Ramadanov–Zabler Safe Zone for Sacroiliac Screw Placement: A CT-Based Computational Pilot Study
Abstract
1. Introduction
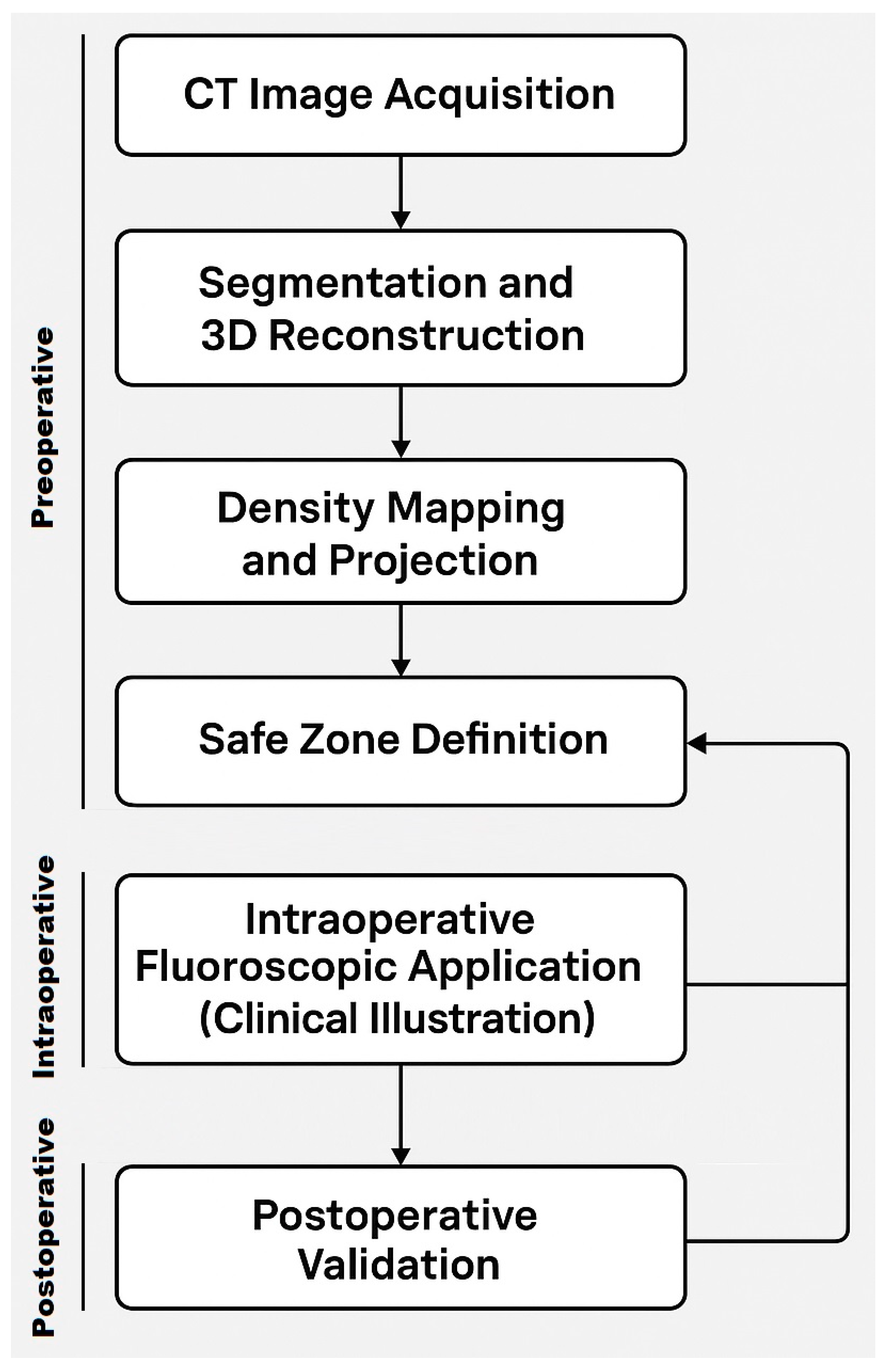
2. Materials and Methods
- CT Data Acquisition: The original CT scan dataset was provided for processing and analysis. The scan quality was sufficient, showing no relevant bony deformities or pathological changes in the sacrum and SI region, allowing for accurate 3D modeling.
