78, 1453–1470. [Google Scholar] [CrossRef]
- Kim, J.H.; Baggish, A.L.; Levine, B.D.; Ackerman, M.J.; Day, S.M.; Dineen, E.H.; Guseh, J.S.; La Gerche, A.; Lampert, R.; Martinez, M.W.; et al. Clinical Considerations for Competitive Sports Participation for Athletes with Cardiovascular Abnormalities: A Scientific Statement from the American Heart Association and American College of Cardiology. Circulation 2025, 151, e716–e761. [Google Scholar] [CrossRef] [PubMed]
- Pelliccia, A.; Sharma, S.; Gati, S.; Bäck, M.; Börjesson, M.; Caselli, S.; Collet, J.-P.; Corrado, D.; Drezner, J.A.; Halle, M.; et al. 2020 ESC Guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur. Heart J. 2021, 42, 17–96. [Google Scholar] [CrossRef]
- Pelliccia, A.; Caselli, S.; Sharma, S.; Basso, C.; Bax, J.J.; Corrado, D.; D’andrea, A.; D’ascenzi, F.; Di Paolo, F.M.; Edvardsen, T.; et al. European Association of Preventive Cardiology (EAPC) and European Association of Cardiovascular Imaging (EACVI) joint position statement: Recommendations for the indication and interpretation of cardiovascular imaging in the evaluation of the athlete. Eur. Heart J. 2017, 39, 1949–1969. [Google Scholar] [CrossRef]
- Szabo, L.; Brunetti, G.; Cipriani, A.; Juhasz, V.; Graziano, F.; Hirschberg, K.; Dohy, Z.; Balla, D.; Drobni, Z.; Marra, M.P.; et al. Certainties and Uncertainties of Cardiac Magnetic Resonance Imaging in Athletes. J. Cardiovasc. Dev. Dis. 2022, 9, 361. [Google Scholar] [CrossRef] [PubMed]
- Prakken, N.H.; Velthuis, B.K.; Teske, A.J.; Mosterd, A.; Mali, W.P.; Cramer, M.J. Cardiac MRI reference values for athletes and nonathletes corrected for body surface area, training hours/week and sex. Eur. J. Prev. Cardiol. 2010, 17, 198–203. [Google Scholar] [CrossRef] [PubMed]
- La Gerche, A.; Burns, A.T.; Taylor, A.J.; MacIsaac, A.I.; Heidbüchel, H.; Prior, D.L. Maximal oxygen consumption is best predicted by measures of cardiac size rather than function in healthy adults. Eur. J. Appl. Physiol. 2011, 112, 2139–2147. [Google Scholar] [CrossRef]
- Letnes, J.M.; Nes, B.M.; Langlo, K.A.R.; Aksetøy, I.-L.A.; Lundgren, K.M.; Skovereng, K.; Sandbakk, Ø.; Wisløff, U.; Dalen, H. Indexing cardiac volumes for peak oxygen uptake to improve differentiation of physiological and pathological remodeling: From elite athletes to heart failure patients. Eur. Hear. J. Cardiovasc. Imaging 2023, 24, 721–729. [Google Scholar] [CrossRef]
- Claessen, G.; De Bosscher, R.; Janssens, K.; Young, P.; Dausin, C.; Claeys, M.; Claus, P.; Goetschalckx, K.; Bogaert, J.; Mitchell, A.M.; et al. Reduced Ejection Fraction in Elite Endurance Athletes: Clinical and Genetic Overlap with Dilated Cardiomyopathy. Circulation 2024, 149, 1405–1415. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, A.; Sheikh, N.; Jongman, J.K.; Gati, S.; Panoulas, V.F.; Carr-White, G.; Papadakis, M.; Sharma, R.; Behr, E.R.; Sharma, S. Clinical differentiation between physiological remodeling and arrhythmogenic right ventricular cardiomyopathy in athletes with marked electrocardiographic repolarization anomalies. J. Am. Coll. Cardiol. 2015, 65, 2702–2711. [Google Scholar] [CrossRef]
- Finocchiaro, G.; Dhutia, H.; D’silva, A.; Malhotra, A.; Steriotis, A.; Millar, L.; Prakash, K.; Narain, R.; Papadakis, M.; Sharma, R.; et al. Effect of Sex and Sporting Discipline on LV Adaptation to Exercise. JACC Cardiovasc. Imaging 2017, 10, 965–972. [Google Scholar] [CrossRef]
- Kramer, C.M.; Barkhausen, J.; Bucciarelli-Ducci, C.; Flamm, S.D.; Kim, R.J.; Nagel, E. Standardized cardiovascular magnetic resonance imaging (CMR) protocols: 2020 update. J. Cardiovasc. Magn. Reson. 2020, 22, 17. [Google Scholar] [CrossRef]
- Pfaffenberger, S.; Bartko, P.; Graf, A.; Pernicka, E.; Babayev, J.; Lolic, E.; Bonderman, D.; Baumgartner, H.; Maurer, G.; Mascherbauer, J. Size matters! Impact of age, sex, height, and weight on the normal heart size. Circ. Cardiovasc. Imaging 2013, 6, 1073–1079. [Google Scholar] [CrossRef]
- Torlasco, C.; Castelletti, S.; Soranna, D.; Volpato, V.; Figliozzi, S.; Menacho, K.; Cernigliaro, F.; Zambon, A.; Kellman, P.; Moon, J.C.; et al. Effective Study: Development and Application of a Question-Driven, Time-Effective Cardiac Magnetic Resonance Scanning Protocol. J. Am. Heart Assoc. 2022, 11, e022605. [Google Scholar] [CrossRef]
- Kanda, T.; Fukusato, T.; Matsuda, M.; Toyoda, K.; Oba, H.; Kotoku, J.; Haruyama, T.; Kitajima, K.; Furui, S. Gadolinium-based Contrast Agent Accumulates in the Brain Even in Subjects without Severe Renal Dysfunction: Evaluation of Autopsy Brain Specimens with Inductively Coupled Plasma Mass Spectroscopy. Radiology 2015, 276, 228–232. [Google Scholar] [CrossRef]
- Grobner, T. Gadolinium—A specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol. Dial. Transplant. 2006, 21, 1104–1108. [Google Scholar] [CrossRef] [PubMed]
- Martino, F.; Amici, G.; Rosner, M.; Ronco, C.; Novara, G. Gadolinium-based contrast media nephrotoxicity in kidney impairment: The physio-pathological conditions for the perfect murder. J. Clin. Med. 2021, 10, 271. [Google Scholar] [CrossRef]
- Bonner, B.P.; Yurista, S.R.; Coll-Font, J.; Chen, S.; Eder, R.A.; Foster, A.N.; Nguyen, K.D.; Caravan, P.; Gale, E.M.; Nguyen, C. Contrast-Enhanced Cardiac Magnetic Resonance Imaging with a Manganese-Based Alternative to Gadolinium for Tissue Characterization of Acute Myocardial Infarction. J. Am. Heart Assoc. 2023, 12, 8. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Collins, J.D.; White, J.A.; Hanneman, K.; Lee, D.C.; Patel, A.R.; Hu, P.; Litt, H.; Weinsaft, J.W.; Davids, R.; et al. SCMR expert consensus statement for cardiovascular magnetic resonance of patients with a cardiac implantable electronic device. J. Cardiovasc. Magn. Reson. 2024, 26, 100995. [Google Scholar] [CrossRef]
- Vuorinen, A.-M.; Lehmonen, L.; Karvonen, J.; Holmström, M.; Kivistö, S.; Kaasalainen, T. Reducing cardiac implantable electronic device–induced artefacts in cardiac magnetic resonance imaging. Eur. Radiol. 2022, 33, 1229–1242. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yang, W.; Zhao, S.; Lu, M. State-of-the-art myocardial strain by CMR feature tracking: Clinical applications and future perspectives. Eur. Radiol. 2022, 32, 5424–5435. [Google Scholar] [CrossRef]
- Cavus, E.; Muellerleile, K.; Schellert, S.; Schneider, J.; Tahir, E.; Chevalier, C.; Jahnke, C.; Radunski, U.K.; Adam, G.; Kirchhof, P.; et al. CMR feature tracking strain patterns and their association with circulating cardiac biomarkers in patients with hypertrophic cardiomyopathy. Clin. Res. Cardiol. 2021, 110, 1757–1769. [Google Scholar] [CrossRef]
- D’ascenzi, F.; Anselmi, F.; Piu, P.; Fiorentini, C.; Carbone, S.F.; Volterrani, L.; Focardi, M.; Bonifazi, M.; Mondillo, S. Cardiac Magnetic Resonance Normal Reference Values of Biventricular Size and Function in Male Athlete’s Heart. JACC Cardiovasc. Imaging 2019, 12, 1755–1765. [Google Scholar] [CrossRef]
- Maceira, A.M.; Monmeneu, J.V.; López, M.P.; García, M.P.; Higueras, L.; Masiá, M.D.; Boraita, A. Reference ventricular dimensions and function parameters by cardiovascular magnetic resonance in highly trained Caucasian athletes. J. Cardiovasc. Magn. Reson. 2023, 25, 1–11. [Google Scholar] [CrossRef]
- Csecs, I.; Czimbalmos, C.; Toth, A.; Dohy, Z.; Suhai, I.F.; Szabo, L.; Kovacs, A.; Lakatos, B.; Sydo, N.; Kheirkhahan, M.; et al. The impact of sex, age and training on biventricular cardiac adaptation in healthy adult and adolescent athletes: Cardiac magnetic resonance imaging study. Eur. J. Prev. Cardiol. 2019, 27, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Geiger, J.; Rahsepar, A.A.; Suwa, K.; Powell, A.; Ghasemiesfe, A.; Barker, A.J.; Collins, J.D.; Carr, J.C.; Markl, M. 4D flow MRI, cardiac function, and T 1 -mapping: Association of valve-mediated changes in aortic hemodynamics with left ventricular remodeling. J. Magn. Reson. Imaging 2017, 48, 121–131. [Google Scholar] [CrossRef]
- Monosilio, S.; Prosperi, S.; Filomena, D.; Lemme, E.; Di Gioia, G.; Mango, R.; Netti, L.; Tonti, G.; Pedrizzetti, G.; Gualdi, G.; et al. Cardiac Magnetic Resonance Feature-Tracking in Olympic Athletes: A myocardial deformation analysis. Eur. J. Prev. Cardiol. 2025, zwaf042, Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Daems, J.J.N.; Wierdsma, S.J.; A Van Diepen, M.; Verwijs, S.M.; Van Hattum, J.C.; Van Luijk, R.D.; Nelissen, J.L.; A Hopman, L.H.G.; Allaart, C.P.; Gotte, M.J.W.; et al. Differences in cardiac magnetic resonance imaging bi-atrial strain between elite athletes and atrial fibrillation patients. Eur. Heart J. 2024, 45, ehae666.3000. [Google Scholar] [CrossRef]
- Pelliccia, A.; Culasso, F.; Di Paolo, F.M.; Maron, B.J. Physiologic Left Ventricular Cavity Dilatation in Elite Athletes. Ann. Intern. Med. 1999, 130, 23–31. [Google Scholar] [CrossRef]
- Abergel, E.; Chatellier, G.; A Hagege, A.; Oblak, A.; Linhart, A.; Ducardonnet, A.; Menard, J. Serial left ventricular adaptations in world-class professional cyclists. J. Am. Coll. Cardiol. 2004, 44, 144–149. [Google Scholar] [CrossRef]
- Teske, A.J.; Prakken, N.H.; De Boeck, B.W.; Velthuis, B.K.; Martens, E.P.; Doevendans, P.A.; Cramer, M.J. Echocardiographic tissue deformation imaging of right ventricular systolic function in endurance athletes. Eur. Heart J. 2008, 30, 969–977. [Google Scholar] [CrossRef]
- Basavarajaiah, S.; Boraita, A.; Whyte, G.; Wilson, M.; Carby, L.; Shah, A.; Sharma, S. Ethnic Differences in Left Ventricular Remodeling in Highly-Trained Athletes. J. Am. Coll. Cardiol. 2008, 51, 2256–2262. [Google Scholar] [CrossRef]
- Pelliccia, A.; Maron, B.J.; Spataro, A.; Proschan, M.A.; Spirito, P. The Upper Limit of Physiologic Cardiac Hypertrophy in Highly Trained Elite Athletes. N. Engl. J. Med. 1991, 324, 295–301. [Google Scholar] [CrossRef]
- Pelliccia, A. Athlete’s Heart in Women. JAMA 1996, 276, 211. [Google Scholar] [CrossRef]
- Rawlins, J.; Carre, F.; Kervio, G.; Papadakis, M.; Chandra, N.; Edwards, C.; Whyte, G.; Sharma, S. Ethnic differences in physiological cardiac adaptation to intense physical exercise in highly trained female athletes. Circulation 2010, 121, 1078–1085. [Google Scholar] [CrossRef] [PubMed]
- Gati, S.; Sharma, S.; Pennell, D. The Role of Cardiovascular Magnetic Resonance Imaging in the Assessment of Highly Trained Athletes. JACC Cardiovasc. Imaging 2018, 11, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Malhotra, A. Cardiac Magnetic Resonance Imaging in Athletes. JACC Cardiovasc. Imaging 2019, 12, 1766–1768. [Google Scholar] [CrossRef] [PubMed]
- Papadakis, M.; Carre, F.; Kervio, G.; Rawlins, J.; Panoulas, V.F.; Chandra, N.; Basavarajaiah, S.; Carby, L.; Fonseca, T.; Sharma, S. The prevalence, distribution, and clinical outcomes of electrocardiographic repolarization patterns in male athletes of African/Afro-Caribbean origin. Eur. Heart J. 2011, 32, 2304–2313. [Google Scholar] [CrossRef]
- Calore, C.; Zorzi, A.; Sheikh, N.; Nese, A.; Facci, M.; Malhotra, A.; Zaidi, A.; Schiavon, M.; Pelliccia, A.; Sharma, S.; et al. Electrocardiographic anterior T-wave inversion in athletes of different ethnicities: Differential diagnosis between athlete’s heart and cardiomyopathy. Eur. Heart J. 2016, 37, 2515–2527. [Google Scholar] [CrossRef]
- Graziano, F.; Genta, O.E.; Manfrin, L.; Corrado, D.; Brusamolin, L.; Giada, F.; Gerbino, L.; Compagno, S.; Zorzi, A. Prevalence and determinants of low QRS voltages and QRS fragmentation in children and adolescents undergoing sports pre-participation screening. Eur. J. Prev. Cardiol. 2024, 31, 1535–1542. [Google Scholar] [CrossRef]
- D’Ascenzi, F.; Anselmi, F.; Berti, B.; Capitani, E.; Chiti, C.; Franchini, A.; Graziano, F.; Nistri, S.; Focardi, M.; Capitani, M.; et al. Prevalence and significance of T-wave inversion in children practicing sport: A prospective, 4-year follow-up study. Int. J. Cardiol. 2018, 279, 100–104. [Google Scholar] [CrossRef]
- Kim, J.H.; Noseworthy, P.A.; McCarty, D.; Yared, K.; Weiner, R.; Wang, F.; Wood, M.J.; Hutter, A.M.; Picard, M.H.; Baggish, A.L. Significance of electrocardiographic right bundle branch block in trained athletes. Am. J. Cardiol. 2011, 107, 1083–1089. [Google Scholar] [CrossRef]
- Bent, R.E.; Wheeler, M.T.; Hadley, D.; Froelicher, V.; Ashley, E.; Perez, M.V. Computerized Q wave dimensions in athletes and hypertrophic cardiomyopathy patients. J. Electrocardiol. 2015, 48, 362–367. [Google Scholar] [CrossRef]
- Kim, J.H.; Baggish, A.L. Electrocardiographic right and left bundle branch block patterns in athletes: Prevalence, pathology, and clinical significance. J. Electrocardiol. 2015, 48, 380–384. [Google Scholar] [CrossRef]
- Calò, L.; Martino, A.; Tranchita, E.; Sperandii, F.; Guerra, E.; Quaranta, F.; Parisi, A.; Nigro, A.; Sciarra, L.; de Ruvo, E.; et al. Electrocardiographic and echocardiographic evaluation of a large cohort of peri-pubertal soccer players during pre-participation screening. Eur. J. Prev. Cardiol. 2019, 26, 1444–1455. [Google Scholar] [CrossRef] [PubMed]
- Abela, M.; Cuschieri, D.; Okoh, S.; Micallef, D.; Cuschieri, A.; Felice, T.; Scerri, C.; Grech, V.; Sammut, M. Low QRS voltages and QRS fragmentation in adolescence—Findings from the BEAT-IT screening program. J. Am. Coll. Cardiol. 2025, 85 (Suppl. S12), 2493. [Google Scholar] [CrossRef]
- Rickers, C.; Wilke, N.M.; Jerosch-Herold, M.; Casey, S.A.; Panse, P.; Panse, N.; Weil, J.; Zenovich, A.G.; Maron, B.J. Utility of cardiac magnetic resonance imaging in the diagnosis of hypertrophic cardiomyopathy. Circulation 2005, 112, 855–861. [Google Scholar] [CrossRef] [PubMed]
- Abela, M.; Calleja, A.; Felice, T.; Yamagata, K. Mitral regurgitation in patients with hypertrophic cardiomyopathy: A case series. Metzner A, Afana AS, Giannopoulos A, Jakstaite AM, Mathai SV, eds. Eur. Heart J. Case Rep. 2025, 9, ytaf113. [Google Scholar] [CrossRef]
- Maron, M.S.; Rowin, E.J.; Lin, D.; Appelbaum, E.; Chan, R.H.; Gibson, C.M.; Lesser, J.R.; Lindberg, J.; Haas, T.S.; Udelson, J.E.; et al. Prevalence and Clinical Profile of Myocardial Crypts in Hypertrophic Cardiomyopathy. Circ. Cardiovasc. Imaging 2012, 5, 441–447. [Google Scholar] [CrossRef]