- Manual Segmentation: Initial segmentation of the SI region was performed manually using Avizo software (version 2020.3, Thermo Fisher Scientific, Waltham, MA, USA). Standard thresholding techniques were attempted; however, due to the relatively low bone density in the SI region, these methods were ineffective in accurately delineating the joint.
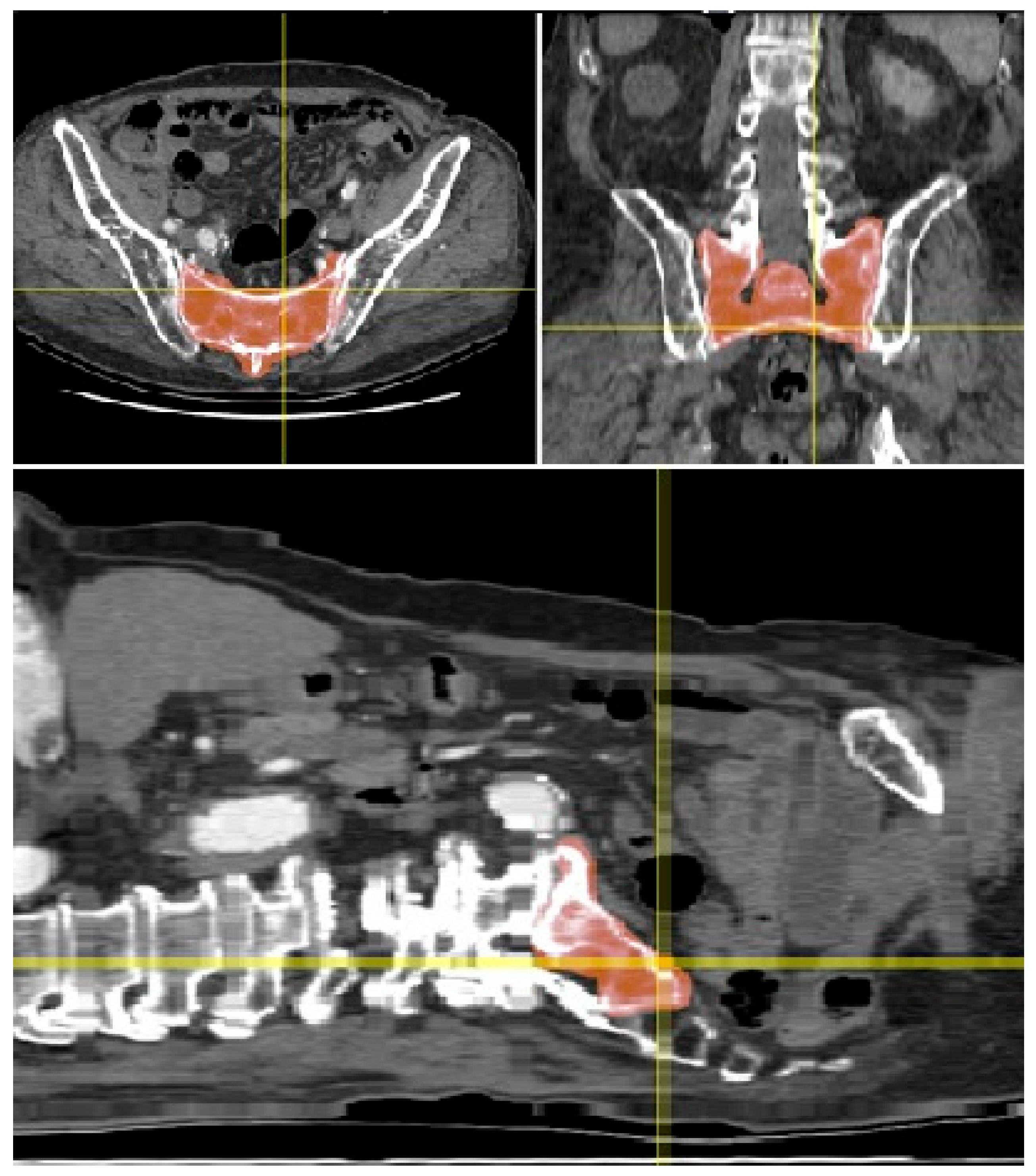
- Freehand Segmentation: To overcome the limitations posed by the low-density bone structure, a freehand segmentation approach was employed. This method, which involved manually tracing the boundaries of the SI region, took approximately one hour to complete. The result was a high-precision binary mask of the SI region, which was then used to create an accurate 3D model of the joint.
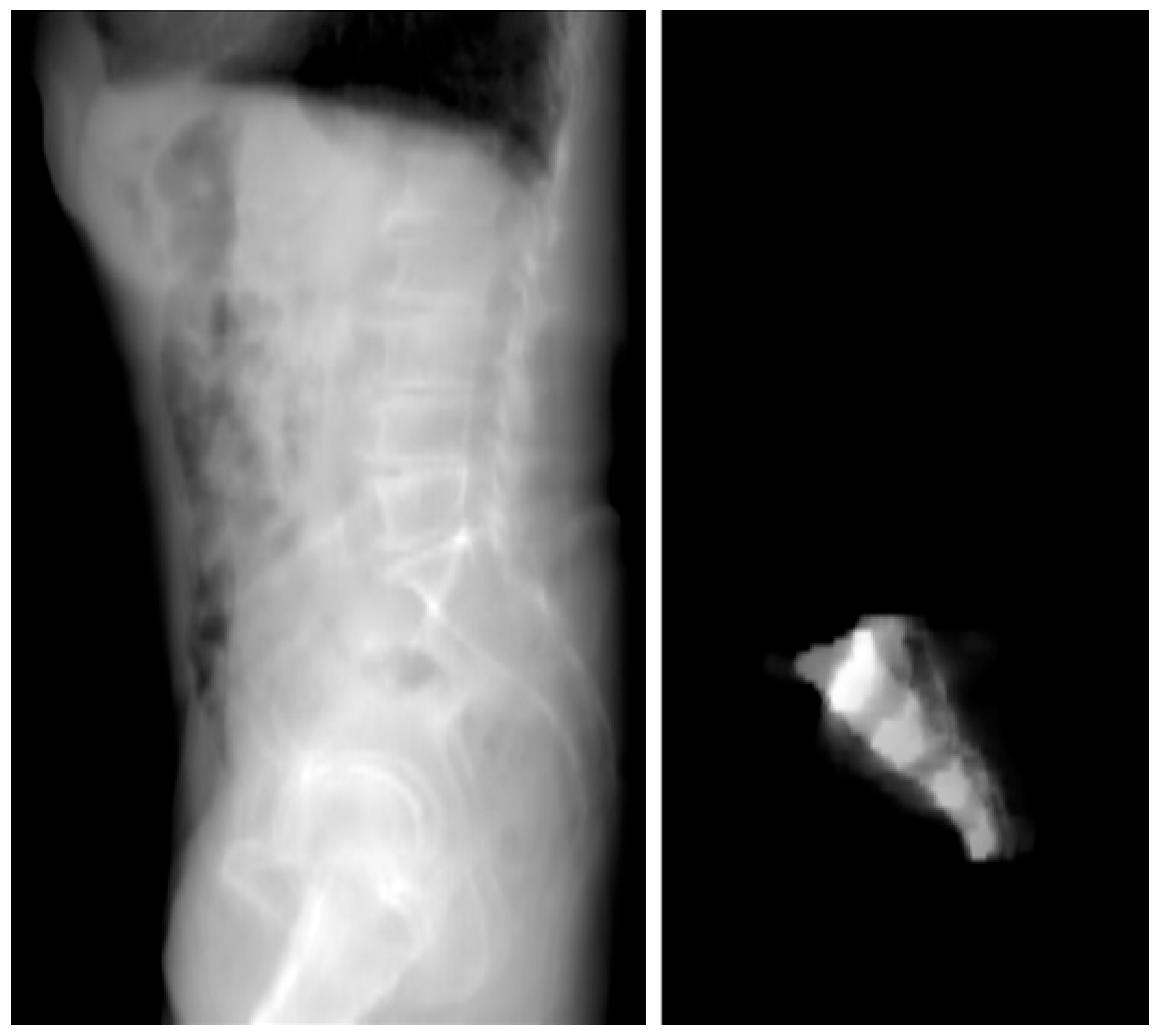
- 2D Projection Creation: From the 3D model, a 2D lateral view of the sacrum was generated by summing the Y-axis slices of the dataset. This 2D projection effectively reflected the bone density distribution within the sacrum and was crucial for visualizing regions of higher bone density. These areas were considered optimal for screw placement, as higher density regions are more likely to provide stable, intraosseous fixation.