- Marian, A.J.; Braunwald, E. Hypertrophic cardiomyopathy: Genetics, pathogenesis, clinical manifestations, diagnosis, and therapy. Circ. Res. 2017, 121, 749–770. [Google Scholar] [CrossRef]
- Ommen, S.R.; Ho, C.Y.; Asif, I.M.; Balaji, S.; Burke, M.A.; Day, S.M.; Dearani, J.A.; Epps, K.C.; Evanovich, L.; Ferrari, V.A.; et al. 2024 AHA/ACC/AMSSM/HRS/PACES/SCMR Guideline for the Management of Hypertrophic Cardiomyopathy: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2024, 149, e1239–e1311. [Google Scholar] [CrossRef]
- Captur, G.; Lopes, L.R.; Patel, V.; Li, C.; Bassett, P.; Syrris, P.; Sado, D.M.; Maestrini, V.; Mohun, T.J.; McKenna, W.J.; et al. Abnormal Cardiac Formation in Hypertrophic Cardiomyopathy. Circ. Cardiovasc. Genet. 2014, 7, 241–248. [Google Scholar] [CrossRef]
- Captur, G.; Lopes, L.R.; Mohun, T.J.; Patel, V.; Li, C.; Bassett, P.; Finocchiaro, G.; Ferreira, V.M.; Esteban, M.T.; Muthurangu, V.; et al. Prediction of Sarcomere Mutations in Subclinical Hypertrophic Cardiomyopathy. Circ. Cardiovasc. Imaging 2014, 7, 863–871. [Google Scholar] [CrossRef]
- Reant, P.; Captur, G.; Mirabel, M.; Nasis, A.; Sado, D.M.; Maestrini, V.; Castelletti, S.; Manisty, C.; Herrey, A.S.; Syrris, P.; et al. Abnormal septal convexity into the left ventricle occurs in subclinical hypertrophic cardiomyopathy. J. Cardiovasc. Magn. Reson. 2015, 17, 64. [Google Scholar] [CrossRef]
- Hughes, R.K.; Camaioni, C.; Augusto, J.B.; Knott, K.; Quinn, E.; Captur, G.; Seraphim, A.; Joy, G.; Syrris, P.; Elliott, P.M.; et al. Myocardial perfusion defects in hypertrophic cardiomyopathy mutation carriers. J. Am. Heart Assoc. 2021, 10, e020227. [Google Scholar] [CrossRef] [PubMed]
- Vigneault, D.M.; Yang, E.; Jensen, P.J.; Tee, M.W.; Farhad, H.; Chu, L.; Noble, J.A.; Day, S.M.; Colan, S.D.; Russell, M.W.; et al. Left Ventricular Strain Is Abnormal in Preclinical and Overt Hypertrophic Cardiomyopathy: Cardiac MR Feature Tracking. Radiology 2019, 290, 640–648. [Google Scholar] [CrossRef]
- Sheikh, N.; Papadakis, M.; Schnell, F.; Panoulas, V.; Malhotra, A.; Wilson, M.; Carré, F.; Sharma, S. Clinical profile of athletes with hypertrophic cardiomyopathy. Circ. Cardiovasc. Imaging 2015, 8, e003454. [Google Scholar] [CrossRef] [PubMed]
- Corona-Villalobos, C.P.; Sorensen, L.L.; Pozios, I.; Chu, L.; Eng, J.; Abraham, M.R.; Abraham, T.P.; Kamel, I.R.; Zimmerman, S.L. Left ventricular wall thickness in patients with hypertrophic cardiomyopathy: A comparison between cardiac magnetic resonance imaging and echocardiography. J. Cardiovasc. Imaging 2016, 32, 945–954. [Google Scholar] [CrossRef] [PubMed]
- Bois, J.P.; Geske, J.B.; Foley, T.A.; Ommen, S.R.; Pellikka, P.A. Comparison of Maximal Wall Thickness in Hypertrophic Cardiomyopathy Differs Between Magnetic Resonance Imaging and Transthoracic Echocardiography. Am. J. Cardiol. 2016, 119, 643–650. [Google Scholar] [CrossRef]
- Weng, Z.; Yao, J.; Chan, R.H.; He, J.; Yang, X.; Zhou, Y.; He, Y. Prognostic Value of LGE-CMR in HCM: A Meta-Analysis. JACC Cardiovasc. Imaging 2016, 9, 1392–1402. [Google Scholar] [CrossRef]
- Chan, R.H.; Maron, B.J.; Olivotto, I.; Pencina, M.J.; Assenza, G.E.; Haas, T.; Lesser, J.R.; Gruner, C.; Crean, A.M.; Rakowski, H.; et al. Prognostic value of quantitative contrast-enhanced cardiovascular magnetic resonance for the evaluation of sudden death risk in patients with hypertrophic cardiomyopathy. Circulation 2014, 130, 484–495. [Google Scholar] [CrossRef]
- Mentias, A.; Raeisi-Giglou, P.; Smedira, N.G.; Feng, K.; Sato, K.; Wazni, O.; Kanj, M.; Flamm, S.D.; Thamilarasan, M.; Popovic, Z.B.; et al. Late Gadolinium Enhancement in Patients with Hypertrophic Cardiomyopathy and Preserved Systolic Function. J. Am. Coll. Cardiol. 2018, 72, 857–870. [Google Scholar] [CrossRef]
- Raja, A.A.; Farhad, H.; Valente, A.M.; Couce, J.-P.; Jefferies, J.L.; Bundgaard, H.; Zahka, K.; Lever, H.; Murphy, A.M.; Ashley, E.; et al. Prevalence and progression of late gadolinium enhancement in children and adolescents with hypertrophic cardiomyopathy. Circulation 2018, 138, 782–792. [Google Scholar] [CrossRef]
- Prosperi, S.; Monosilio, S.; Lemme, E.; Filomena, D.; Penza, M.; Birtolo, L.I.; Mango, R.; Di Gioia, G.; Gualdi, G.; Squeo, M.R.; et al. CMR native T1 and T2 mapping in Olympic athletes: The influence of sports discipline and sex. Eur. Hear. J. Cardiovasc. Imaging 2024, 26, 89–95. [Google Scholar] [CrossRef]
- McDiarmid, A.K.; Swoboda, P.P.; Erhayiem, B.; Lancaster, R.E.; Lyall, G.K.; Broadbent, D.A.; Dobson, L.E.; Musa, T.A.; Ripley, D.P.; Garg, P.; et al. Athletic Cardiac Adaptation in Males Is a Consequence of Elevated Myocyte Mass. Circ. Cardiovasc. Imaging 2016, 9, e003579. [Google Scholar] [CrossRef] [PubMed]
- Swoboda, P.P.; Garg, P.; Levelt, E.; Broadbent, D.A.; Zolfaghari-Nia, A.; Foley, A.J.R.; Fent, G.J.; Chew, P.G.; Brown, L.A.; Saunderson, C.E.; et al. Regression of Left Ventricular Mass in Athletes Undergoing Complete Detraining Is Mediated by Decrease in Intracellular but Not Extracellular Compartments. Circ. Cardiovasc. Imaging 2019, 12, e009417. [Google Scholar] [CrossRef]
- Swoboda, P.P.; McDiarmid, A.K.; Erhayiem, B.; Broadbent, D.A.; Dobson, L.E.; Garg, P.; Ferguson, C.; Page, S.P.; Greenwood, J.P.; Plein, S. Assessing Myocardial Extracellular Volume by T1 Mapping to Distinguish Hypertrophic Cardiomyopathy from Athlete’s Heart. J. Am. Coll. Cardiol. 2016, 67, 2189–2190. [Google Scholar] [CrossRef]
- Arbelo, E.; Protonotarios, A.; Gimeno, J.R.; Arbustini, E.; Barriales-Villa, R.; Basso, C.; Bezzina, C.R.; Biagini, E.; A Blom, N.; A de Boer, R.; et al. 2023 ESC Guidelines for the management of cardiomyopathies. Eur. Heart J. 2023, 44, 3503–3626. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.K.; Lee, S.-C.; Chang, S.-A.; Jang, S.-Y.; Kim, S.M.; Park, S.-J.; Choi, J.-O.; Park, S.W.; Jeon, E.-S.; Choe, Y.H. Prevalence and clinical significance of cardiovascular magnetic resonance adenosine stress-induced myocardial perfusion defect in hypertrophic cardiomyopathy. J. Cardiovasc. Magn. Reson. 2020, 22, 30. [Google Scholar] [CrossRef]
- Radvan, J.; Choudhury, L.; Sheridan, D.J.; Camici, P.G. Comparison of Coronary Vasodilator Reserve in Elite Rowing Athletes Versus Hypertrophic Cardiomyopathy. Am. J. Cardiol. 1997, 80, 1621–1623. [Google Scholar] [CrossRef] [PubMed]
- Ipek, R.; Holland, J.; Cramer, M.; Rider, O. CMR to characterize myocardial structure and function in heart failure with preserved left ventricular ejection fraction. Eur. Heart J. Cardiovasc. Imaging 2024, 25, 1491–1504. [Google Scholar] [CrossRef]
- Halliday, B.P.; Baksi, A.J.; Gulati, A.; Ali, A.; Newsome, S.; Izgi, C.; Arzanauskaite, M.; Lota, A.; Tayal, U.; Vassiliou, V.S.; et al. Outcome in Dilated Cardiomyopathy Related to the Extent, Location, and Pattern of Late Gadolinium Enhancement. JACC Cardiovasc. Imaging 2019, 12 Pt 2, 1645–1655. [Google Scholar] [CrossRef]