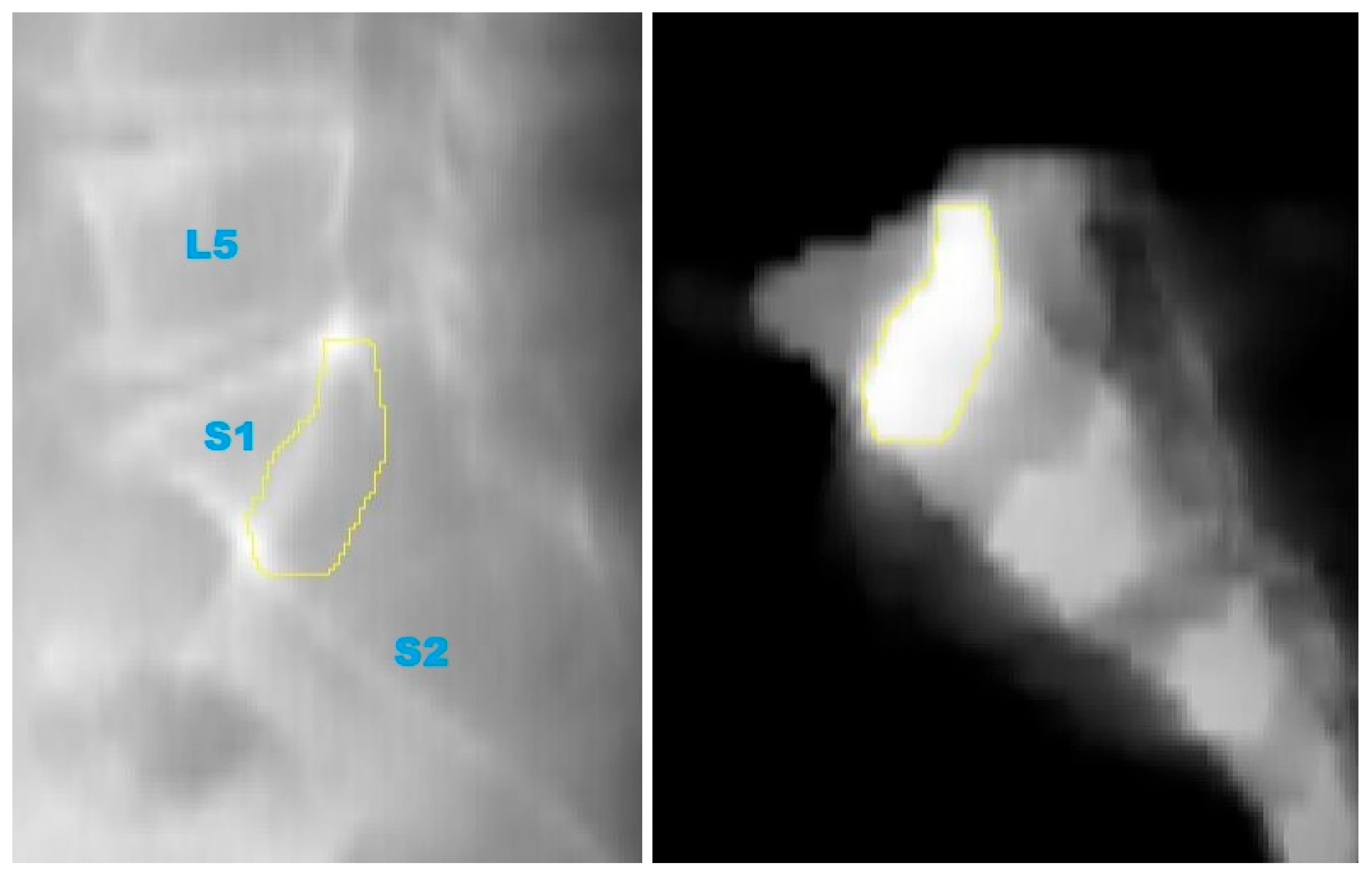
- Generation of Screw Trajectory Target Zone: Using the 2D projection, a patient-specific safe zone for screw placement was identified through a reproducible computational technique developed in this study. The resulting zone is referred to as the Ramadanov–Zabler Safe Zone for reference purposes. Regions with higher bone density were targeted as