- Allwood, R.P.; Papadakis, M.; Androulakis, E. Myocardial Fibrosis in Young and Veteran Athletes: Evidence from a Systematic Review of the Current Literature. J. Clin. Med. 2024, 13, 4536. [Google Scholar] [CrossRef]
- Małek, Ł.A.; Bucciarelli-Ducci, C. Myocardial fibrosis in athletes—Current perspective. Clin. Cardiol. 2020, 43, 882–888. [Google Scholar] [CrossRef]
- Mandawat, A.; Chattranukulchai, P.; Mandawat, A.; Blood, A.J.; Ambati, S.; Hayes, B.; Rehwald, W.; Kim, H.W.; Heitner, J.F.; Shah, D.J.; et al. Progression of Myocardial Fibrosis in Nonischemic DCM and Association with Mortality and Heart Failure Outcomes. JACC Cardiovasc. Imaging 2021, 14, 1338–1350. [Google Scholar] [CrossRef]
- Di Marco, A.; Anguera, I.; Schmitt, M.; Klem, I.; Neilan, T.G.; White, J.A.; Sramko, M.; Masci, P.G.; Barison, A.; Mckenna, P.; et al. Late Gadolinium Enhancement and the Risk for Ventricular Arrhythmias or Sudden Death in Dilated Cardiomyopathy. JACC Heart Fail. 2017, 5, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Qian, W.; Zhang, X.; Li, D.; Qian, Z.; Xu, H.; Liao, S.; Chen, X.; Wang, Y.; Hou, X.; et al. Ring-like late gadolinium enhancement for predicting ventricular tachyarrhythmias in non-ischaemic dilated cardiomyopathy. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 1130–1138. [Google Scholar] [CrossRef]
- Shah, R.A.; Asatryan, B.; Dabbagh, G.S.; Aung, N.; Khanji, M.Y.; Lopes, L.R.; van Duijvenboden, S.; Holmes, A.; Muser, D.; Landstrom, A.P.; et al. Frequency, Penetrance, and Variable Expressivity of Dilated Cardiomyopathy-Associated Putative Pathogenic Gene Variants in UK Biobank Participants. Circulation 2022, 146, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Laws, J.L.; Lancaster, M.C.; Ben Shoemaker, M.; Stevenson, W.G.; Hung, R.R.; Wells, Q.; Brinkley, D.M.; Hughes, S.; Anderson, K.; Roden, D.; et al. Arrhythmias as presentation of genetic cardiomyopathy. Circ. Res. 2022, 130, 1698–1722. [Google Scholar] [CrossRef]
- Franzen, E.; Mangold, S.; Erz, G.; Claussen, C.D.; Niess, A.M.; Kramer, U.; Burgstahler, C. Comparison of morphological and functional adaptations of the heart in highly trained triathletes and long-distance runners using cardiac magnetic resonance imaging. Heart Vessel. 2012, 28, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Breuckmann, F.; Möhlenkamp, S.; Nassenstein, K.; Lehmann, N.; Ladd, S.; Schmermund, A.; Sievers, B.; Schlosser, T.; Jöckel, K.-H.; Heusch, G.; et al. Myocardial Late Gadolinium Enhancement: Prevalence, Pattern, and Prognostic Relevance in Marathon Runners. Radiology 2009, 251, 50–57. [Google Scholar] [CrossRef]
- Erz, G.; Mangold, S.; Franzen, E.; Claussen, C.D.; Niess, A.M.; Burgstahler, C.; Kramer, U. Correlation between ECG abnormalities and cardiac parameters in highly trained asymptomatic male endurance athletes: Evaluation using cardiac magnetic resonance imaging. Int. J. Cardiovasc. Imaging 2012, 29, 325–334. [Google Scholar] [CrossRef]
- Zorzi, A.; Marra, M.P.; Rigato, I.; De Lazzari, M.; Susana, A.; Niero, A.; Pilichou, K.; Migliore, F.; Rizzo, S.; Giorgi, B.; et al. Nonischemic left ventricular scar as a substrate of life-threatening ventricular arrhythmias and sudden cardiac death in competitive athletes. Circ. Arrhythm Electrophysiol. 2016, 9, e004229. [Google Scholar] [CrossRef]
- Farooq, M.; Brown, L.A.E.; Fitzpatrick, A.; Broadbent, D.A.; Wahab, A.; Klassen, J.R.L.; Farley, J.; Saunderson, C.E.D.; Das, A.; Craven, T.; et al. Identification of non-ischaemic fibrosis in male veteran endurance athletes, mechanisms and association with premature ventricular beats. Sci. Rep. 2023, 13, 14640. [Google Scholar] [CrossRef]
- Leary, R.J.; Kinde, I.; Diehl, F.; Schmidt, K.; Clouser, C.; Duncan, C.; Antipova, A.; Lee, C.; McKernan, K.; De La Vega, F.M.; et al. Exercise Increases Age-Related Penetrance and Arrhythmic Risk in Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy Associated Desmosomal Mutation Carriers. JACC 2013, 62, 1290–1297. [Google Scholar] [CrossRef]
- Saberniak, J.; Hasselberg, N.E.; Borgquist, R.; Platonov, P.G.; Sarvari, S.I.; Smith, H.; Ribe, M.; Holst, A.G.; Edvardsen, T.; Haugaa, K.H. Vigorous physical activity impairs myocardial function in patients with arrhythmogenic right ventricular cardiomyopathy and in mutation positive family members. Eur. J. Heart Fail. 2014, 16, 1337–1344. [Google Scholar] [CrossRef]
- Ruwald, A.-C.; Marcus, F.; Estes, N.M.; Link, M.; McNitt, S.; Polonsky, B.; Calkins, H.; Towbin, J.A.; Moss, A.J.; Zareba, W. Association of competitive and recreational sport participation with cardiac events in patients with arrhythmogenic right ventricular cardiomyopathy: Results from the North American multidisciplinary study of arrhythmogenic right ventricular cardiomyopathy. Eur. Heart J. 2015, 36, 1735–1743. [Google Scholar] [CrossRef]
- Towbin, J.A.; McKenna, W.J.; Abrams, D.J.; Ackerman, M.J.; Calkins, H.; Darrieux, F.C.; Daubert, J.P.; de Chillou, C.; DePasquale, E.C.; Desai, M.Y.; et al. 2019 HRS expert consensus statement on evaluation, risk stratification, and management of arrhythmogenic cardiomyopathy. Heart Rhythm. 2019, 16, e301–e372. [Google Scholar] [CrossRef] [PubMed]
- D’ascenzi, F.; Solari, M.; Corrado, D.; Zorzi, A.; Mondillo, S. Diagnostic Differentiation Between Arrhythmogenic Cardiomyopathy and Athlete’s Heart by Using Imaging. JACC Cardiovasc. Imaging 2018, 11, 1327–1339. [Google Scholar] [CrossRef] [PubMed]
- Corrado, D.; Perazzolo Marra, M.; Zorzi, A.; Beffagna, G.; Cipriani, A.; Lazzari, M.; Migliore, F.; Pilichou, K.; Rampazzo, A.; Rigato, I.; et al. Diagnosis of arrhythmogenic cardiomyopathy: The Padua criteria. Int. J. Cardiol. 2020, 319, 106–114. [Google Scholar] [CrossRef]
- Gati, S.; Chandra, N.; Bennett, R.L.; Reed, M.; Kervio, G.; Panoulas, V.F.; Ghani, S.; Sheikh, N.; Zaidi, A.; Wilson, M.; et al. Increased left ventricular trabeculation in highly trained athletes: Do we need more stringent criteria for the diagnosis of left ventricular non-compaction in athletes? Heart 2013, 99, 401–408. [Google Scholar] [CrossRef]
- Abela, M.; D’silva, A. Left Ventricular Trabeculations in Athletes: Epiphenomenon or Phenotype of Disease? Curr. Treat. Options Cardiovasc. Med. 2018, 20, 1–16. [Google Scholar] [CrossRef]
- Devereux, R.B.; Jones, E.C.; Roman, M.J.; Howard, B.V.; Fabsitz, R.R.; E Liu, J.; Palmieri, V.; Welty, T.K.; Lee, E.T. Prevalence and correlates of mitral valve prolapse in a population-based sample of American Indians: The strong heart study. Am. J. Med. 2001, 111, 679–685. [Google Scholar] [CrossRef]
- Caselli, S.; Mango, F.; Clark, J.; Pandian, N.G.; Corrado, D.; Autore, C.; Pelliccia, A. Prevalence and Clinical Outcome of Athletes with Mitral Valve Prolapse. Circulation 2018, 137, 2080–2082. [Google Scholar] [CrossRef]
- Nalliah, C.J.; Mahajan, R.; Elliott, A.D.; Haqqani, H.; Lau, D.H.; Vohra, J.K.; Morton, J.B.; Semsarian, C.; Marwick, T.; Kalman, J.M.; et al. Mitral valve prolapse and sudden cardiac death: A systematic review and meta-analysis. Heart 2018, 105, 144–151. [Google Scholar] [CrossRef]
- Vriz, O.; Landi, I.; Eltayeb, A.; Limongelli, G.; Mos, L.; Delise, P.; Bossone, E.; D’andrea, A. Mitral Valve Prolapse and Sudden Cardiac Death in Athletes at High Risk. Curr. Cardiol. Rev. 2023, 19, e201222212066. [Google Scholar] [CrossRef] [PubMed]
- Beaufils, A.-L.C.D.; Huttin, O.; Jobbe-Duval, A.; Senage, T.; Filippetti, L.; Piriou, N.; Cueff, C.; Venner, C.; Mandry, D.; Sellal, J.-M.; et al. Replacement Myocardial Fibrosis in Patients with Mitral Valve Prolapse: Relation to Mitral Regurgitation, Ventricular Remodeling, and Arrhythmia. Circulation 2021, 143, 1763–1774. [Google Scholar] [CrossRef] [PubMed]
- Basso, C.; Marra, M.P.; Rizzo, S.; De Lazzari, M.; Giorgi, B.; Cipriani, A.; Frigo, A.C.; Rigato, I.; Migliore, F.; Pilichou, K.; et al. Arrhythmic Mitral Valve Prolapse and Sudden Cardiac Death. Circulation 2015, 132, 556–566. [Google Scholar] [CrossRef]
- Marra, M.P.; Basso, C.; De Lazzari, M.; Rizzo, S.; Cipriani, A.; Giorgi, B.; Lacognata, C.; Rigato, I.; Migliore, F.; Pilichou, K.; et al. Morphofunctional abnormalities of mitral annulus and arrhythmic mitral valve prolapse. Circ. Cardiovasc. Imaging 2016, 9, e005030. [Google Scholar] [CrossRef]
- Mantegazza, V.; Volpato, V.; Gripari, P.; Ali, S.G.; Fusini, L.; Italiano, G.; Muratori, M.; Pontone, G.; Tamborini, G.; Pepi, M. Multimodality imaging assessment of mitral annular disjunction in mitral valve prolapse. Heart 2021, 107, 25–32. [Google Scholar] [CrossRef]
- Pavon, A.G.; Arangalage, D.; Pascale, P.; Hugelshofer, S.; Rutz, T.; Porretta, A.P.; Le Bloa, M.; Muller, O.; Pruvot, E.; Schwitter, J.; et al. Myocardial extracellular volume by T1 mapping: A new marker of arrhythmia in mitral valve prolapse. J. Cardiovasc. Magn. Reson. 2021, 23, 102. [Google Scholar] [CrossRef]
- Losenno, K.L.; Goodman, R.L.; Chu, M.W.A. Bicuspid aortic valve disease and ascending aortic aneurysms: Gaps in knowledge. Cardiol. Res. Pract. 2012, 2012, 1–16. [Google Scholar] [CrossRef]
- Verma, S.; Siu, S.C. Aortic Dilatation in Patients with Bicuspid Aortic Valve. N. Engl. J. Med. 2014, 370, 1920–1929. [Google Scholar] [CrossRef]
- Thiene, G.; Rizzo, S.; Basso, C. Bicuspid aortic valve: The most frequent and not so benign congenital heart disease. Cardiovasc. Pathol. 2024, 70, 107604. [Google Scholar] [CrossRef]
- Wassmuth, R.; von Knobelsdorff-Brenkenhoff, F.; Gruettner, H.; Utz, W.; Schulz-Menger, J. Cardiac magnetic resonance imaging of congenital bicuspid aortic valves and associated aortic pathologies in adults. Eur. Heart J. Cardiovasc. Imaging 2014, 15, 673–679. [Google Scholar] [CrossRef] [PubMed]
- De Bosscher, R.; Claeys, M.; Dausin, C.; Goetschalckx, K.; Claus, P.; Herbots, L.; Ghekiere, O.; Van De Heyning, C.; Paelinck, B.P.; Janssens, K.; et al. Three-dimensional echocardiography of the athlete’s heart: A comparison with cardiac magnetic resonance imaging. Int. J. Cardiovasc. Imaging 2022, 39, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Yang, K.; Chen, X.; Li, R.; Su, G.; Yin, G.; Wang, K.; Lu, M.; Zhao, S. Prognostic significance of myocardial fibrosis and CMR characteristics in bicuspid aortic valve with moderate and severe aortic insufficiency. Eur. Radiol. 2021, 31, 7262–7272. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Park, J.-B.; Yoon, Y.E.; Park, E.-A.; Kim, H.-K.; Lee, W.; Kim, Y.-J.; Cho, G.-Y.; Sohn, D.-W.; Greiser, A.; et al. Noncontrast Myocardial T1 Mapping by Cardiac Magnetic Resonance Predicts Outcome in Patients with Aortic Stenosis. JACC Cardiovasc. Imaging 2018, 11, 974–983. [Google Scholar] [CrossRef]
- Siu, S.C.; Silversides, C.K. Bicuspid Aortic Valve Disease. J. Am. Coll. Cardiol. 2010, 55, 2789–2800. [Google Scholar] [CrossRef]
- Bhave, N.M.; Nienaber, C.A.; Clough, R.E.; Eagle, K.A. Multimodality Imaging of Thoracic Aortic Diseases in Adults. JACC Cardiovasc. Imaging 2018, 11, 902–919. [Google Scholar] [CrossRef]
- Harris, K.M.; Tung, M.; Haas, T.S.; Maron, B.J. Under-Recognition of Aortic and Aortic Valve Disease and the Risk for Sudden Death in Competitive Athletes. J. Am. Coll. Cardiol. 2015, 65, 860–862. [Google Scholar] [CrossRef]
- Kim, J.H.; Dickert, N.W. Athletes with Cardiovascular Disease and Competitive Sports Eligibility. JAMA Cardiol. 2022, 7, 663. [Google Scholar] [CrossRef]
- Groves, E.M.; Bireley, W.; Dill, K.; Carroll, T.J.; Carr, J.C. Quantitative Analysis of ECG-Gated High-Resolution Contrast-Enhanced MR Angiography of the Thoracic Aorta. Am. J. Roentgenol. 2007, 188, 522–528. [Google Scholar] [CrossRef]
- Isselbacher, E.M.; Preventza, O.; Black, J.H., 3rd; Augoustides, J.G.; Beck, A.W.; Bolen, M.A.; Braverman, A.C.; Bray, B.E.; Brown-Zimmerman, M.M.; Chen, E.P.; et al. 2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2022, 146, E334–E482. [Google Scholar] [CrossRef]
- Rodríguez-Palomares, J.F.; Dux-Santoy, L.; Guala, A.; Kale, R.; Maldonado, G.; Teixidó-Turà, G.; Galian, L.; Huguet, M.; Valente, F.; Gutierrez, L.; et al. Aortic Root Dilatation in Athletic Population. Prog. Cardiovasc. Dis. 2012, 54, 432–437. [Google Scholar] [CrossRef]
- Rodríguez-Palomares, J.F.; Dux-Santoy, L.; Guala, A.; Kale, R.; Maldonado, G.; Teixidó-Turà, G.; Galian, L.; Huguet, M.; Valente, F.; Gutierrez, L.; et al. Aortic flow patterns and wall shear stress maps by 4D-flow cardiovascular magnetic resonance in the assessment of aortic dilatation in bicuspid aortic valve disease. J. Cardiovasc. Magn. Reson. 2018, 20, 28. [Google Scholar] [CrossRef]
- Hope, T.A.; Markl, M.; Wigström, L.; Alley, M.T.; Miller, D.C.; Herfkens, R.J. Comparison of flow patterns in ascending aortic aneurysms and volunteers using four-dimensional magnetic resonance velocity mapping. J. Magn. Reson. Imaging 2007, 26, 1471–1479. [Google Scholar] [CrossRef]
- D’Ascenzi, F.; Cavigli, L.; Cameli, M.; Claessen, G.; van Craenenbroeck, E.M.; Cavarretta, E.; D’Andrea, A.; De la Garza, M.S.; Eijsvogels, T.M.H.; van Kimmenade, R.R.J.; et al. Sport PRactice and its Effects on aortic size and valve function in bicuspid Aortic valve Disease: A cross-sectional report from the SPREAD study. Br. J. Sports Med. 2024, 58, 1419–1425. [Google Scholar] [CrossRef] [PubMed]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef]
- van Buuren, F.; Gati, S.; Sharma, S.; Papadakis, M.; Adami, P.E.; Niebauer, J.; Pelliccia, A.; Rudolph, V.; Börjesson, M.; Carre, F.; et al. Athletes with valvular heart disease and competitive sports: A position statement of the Sport Cardiology Section of the European Association of Preventive Cardiology. Eur. J. Prev. Cardiol. 2021, 28, 1569–1578. [Google Scholar] [CrossRef] [PubMed]
- Grothues, F.; Moon, J.C.; Bellenger, N.G.; Smith, G.S.; Klein, H.U.; Pennell, D.J. Interstudy reproducibility of right ventricular volumes, function, and mass with cardiovascular magnetic resonance. Am. Heart J. 2004, 147, 218–223. [Google Scholar] [CrossRef]