they suggested a greater likelihood that the screw would remain strictly intraosseous, thereby reducing the risk of cortical breaches or screw misplacement. The target zone served as a guide for the ideal screw trajectory during percutaneous SI screw fixation.
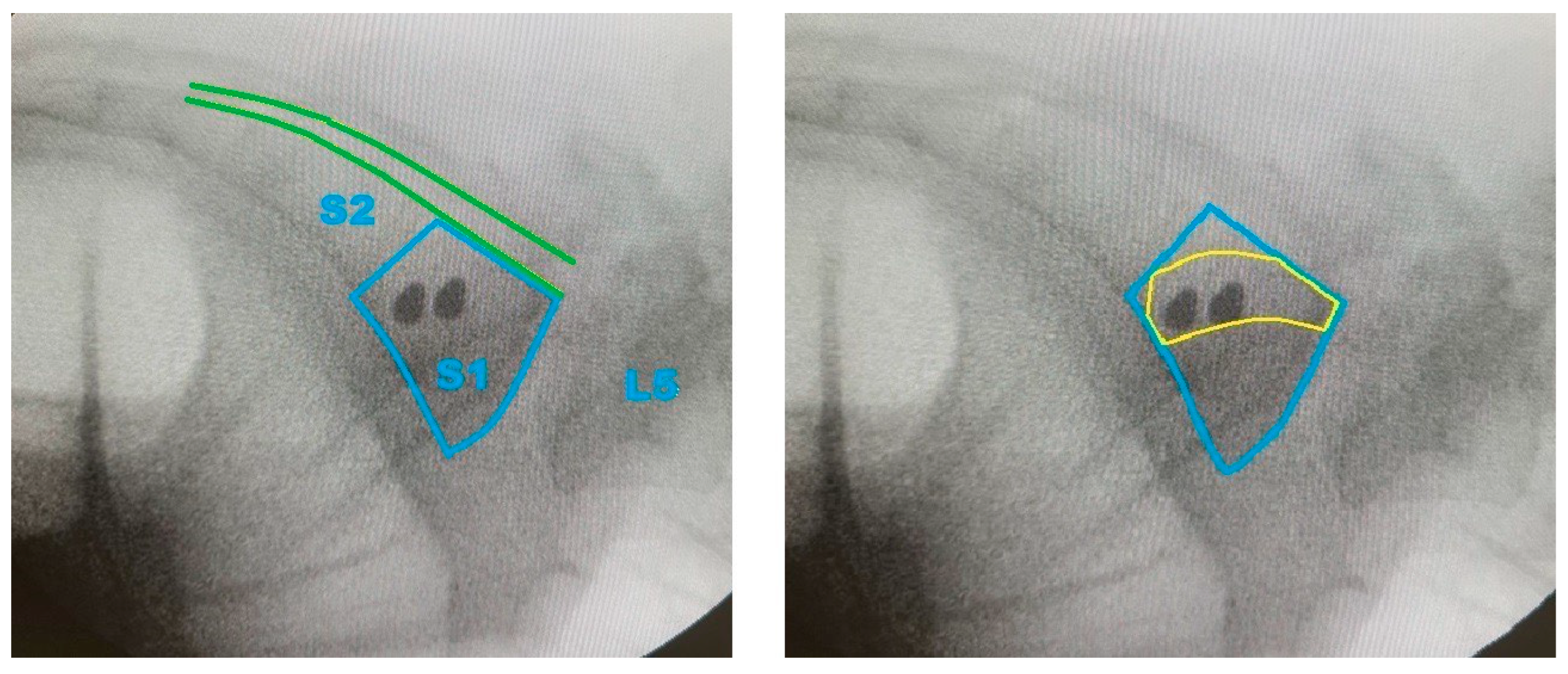
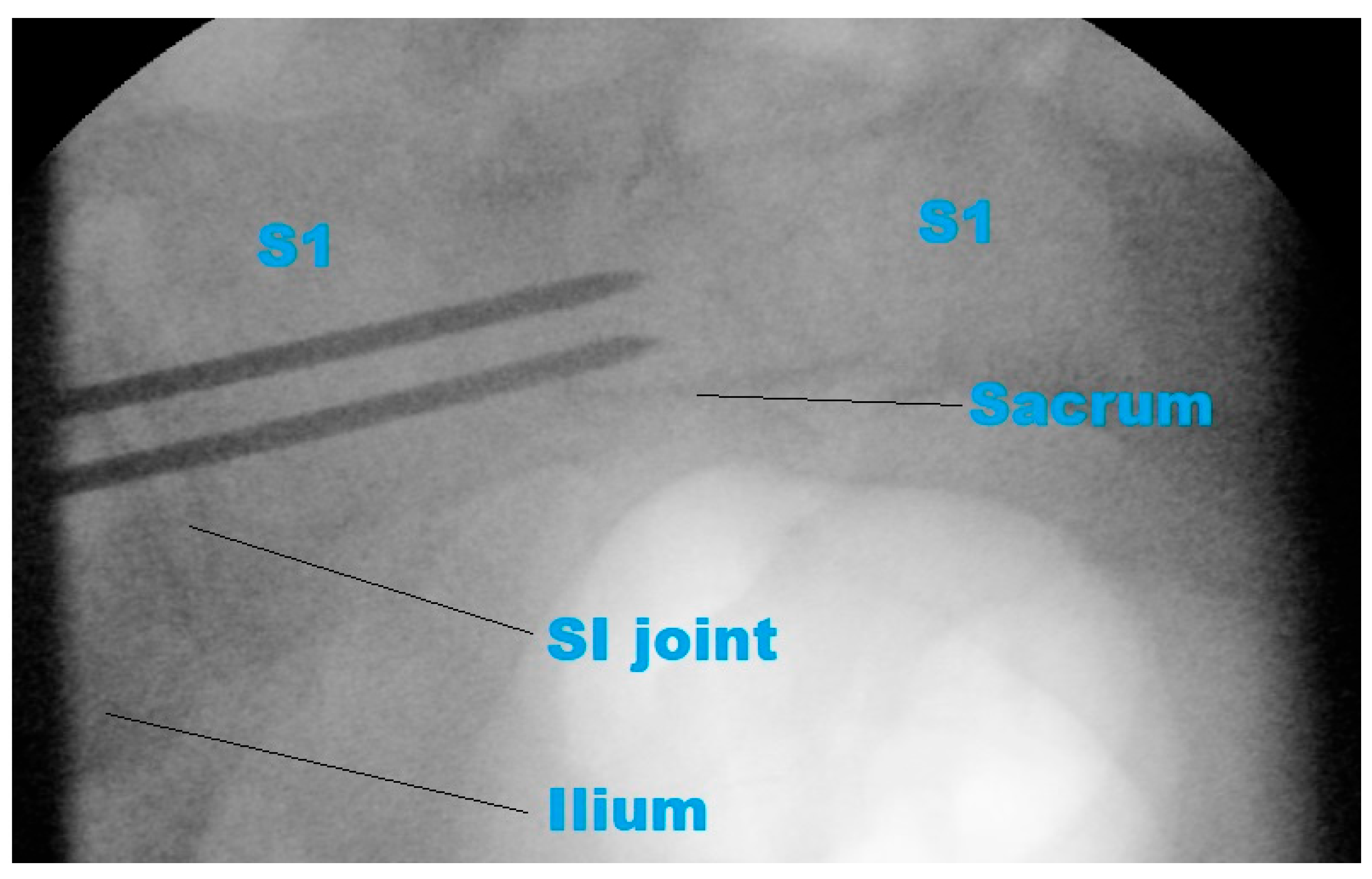
- To support the translational relevance of the proposed technique in a clinical illustration case, we included intraoperative and postoperative imaging from a representative patient with a B-type pelvic injury. The patient underwent standard percutaneous sacroiliac screw fixation. A lateral fluoroscopic image was obtained intraoperatively after guidewire placement, and postoperative CT scans (sagittal and axial) were acquired routinely.