- Hundley, W.G.; Bluemke, D.A.; Finn, J.P.; Flamm, S.D.; Fogel, M.A.; Friedrich, M.G.; Ho, V.B.; Jerosch-Herold, M.; Kramer, C.M.; Manning, W.J.; et al. ACCF/ACR/AHA/NASCI/SCMR 2010 Expert Consensus Document on Cardiovascular Magnetic Resonance. J. Am. Coll. Cardiol. 2010, 55, 2614–2662. [Google Scholar] [CrossRef]
- Bing, R.; Cavalcante, J.L.; Everett, R.J.; Clavel, M.-A.; Newby, D.E.; Dweck, M.R. Imaging and Impact of Myocardial Fibrosis in Aortic Stenosis. JACC Cardiovasc. Imaging 2019, 12, 283–296. [Google Scholar] [CrossRef]
- Myerson, S.G. CMR in Evaluating Valvular Heart Disease. JACC Cardiovasc. Imaging 2021, 14, 2020–2032. [Google Scholar] [CrossRef]
- Gatehouse, P.D.; Keegan, J.; Crowe, L.A.; Masood, S.; Mohiaddin, R.H.; Kreitner, K.-F.; Firmin, D.N. Applications of phase-contrast flow and velocity imaging in cardiovascular MRI. Eur. Radiol. 2005, 15, 2172–2184. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, S.; Zühlke, L.; Black, G.C.; Choy, M.-K.; Li, N.; Keavney, B.D. Global birth prevalence of congenital heart defects 1970–2017: Updated systematic review and meta-analysis of 260 studies. Int. J. Epidemiol. 2019, 48, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Geva, T. Is MRI the Preferred Method for Evaluating Right Ventricular Size and Function in Patients with Congenital Heart Disease? Circ. Cardiovasc. Imaging 2014, 7, 190–197. [Google Scholar] [CrossRef]
- Baumgartner, H.; De Backer, J.; Babu-Narayan, S.V.; Budts, W.; Chessa, M.; Diller, G.-P.; Lung, B.; Kluin, J.; Lang, I.M.; Meijboom, F.; et al. 2020 ESC Guidelines for the management of adult congenital heart disease. Eur. Heart J. 2021, 42, 563–645. [Google Scholar] [CrossRef]
- Kilner, P.J.; Geva, T.; Kaemmerer, H.; Trindade, P.T.; Schwitter, J.; Webb, G.D. Recommendations for cardiovascular magnetic resonance in adults with congenital heart disease from the respective working groups of the European Society of Cardiology. Eur. Heart J. 2010, 31, 794–805. [Google Scholar] [CrossRef]
- Babu-Narayan, S.V.; Kilner, P.J.; Li, W.; Moon, J.C.; Goktekin, O.; Davlouros, P.A.; Khan, M.; Ho, S.Y.; Pennell, D.J.; Gatzoulis, M.A. Ventricular Fibrosis Suggested by Cardiovascular Magnetic Resonance in Adults with Repaired Tetralogy of Fallot and Its Relationship to Adverse Markers of Clinical Outcome. Circulation 2006, 113, 405–413. [Google Scholar] [CrossRef]
- Devos, D.G.H.; Kilner, P.J. Calculations of cardiovascular shunts and regurgitation using magnetic resonance ventricular volume and aortic and pulmonary flow measurements. Eur. Radiol. 2009, 20, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Pelliccia, A.; Fagard, R.; Bjørnstad, H.H.; Anastassakis, A.; Arbustini, E.; Assanelli, D.; Thiene, G. Recommendations for competitive sports participation in athletes with cardiovascular disease. Eur. Heart J. 2005, 26, 1422–1445. [Google Scholar] [CrossRef]
- De Bosscher, R.; Dausin, C.; Claus, P.; Bogaert, J.; Dymarkowski, S.; Goetschalckx, K.; Ghekiere, O.; Van De Heyning, C.M.; Van Herck, P.; Paelinck, B.; et al. Lifelong endurance exercise and its relation with coronary atherosclerosis. Eur. Heart J. 2023, 44, 2388–2399. [Google Scholar] [CrossRef]
- Merghani, A.; Maestrini, V.; Rosmini, S.; Cox, A.T.; Dhutia, H.; Bastiaenan, R.; David, S.; Yeo, T.J.; Narain, R.; Malhotra, A.; et al. Prevalence of Subclinical Coronary Artery Disease in Masters Endurance Athletes with a Low Atherosclerotic Risk Profile. Circulation 2017, 136, 126–137. [Google Scholar] [CrossRef]
- Gulati, M.; Levy, P.D.; Mukherjee, D.; Amsterdam, E.; Bhatt, D.L.; Birtcher, K.K.; Blankstein, R.; Boyd, J.; Bullock-Palmer, R.P.; Conejo, T.; et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR Guideline for the Evaluation and Diagnosis of Chest Pain: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 144, E368–E454. [Google Scholar] [CrossRef] [PubMed]
- Ripley, D.P.; Motwani, M.; Brown, J.M.; Nixon, J.; Everett, C.C.; Bijsterveld, P.; Maredia, N.; Plein, S.; Greenwood, J.P. Individual component analysis of the multi-parametric cardiovascular magnetic resonance protocol in the CE-MARC trial. J. Cardiovasc. Magn. Reson. 2015, 17, 59. [Google Scholar] [CrossRef]
- Vrints, C.; Vrints, C.; Andreotti, F.; Andreotti, F.; Koskinas, K.C.; Koskinas, K.C.; Rossello, X.; Rossello, X.; Adamo, M.; Adamo, M.; et al. 2024 ESC Guidelines for the management of chronic coronary syndromes. Eur. Heart J. 2024, 45, 3415–3537. [Google Scholar] [CrossRef]
- Wu, H.D.; Kwong, R.Y. Cardiac magnetic resonance imaging in patients with coronary disease. Curr. Treat. Options Cardiovasc. Med. 2008, 10, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Menadas, J.V.M.; Gonzalez, M.P.G.; Lopez-Lereu, M.P.; Ortega, L.H.; Gonzalez, A.M.M. Safety and tolerability of regadenoson in comparison with adenosine stress cardiovascular magnetic resonance: Data from a multicentre prospective registry. Int. J. Cardiovasc. Imaging 2022, 38, 195–209. [Google Scholar] [CrossRef] [PubMed]
- Schieda, N.; Blaichman, J.I.; Costa, A.F.; Glikstein, R.; Hurrell, C.; James, M.; Maralani, P.J.; Shabana, W.; Tang, A.; Tsampalieros, A.; et al. Gadolinium-Based Contrast Agents in Kidney Disease: A Comprehensive Review and Clinical Practice Guideline Issued by the Canadian Association of Radiologists. Can. J. Kidney Health Dis. 2018, 5, 2054358118778573. [Google Scholar] [CrossRef]
- Kefer, J.; Coche, E.; Legros, G.; Pasquet, A.; Grandin, C.; Van Beers, B.E.; Vanoverschelde, J.-L.; Gerber, B.L. Head-to-Head Comparison of Three-Dimensional Navigator-Gated Magnetic Resonance Imaging and 16-Slice Computed Tomography to Detect Coronary Artery Stenosis in Patients. J. Am. Coll. Cardiol. 2005, 46, 92–100. [Google Scholar] [CrossRef]
- Di Leo, G.; Fisci, E.; Secchi, F.; Alì, M.; Ambrogi, F.; Sconfienza, L.M.; Sardanelli, F. Diagnostic accuracy of magnetic resonance angiography for detection of coronary artery disease: A systematic review and meta-analysis. Eur. Radiol. 2016, 26, 3706–3718. [Google Scholar] [CrossRef]
- Sheppard, M.N. Aetiology of sudden cardiac death in sport: A histopathologist’s perspective. Br. J. Sports Med. 2012, 46 (Suppl. S1), i15–i21. [Google Scholar] [CrossRef]
- Haberkorn, S.M.; Haberkorn, S.I.; Bönner, F.; Kelm, M.; Hopkin, G.; Petersen, S.E. Vasodilator Myocardial Perfusion Cardiac Magnetic Resonance Imaging Is Superior to Dobutamine Stress Echocardiography in the Detection of Relevant Coronary Artery Stenosis: A Systematic Review and Meta-Analysis on Their Diagnostic Accuracy. Front. Cardiovasc. Med. 2021, 8, 630846. [Google Scholar] [CrossRef]
- Strach, K.; Meyer, C.; Schild, H.; Sommer, T. Cardiac stress MR imaging with dobutamine. Eur. Radiol. 2006, 16, 2728–2738. [Google Scholar] [CrossRef]