3. Results
3.1. Three-Dimensional Model Creation and Segmentation
3.2. Two-Dimensional Projection Analysis
3.3. Safe Zone Identification
3.4. Preliminary Clinical Illustration
4. Discussion
4.1. Limitations and Strengths
4.2. Suggestions for Future Research
4.3. Pilot Application in Surgery
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 2D | two-dimensional |
| 3D | three-dimensional |
| CT | computed tomography |
| DICOM | digital imaging and communications in medicine |
| FEA | finite element analysis |
| HU | Hounsfield Unit |
| MSCT | multislice computed tomography |
| SI | sacroiliac |
References
- de Ridder, V.A.; Whiting, P.S.; Balogh, Z.J.; Mir, H.R.; Schultz, B.J.; Routt, M.C. Pelvic ring injuries: Recent advances in diagnosis and treatment. OTA Int. 2023, 6 (Suppl. S3), e261. [Google Scholar] [CrossRef]
- Matta, J.M.; Saucedo, T. Internal fixation of pelvic ring fractures. Clin. Orthop. Relat. Res. 1989, 242, 83–97. [Google Scholar] [CrossRef]
- Yin, Y.; Hou, Z.; Zhang, R.; Jin, L.; Chen, W.; Zhang, Y. Percutaneous Placement of Iliosacral Screws Under the Guidance of Axial View Projection of the S1 Pedicle: A Case Series. Sci. Rep. 2017, 7, 7925. [Google Scholar] [CrossRef] [PubMed]
- Day, C.S.; Prayson, M.J.; Shuler, T.E.; Towers, J.; Gruen, G.S. Transsacral versus modified pelvic landmarks for percutaneous iliosacral screw placement—A computed tomographic analysis and cadaveric study. Am. J. Orthop. 2000, 29 (Suppl. S9), 16–21. [Google Scholar]
- Ebraheim, N.A.; Lin, D.; Xu, R.; Yeasting, R.A. Evaluation of the upper sacrum by three-dimensional computed tomography. Am. J. Orthop. 1999, 28, 578–582. [Google Scholar]
- Chen, J.P.; Tsai, P.J.; Su, C.Y.; Tseng, I.C.; Chou, Y.C.; Chen, I.J.; Lee, P.W.; Yu, Y.H. Percutaneous Iliosacral Screw and Trans-Iliac Trans-Sacral Screw with Single C-Arm Fluoroscope Intensifier Is a Safe Treatment for Pelvic Ring Injuries. Sci. Rep. 2022, 12, 368. [Google Scholar] [CrossRef] [PubMed]
- Hilgert, R.E.; Finn, J.; Egbers, H.J. Technik der perkutanen SI-Verschraubung mit Unterstützung durch konventionellen C-Bogen. Unfallchirurg 2005, 108, 954–960. (In German) [Google Scholar] [CrossRef] [PubMed]
- Tosounidis, G.; Culemann, U.; Wirbel, R.; Holstein, J.H.; Pohlemann, T. Die perkutane transiliosakrale Zugschraubenosteosynthese des hinteren Beckenrings. Unfallchirurg 2007, 110, 669–674. (In German) [Google Scholar] [CrossRef] [PubMed]
- Tejwani, N.C.; Raskolnikov, D.; McLaurin, T.; Takemoto, R. The role of computed tomography for postoperative evaluation of percutaneous sacroiliac screw fixation and description of a “safe zone”. Am. J. Orthop. 2014, 43, 513–516. [Google Scholar] [PubMed]