- Villa, A.D.M.; Corsinovi, L.; Ntalas, I.; Milidonis, X.; Scannell, C.; Di Giovine, G.; Child, N.; Ferreira, C.; Nazir, M.S.; Karady, J.; et al. Importance of operator training and rest perfusion on the diagnostic accuracy of stress perfusion cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2018, 20, 74. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.R.; Salerno, M.; Kwong, R.Y.; Singh, A.; Heydari, B.; Kramer, C.M. Stress Cardiac Magnetic Resonance Myocardial Perfusion Imaging: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2021, 78, 1655–1668. [Google Scholar] [CrossRef]
- A Byrne, R.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.-A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef]
- Balakrishna, A.M.; Ismayl, M.; Thandra, A.; Walters, R.; Ganesan, V.; Anugula, D.; Shah, D.J.; Aboeata, A. Diagnostic Value of Cardiac Magnetic Resonance Imaging and Intracoronary Optical Coherence Tomography in Patients with a Working Diagnosis of Myocardial Infarction with Non-obstructive Coronary Arteries—A Systematic Review and Meta-analysis. Curr. Probl. Cardiol. 2022, 48, 101126. [Google Scholar] [CrossRef] [PubMed]
- Agewall, S.; Beltrame, J.F.; Reynolds, H.R.; Niessner, A.; Rosano, G.; Caforio, A.L.P.; De Caterina, R.; Zimarino, M.; Roffi, M.; Kjeldsen, K.; et al. ESC working group position paper on myocardial infarction with non-obstructive coronary arteries. Eur. Heart J. 2016, 38, ehw149. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.-Q.; Abdu, F.A.; Liu, L.; Yin, G.; Mareai, R.M.; Xu, Y.; Che, W. Coronary microvascular dysfunction and myocardial infarction with non-obstructive coronary arteries: Where do we stand? Eur. J. Intern. Med. 2023, 117, 8–20. [Google Scholar] [CrossRef]
- Kotecha, T.; Martinez-Naharro, A.; Boldrini, M.; Knight, D.; Hawkins, P.; Kalra, S.; Patel, D.; Coghlan, G.; Moon, J.; Plein, S.; et al. Automated Pixel-Wise Quantitative Myocardial Perfusion Mapping by CMR to Detect Obstructive Coronary Artery Disease and Coronary Microvascular Dysfunction. JACC Cardiovasc. Imaging 2019, 12, 1958–1969. [Google Scholar] [CrossRef]
- Johansson, R.S.; Tornvall, P.; Sörensson, P.; Nickander, J. Reduced stress perfusion in myocardial infarction with nonobstructive coronary arteries. Sci. Rep. 2023, 13, 22094. [Google Scholar] [CrossRef]
- Dreyer, R.P.; Tavella, R.; Curtis, J.P.; Wang, Y.; Pauspathy, S.; Messenger, J.; Rumsfeld, J.S.; Maddox, T.M.; Krumholz, H.M.; A Spertus, J.; et al. Myocardial infarction with non-obstructive coronary arteries as compared with myocardial infarction and obstructive coronary disease: Outcomes in a Medicare population. Eur. Heart J. 2020, 41, 870–878. [Google Scholar] [CrossRef]
- Konst, R.E.; Parker, M.; Bhatti, L.; Kaolawanich, Y.; Alenezi, F.; Elias-Smale, S.E.; Nijveldt, R.; Kim, R.J. Prognostic Value of Cardiac Magnetic Resonance Imaging in Patients with a Working Diagnosis of MINOCA—An Outcome Study with up to 10 Years of Follow-Up. Circ. Cardiovasc. Imaging 2023, 16, e014454-642. [Google Scholar] [CrossRef] [PubMed]
- Angelini, P. Normal and anomalous coronary arteries: Definitions and classification. Am. Heart J. 1989, 117, 418–434. [Google Scholar] [CrossRef] [PubMed]
- Sternheim, D.; Power, D.A.; Samtani, R.; Kini, A.; Fuster, V.; Sharma, S. Myocardial Bridging: Diagnosis, Functional Assessment, and Management: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 78, 2196–2212. [Google Scholar] [CrossRef]
- Angelini, P.; Cheong, B.Y.; De Rosen, V.V.L.; Lopez, J.A.; Uribe, C.; Masso, A.H.; Ali, S.W.; Davis, B.R.; Muthupillai, R.; Willerson, J.T. Magnetic Resonance Imaging–Based Screening Study in a General Population of Adolescents. J. Am. Coll. Cardiol. 2018, 71, 579–580. [Google Scholar] [CrossRef]
- Frommelt, P.; Lopez, L.; Dimas, V.V.; Eidem, B.; Han, B.K.; Ko, H.H.; Lorber, R.; Nii, M.; Printz, B.; Srivastava, S.; et al. Recommendations for Multimodality Assessment of Congenital Coronary Anomalies: A Guide from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2020, 33, 259–294. [Google Scholar] [CrossRef]
- Harmon, K.G.; Asif, I.M.; Maleszewski, J.J.; Owens, D.S.; Prutkin, J.M.; Salerno, J.C.; Zigman, M.L.; Ellenbogen, R.; Rao, A.L.; Ackerman, M.J.; et al. Incidence, cause, and comparative frequency of sudden cardiac death in national collegiate athletic association athletes a decade in review. Circulation 2015, 132, 10–19. [Google Scholar] [CrossRef]
- Maron, B.J.; Doerer, J.J.; Haas, T.S.; Tierney, D.M.; Mueller, F.O. Sudden Deaths in Young Competitive Athletes: Analysis of 1866 deaths in the United States, 1980-2006. Circulation 2009, 119, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Basso, C.; Maron, B.J.; Corrado, D.; Thiene, G. Clinical profile of congenital coronary artery anomalies with origin from the wrong aortic sinus leading to sudden death in young competitive athletes. J. Am. Coll. Cardiol. 2000, 35, 1493–1501. [Google Scholar] [CrossRef]
- Gräni, C.; Buechel, R.R.; Kaufmann, P.A.; Kwong, R.Y. Multimodality Imaging in Individuals with Anomalous Coronary Arteries. JACC Cardiovasc. Imaging 2017, 10, 471–481. [Google Scholar] [CrossRef]
- Gräni, C.; Benz, D.C.; Steffen, D.A.; Giannopoulos, A.A.; Messerli, M.; Pazhenkottil, A.P.; Gaemperli, O.; Gebhard, C.; Schmied, C.; Kaufmann, P.A.; et al. Sports Behavior in Middle-Aged Individuals with Anomalous Coronary Artery from the Opposite Sinus of Valsalva. Cardiology 2018, 139, 222–230. [Google Scholar] [CrossRef]
- Van Hare, G.F.; Ackerman, M.J.; Evangelista, J.-A.K.; Kovacs, R.J.; Myerburg, R.J.; Shafer, K.M.; Warnes, C.A.; Washington, R.L. Eligibility and Disqualification Recommendations for Competitive Athletes with Cardiovascular Abnormalities: Task Force 4: Congenital Heart Disease. Circulation 2015, 132, 22. [Google Scholar] [CrossRef] [PubMed]
- Freeman, R.V.; Otto, C.M. Bicuspid Aortic Valve and Aortopathy: See the First, Then Look at the Second. JACC Cardiovasc. Imaging 2013, 6, 162–164. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tangcharoen, T.; Bell, A.; Hegde, S.; Hussain, T.; Beerbaum, P.; Schaeffter, T.; Razavi, R.; Botnar, R.M.; Greil, G.F. Detection of Coronary Artery Anomalies in Infants and Young Children with Congenital Heart Disease by Using MR Imaging. Radiology 2011, 259, 240–247. [Google Scholar] [CrossRef]
- Bluemke, D.A.; Achenbach, S.; Budoff, M.; Gerber, T.C.; Gersh, B.; Hillis, L.D.; Hundley, W.G.; Manning, W.J.; Printz, B.F.; Stuber, M.; et al. Noninvasive Coronary Artery Imaging. Circulation 2008, 118, 586–606. [Google Scholar] [CrossRef]
- Wierzbowska-Drabik, K.; Kasprzak, J.D.; Mrozowska-Peruga, E.; Peruga, J.Z. Circumflex Origin from Right Coronary Artery—The Anomaly That Should Not Be Omitted during Echocardiography—”Crossed Aorta” and “Bleb Sign” Presentation after Stents Implantation. Echocardiography 2016, 33, 659–660. [Google Scholar] [CrossRef] [PubMed]
- Witt, C.M.; Elvert, L.A.; Konik, E.A.; Ammash, N.M.; Foley, D.A.; Foley, T.A. The RAC Sign. JACC Cardiovasc. Imaging 2018, 11, 648–649. [Google Scholar] [CrossRef]
- Doan, T.T.; Molossi, S.; Sachdeva, S.; Wilkinson, J.C.; Loar, R.W.; Weigand, J.D.; Schlingmann, T.R.; Reaves-O’Neal, D.L.; Pednekar, A.S.; Masand, P.; et al. Dobutamine stress cardiac MRI is safe and feasible in pediatric patients with anomalous aortic origin of a coronary artery (AAOCA). Int. J. Cardiol. 2021, 334, 42–48. [Google Scholar] [CrossRef]