- Shrestha, D.; Dhoju, D.; Shrestha, R.; Sharma, V. Percutaneous Ilio-Sacral Screw Fixation in Supine Position Under Fluoroscopy Guidance. Kathmandu Univ. Med. J. KUMJ 2015, 13, 56–60. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Herman, A.; Keener, E.; Dubose, C.; Lowe, J.A. Simple mathematical model of sacroiliac screws safe-zone—Easy to implement by pelvic inlet and outlet views. J. Orthop. Res. 2017, 35, 1478–1484. [Google Scholar] [CrossRef] [PubMed]
- McLaren, D.A.; Busel, G.A.; Parikh, H.R.; Only, A.; Patterson, J.; Gaston, B.T.; McLemore, R.; Cunningham, B. Corridor-diameter-dependent angular tolerance for safe transiliosacral screw placement: An anatomic study of 433 pelves. Eur. J. Orthop. Surg. Traumatol. 2021, 31, 1485–1492. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Liu, G.; Tang, J.; Li, G.; Qin, X.; Hu, J. TiRobot-assisted percutaneous sacroiliac cannulated screw fixation for posterior pelvic ring injury with sacral variations. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2022, 36, 940–945. (In Chinese) [Google Scholar] [PubMed]
- Aregger, F.C.; Gewiess, J.; Albers, C.E.; Deml, M.C.; Schaible, S.; Hoppe, S.; Tinner, C. Evaluation of the true lateral fluoroscopic projection for the relation of the S1 recess/foramen to safe corridors in transiliac-transsacral screw placement in human cadaveric pelves. Eur. J. Orthop. Surg. Traumatol. 2024, 35, 31. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, F.; Gligor, A.; Bataga, T. Structured Integration and Alignment Algorithm: A Tool for Personalized Surgical Treatment of Tibial Plateau Fractures. J. Pers. Med. 2021, 11, 190. [Google Scholar] [CrossRef] [PubMed]
- Solyom, A.; Moldovan, F.; Moldovan, L.; Strnad, G.; Fodor, P. Clinical Workflow Algorithm for Preoperative Planning, Reduction and Stabilization of Complex Acetabular Fractures with the Support of Three-Dimensional Technologies. J. Clin. Med. 2024, 13, 3891. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramadanov, N.; Zabler, S. Ramadanov–Zabler Safe Zone for Sacroiliac Screw Placement: A CT-Based Computational Pilot Study. J. Clin. Med. 2025, 14, 3567. https://doi.org/10.3390/jcm14103567
Ramadanov N, Zabler S. Ramadanov–Zabler Safe Zone for Sacroiliac Screw Placement: A CT-Based Computational Pilot Study. Journal of Clinical Medicine. 2025; 14(10):3567. https://doi.org/10.3390/jcm14103567
Chicago/Turabian StyleRamadanov, Nikolai, and Simon Zabler. 2025. "Ramadanov–Zabler Safe Zone for Sacroiliac Screw Placement: A CT-Based Computational Pilot Study" Journal of Clinical Medicine 14, no. 10: 3567. https://doi.org/10.3390/jcm14103567
APA StyleRamadanov, N., & Zabler, S. (2025). Ramadanov–Zabler Safe Zone for Sacroiliac Screw Placement: A CT-Based Computational Pilot Study. Journal of Clinical Medicine, 14(10), 3567. https://doi.org/10.3390/jcm14103567