- Noel, C. Cardiac stress MRI evaluation of anomalous aortic origin of a coronary artery. Congenit. Heart Dis. 2017, 12, 627–629. [Google Scholar] [CrossRef]
- Cipriani, A.; Rito, M.L.; Pica, S.; De Gaspari, M.; Rigato, I.; Marra, M.P.; De Conti, G.; Corradin, S.; Motta, R.; Pergola, V.; et al. Cardiac magnetic resonance in the assessment of the anomalous right coronary artery originating from the left sinus of Valsalva. Eur. Heart J. 2024, 45, 2098–2100. [Google Scholar] [CrossRef]
- Sachdeva, S.; Ferrino, L.; Doan, T.T.; Reaves-O’neal, D.; Dolgner, S.; Cipriani, A.; Basso, C.; A Padalino, M.; Corrado, D.; Masand, P.; et al. Late gadolinium enhancement and anomalous coronary aortic origin in a large paediatric cohort. Eur. Heart J. 2024, 45, 4862–4864. [Google Scholar] [CrossRef]
- Gaudino, M.; Di Franco, A.; Arbustini, E.; Bacha, E.; Bates, E.R.; Cameron, D.E.; Cao, D.; David, T.E.; De Paulis, R.; El-Hamamsy, I.; et al. Management of Adults with Anomalous Aortic Origin of the Coronary Arteries: State-of-the-Art Review. J. Am. Coll. Cardiol. 2023, 82, 2034–2053. [Google Scholar] [CrossRef] [PubMed]
- Golpour, A.; Patriki, D.; Hanson, P.J.; McManus, B.; Heidecker, B. Epidemiological Impact of Myocarditis. J. Clin. Med. 2021, 10, 603. [Google Scholar] [CrossRef]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, M.G.; Sechtem, U.; Schulz-Menger, J.; Holmvang, G.; Alakija, P.; Cooper, L.T.; White, J.A.; Abdel-Aty, H.; Gutberlet, M.; Prasad, S.; et al. Cardiovascular Magnetic Resonance in Myocarditis: A JACC White Paper. J. Am. Coll. Cardiol. 2009, 53, 1475–1487. [Google Scholar] [CrossRef]
- Leone, O.; Veinot, J.P.; Angelini, A.; Baandrup, U.T.; Basso, C.; Berry, G.; Bruneval, P.; Burke, M.; Butany, J.; Calabrese, F.; et al. 2011 Consensus statement on endomyocardial biopsy from the Association for European Cardiovascular Pathology and the Society for Cardiovascular Pathology. Cardiovasc. Pathol. 2012, 21, 245–274. [Google Scholar] [CrossRef] [PubMed]
- Drazner, M.H.; Bozkurt, B.; Cooper, L.T.; Aggarwal, N.R.; Basso, C.; Bhave, N.M.; Caforio, A.L.; Ferreira, V.M.; Heidecker, B.; Kontorovich, A.R.; et al. 2024 ACC Expert Consensus Decision Pathway on Strategies and Criteria for the Diagnosis and Management of Myocarditis. J. Am. Coll. Cardiol. 2025, 85, 391–431. [Google Scholar] [CrossRef]
- Bassetto, G.; Angriman, F.; Gava, C.P.L.D.; Paldino, A.; Perotto, M.; Bordignon, L.; Gigli, M.; Ferro, M.D.; Massa, L.; Altinier, A.; et al. Hot Phases Cardiomyopathy: Pathophysiology, Diagnostic Challenges, and Emerging Therapies. Curr. Cardiol. Rep. 2025, 27, 11. [Google Scholar] [CrossRef]
- Bariani, R.; Cipriani, A.; Rizzo, S.; Celeghin, R.; Marinas, M.B.; Giorgi, B.; De Gaspari, M.; Rigato, I.; Leoni, L.; Zorzi, A.; et al. ‘Hot phase’ clinical presentation in arrhythmogenic cardiomyopathy. EP Eur. 2021, 23, 907–917. [Google Scholar] [CrossRef]
- Smith, E.D.; Lakdawala, N.K.; Papoutsidakis, N.; Aubert, G.; Mazzanti, A.; McCanta, A.C.; Agarwal, P.P.; Arscott, P.; Dellefave-Castillo, L.M.; Vorovich, E.E.; et al. Desmoplakin Cardiomyopathy, a Fibrotic and Inflammatory Form of Cardiomyopathy Distinct from Typical Dilated or Arrhythmogenic Right Ventricular Cardiomyopathy. Circulation 2020, 141, 1872–1884. [Google Scholar] [CrossRef]
- Yang, Y.; Wei, X.; Lu, G.; Xie, J.; Tan, Z.; Du, Z.; Ye, W.; Xu, H.; Li, X.; Liu, E.; et al. Ringlike late gadolinium enhancement provides incremental prognostic value in non-classical arrhythmogenic cardiomyopathy. J. Cardiovasc. Magn. Reson. 2023, 25, 72. [Google Scholar] [CrossRef]
- Ferreira, V.M.; Schulz-Menger, J.; Holmvang, G.; Kramer, C.M.; Carbone, I.; Sechtem, U.; Kindermann, I.; Gutberlet, M.; Cooper, L.T.; Liu, P.; et al. Cardiovascular Magnetic Resonance in Nonischemic Myocardial Inflammation. J. Am. Coll. Cardiol. 2018, 72, 3158–3176. [Google Scholar] [CrossRef]
- Friedrich, M.G.; Marcotte, F. Cardiac Magnetic Resonance Assessment of Myocarditis. Circ. Cardiovasc. Imaging 2013, 6, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Daniels, C.J.; Rajpal, S.; Greenshields, J.T.; Rosenthal, G.L.; Chung, E.H.; Terrin, M.; Jeudy, J.; Mattson, S.E.; Law, I.H.; Borchers, J.; et al. Prevalence of Clinical and Subclinical Myocarditis in Competitive Athletes with Recent SARS-CoV-2 Infection: Results from the Big Ten COVID-19 Cardiac Registry. JAMA Cardiol. 2021, 6, 1078–1087. [Google Scholar] [CrossRef] [PubMed]
- Pelliccia, A.; Solberg, E.E.; Papadakis, M.; Adami, P.E.; Biffi, A.; Caselli, S.; La Gerche, A.; Niebauer, J.; Pressler, A.; Schmied, C.M.; et al. Recommendations for participation in competitive and leisure time sport in athletes with cardiomyopathies, myocarditis, and pericarditis: Position statement of the Sport Cardiology Section of the European Association of Preventive Cardiology (EAPC). Eur. Heart J. 2019, 40, 19–33. [Google Scholar] [CrossRef]
- Kivity, S.; Baran, T.Z.; Reuveni, M.M.; Irony, A.; Adler, L.; Alder, Y.; Parikh, R.; Kivity, S. The Longitudinal Incidence of Pericarditis in 1.6 Million Patients: A 20-Year Study. Am. J. Cardiol. 2024, 223, 70–72. [Google Scholar] [CrossRef] [PubMed]
- Adler, Y.; Charron, P.; Imazio, M.; Badano, L.; Barón-Esquivias, G.; Bogaert, J.; Brucato, A.; Gueret, P.; Klingel, K.; Lionis, C.; et al. 2015 ESC Guidelines for the diagnosis and management of pericardial diseases. Eur. Heart J. 2015, 36, 2921–2964. [Google Scholar] [CrossRef]
- Cremer, P.C.; Kumar, A.; Kontzias, A.; Tan, C.D.; Rodriguez, E.R.; Imazio, M.; Klein, A.L. Complicated Pericarditis: Understanding Risk Factors and Pathophysiology to Inform Imaging and Treatment. J. Am. Coll. Cardiol. 2016, 68, 2311–2328. [Google Scholar] [CrossRef]


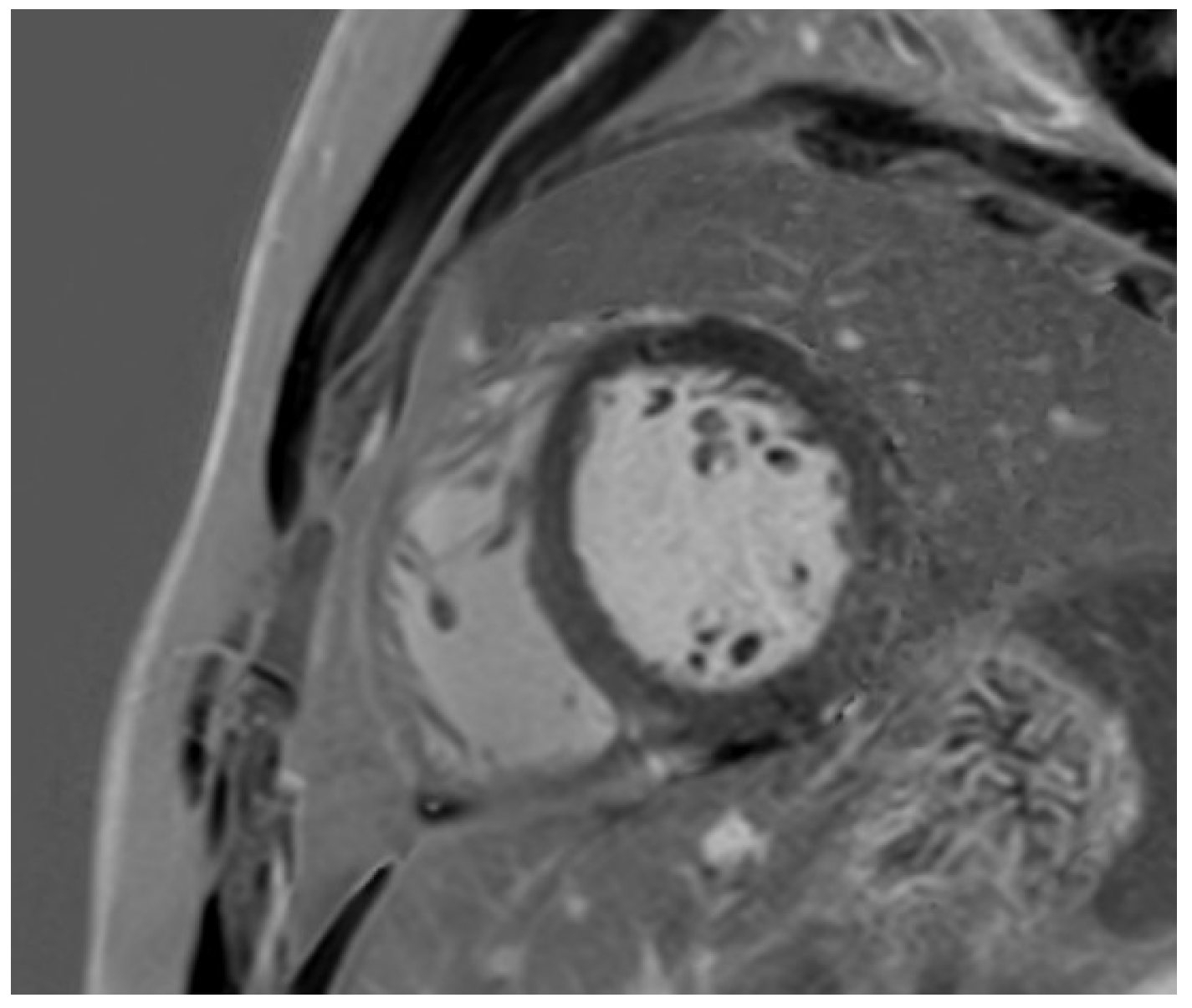

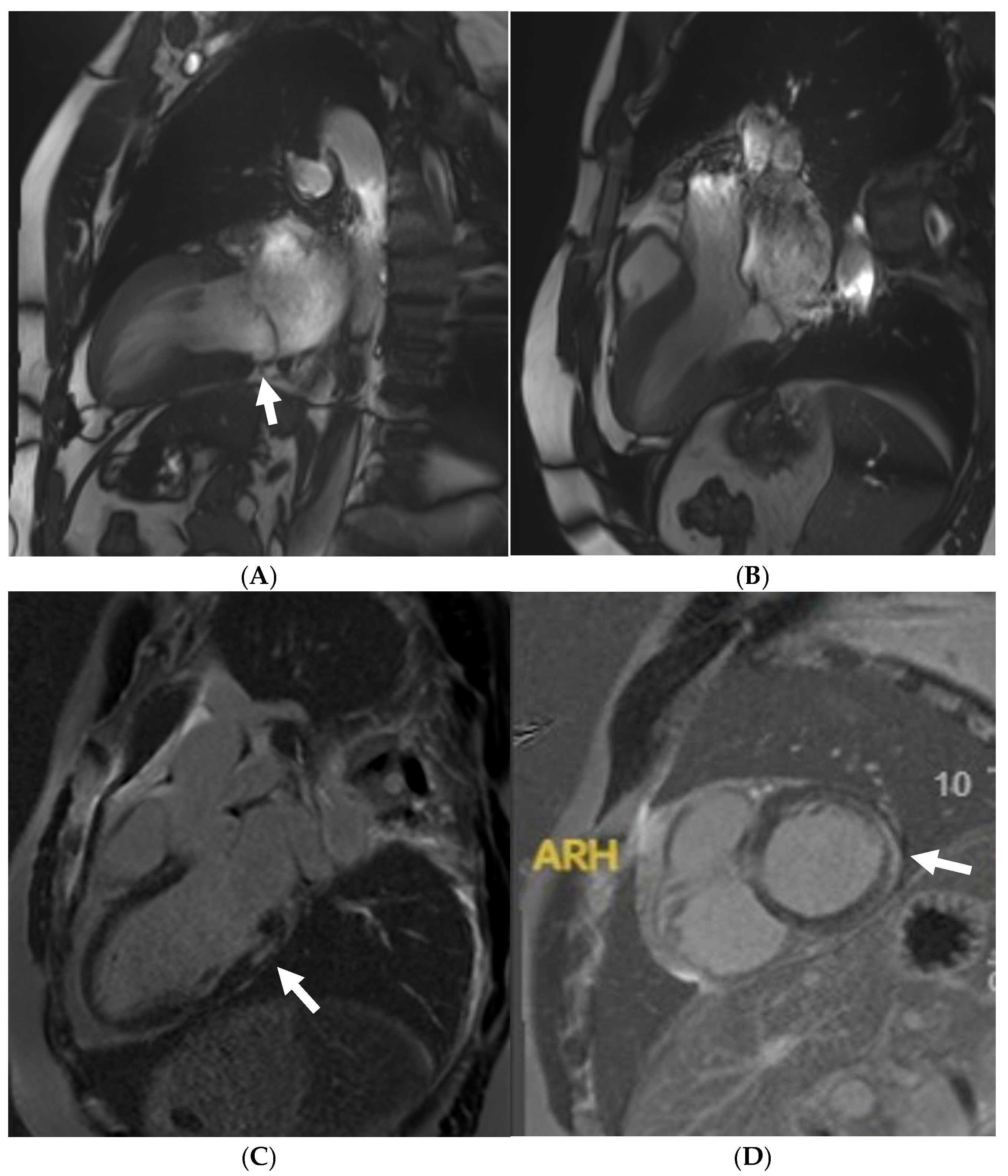
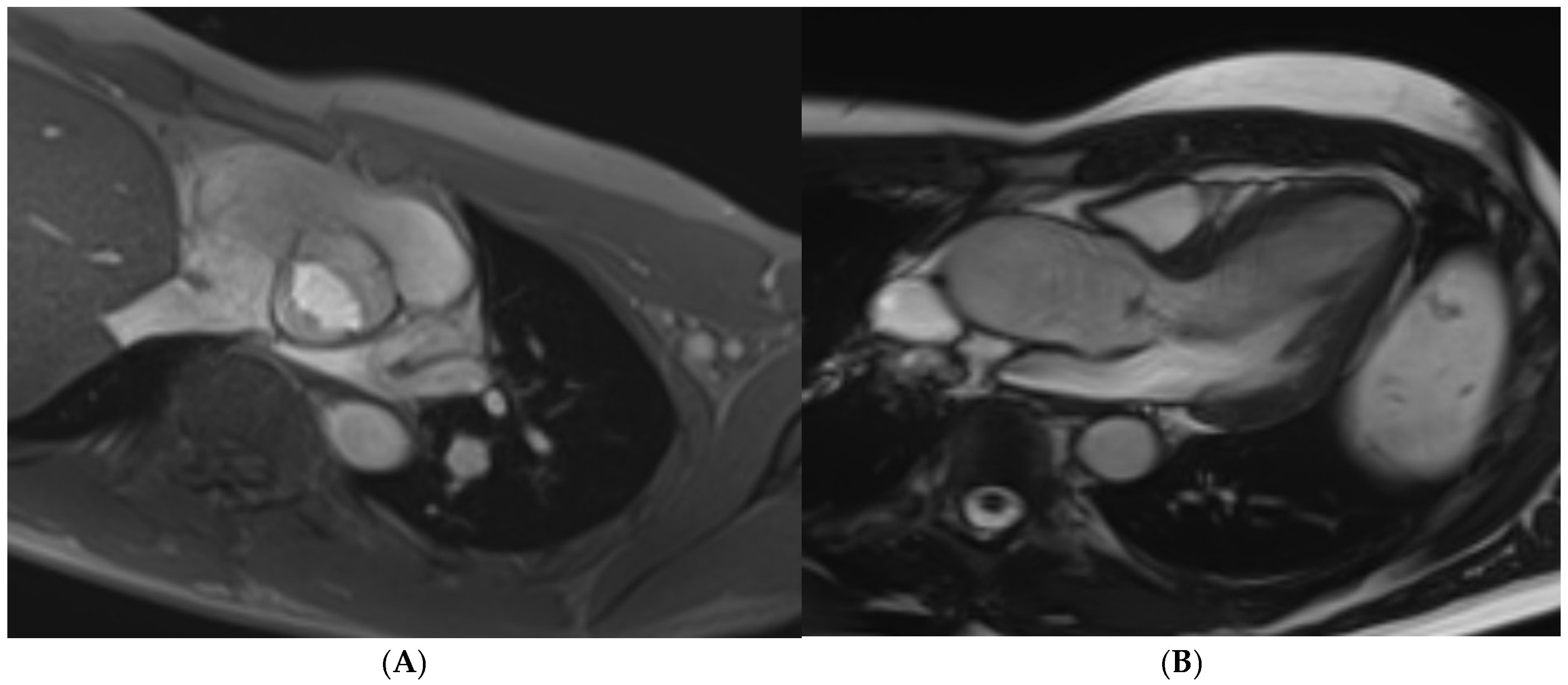

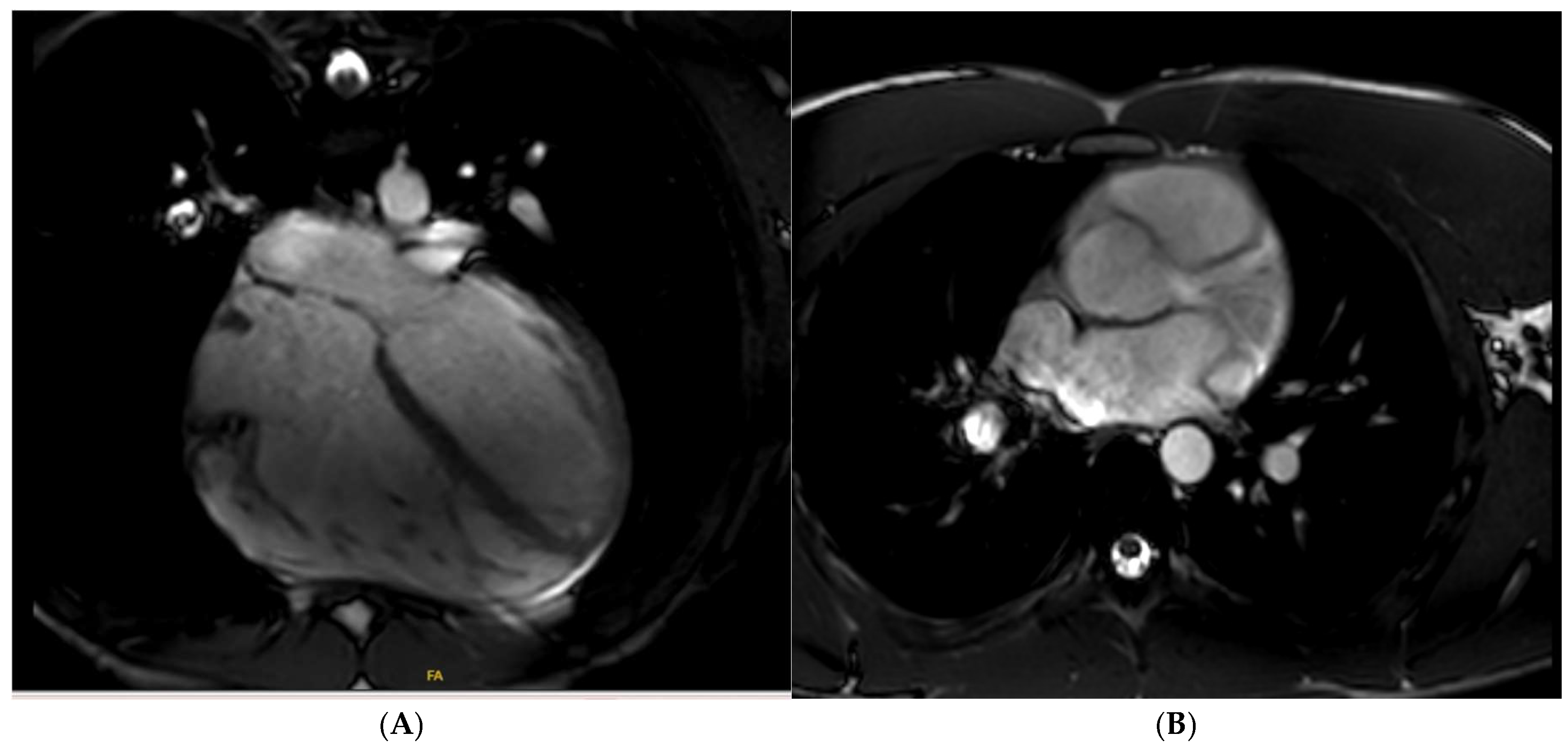
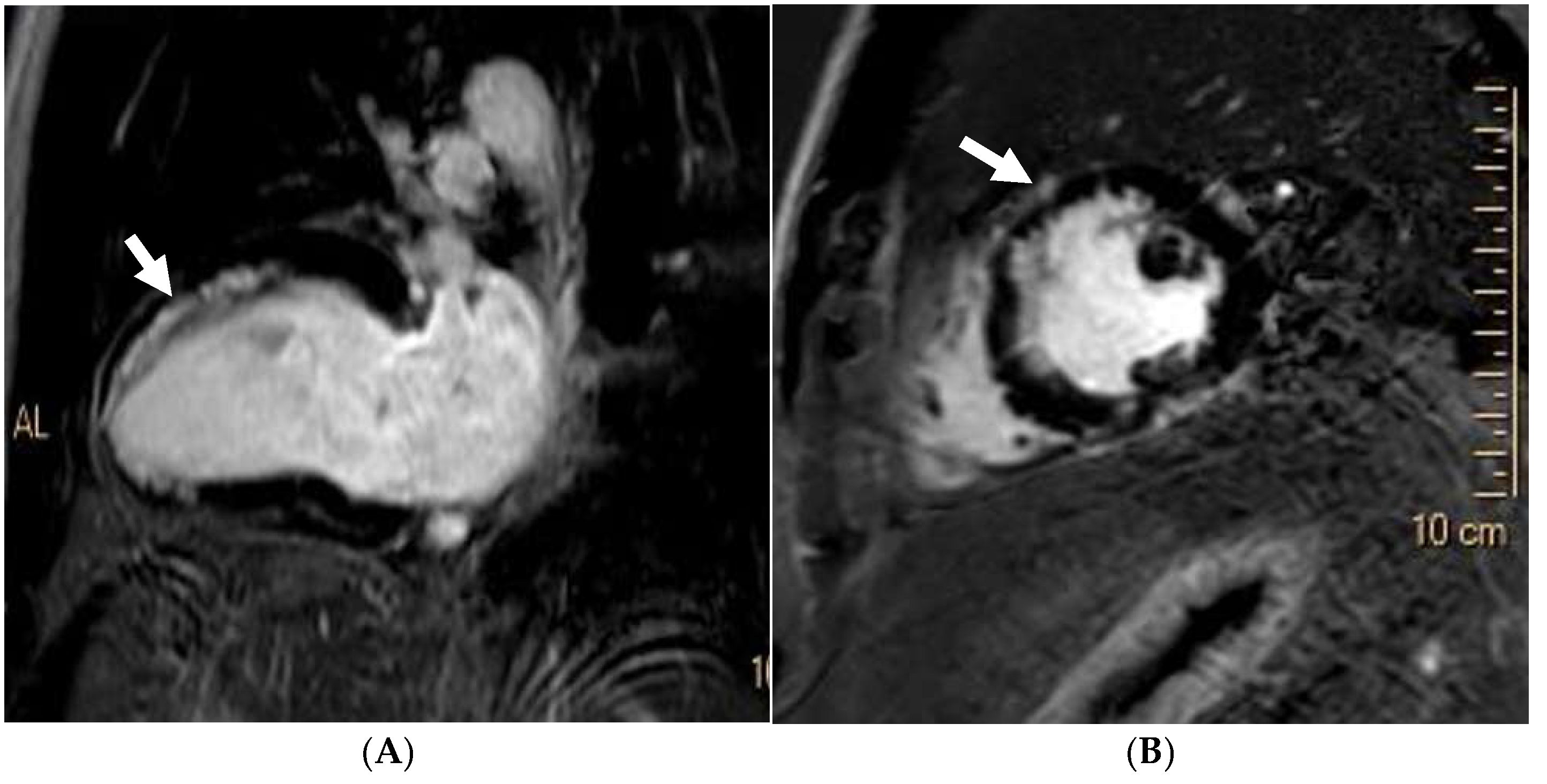
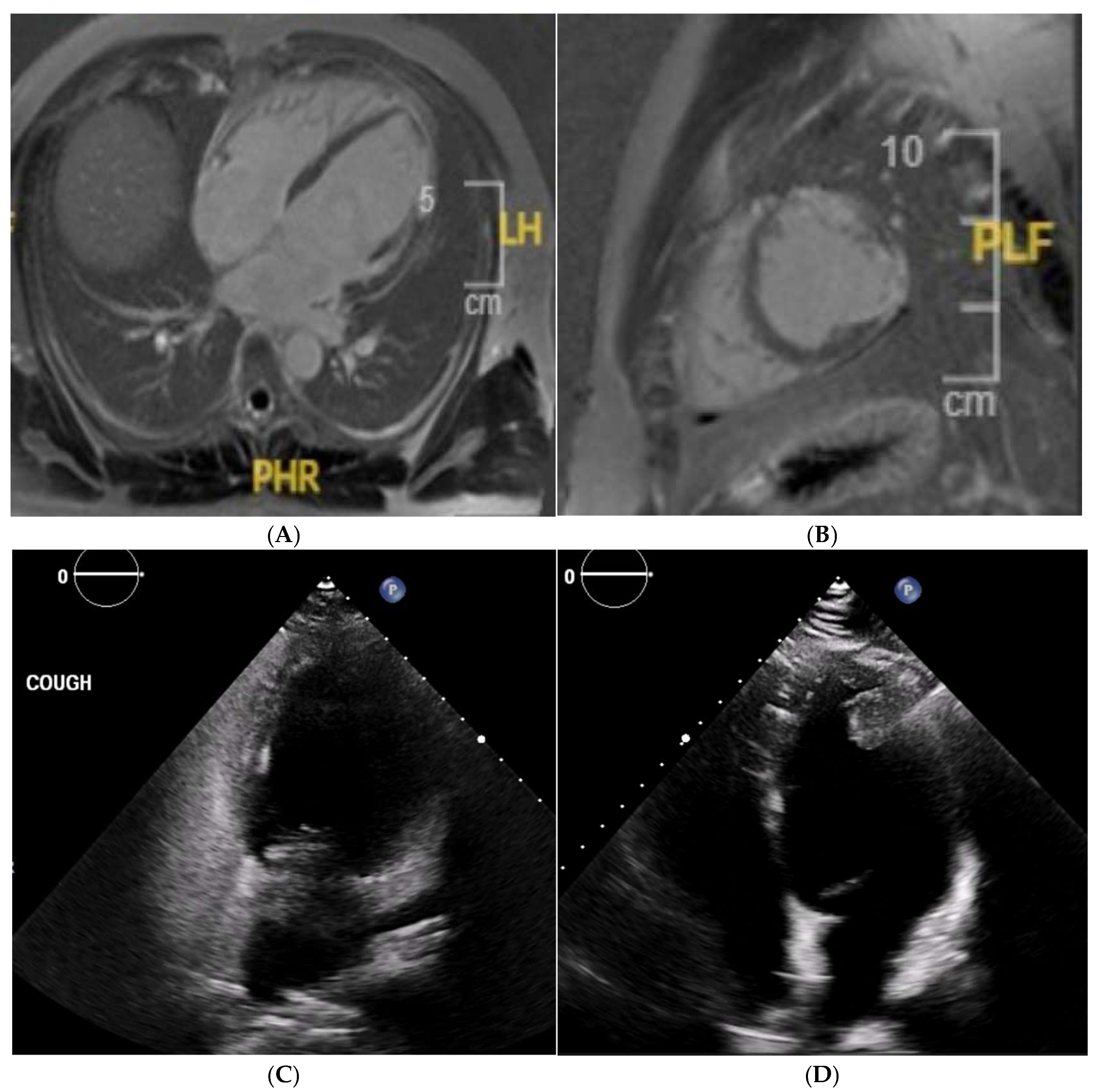
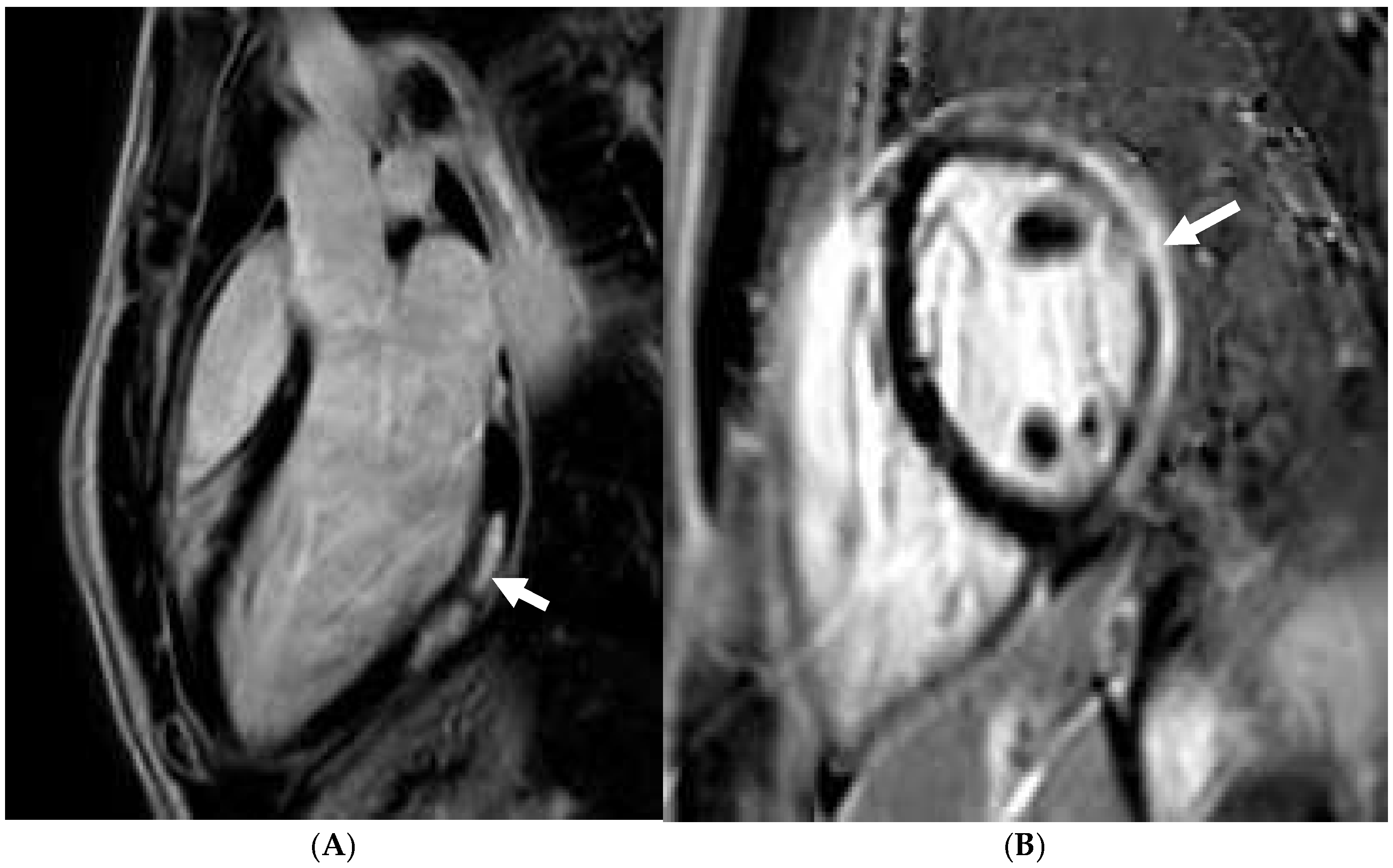

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grech, N.; Abela, M. The Role of Cardiovascular Magnetic Resonance Imaging in Athletic Individuals—A Narrative Review. J. Clin. Med. 2025, 14, 3576. https://doi.org/10.3390/jcm14103576
Grech N, Abela M. The Role of Cardiovascular Magnetic Resonance Imaging in Athletic Individuals—A Narrative Review. Journal of Clinical Medicine. 2025; 14(10):3576. https://doi.org/10.3390/jcm14103576
Chicago/Turabian StyleGrech, Neil, and Mark Abela. 2025. "The Role of Cardiovascular Magnetic Resonance Imaging in Athletic Individuals—A Narrative Review" Journal of Clinical Medicine 14, no. 10: 3576. https://doi.org/10.3390/jcm14103576
APA StyleGrech, N., & Abela, M. (2025). The Role of Cardiovascular Magnetic Resonance Imaging in Athletic Individuals—A Narrative Review. Journal of Clinical Medicine, 14(10), 3576. https://doi.org/10.3390/jcm14103576






