Remineralizing Treatments for Dental Erosion and Sensitivity in Patients Suffering from Gastroesophageal Reflux Disease (GERD): Randomized Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Trial Designs
2.2. Participants
2.3. Interventions and Outcomes
- The Basic Erosive Wear Examination Index (BEWE) presents criteria for evaluation based on 4 scores (0 to 3) assigned according to the amount of hard tissue lost and the ability to visually identify it [30];
- The Schiff Air Index (SAI) quantifies the pain of dental hypersensitivity in response to the evaporative stimulus (no pain, response to stimulation, response to stimulation and removal, accentuated pain response) by assigning a score ranging from 0 to 3 [31];
- The Plaque Index (PI) is recorded during the clinical periodontal examination at 4 sites for each tooth element present, using circumferential probing with a manual periodontal probe [32];
- The Bleeding Score (BS) is based on the amount of bleeding after periodontal probing; the index ranges from no bleeding to profuse and copious bleeding [33].
- in the Trial group, a zinc hydroxyapatite-based toothpaste (Biorepair Plus Total Protection, Biorepair®, Coswell S.p.A., Funo di Argelato, Italy) treatment was applied with a toothbrush (manual or electric) to the buccal and lingual surfaces of the dental elements twice daily for the duration of the study. In addition, a hydroxyapatite-based paste was applied (Biorepair Plus Enamel Repair Intensive Treatment, Biorepair® Coswell, S.p.A., Funo di Argelato, Italy). After the oral hygiene procedures, the treatment was applied to the inner surface of a bite guard, which was then placed in the patient’s mouth. The patients were instructed to close their teeth naturally, allowing the product to act for 10 min. The treatment was repeated for 7 days per month for the entire length of the study.
- Zinc hydroxyapatite-based toothpaste (Biorepair Plus Total Protection) was applied in the Control group as indicated for the Trial group. No additional paste or treatment was applied.
2.4. Sample Size
2.5. Randomization and Blinding
2.6. Statistical Analysis
3. Results
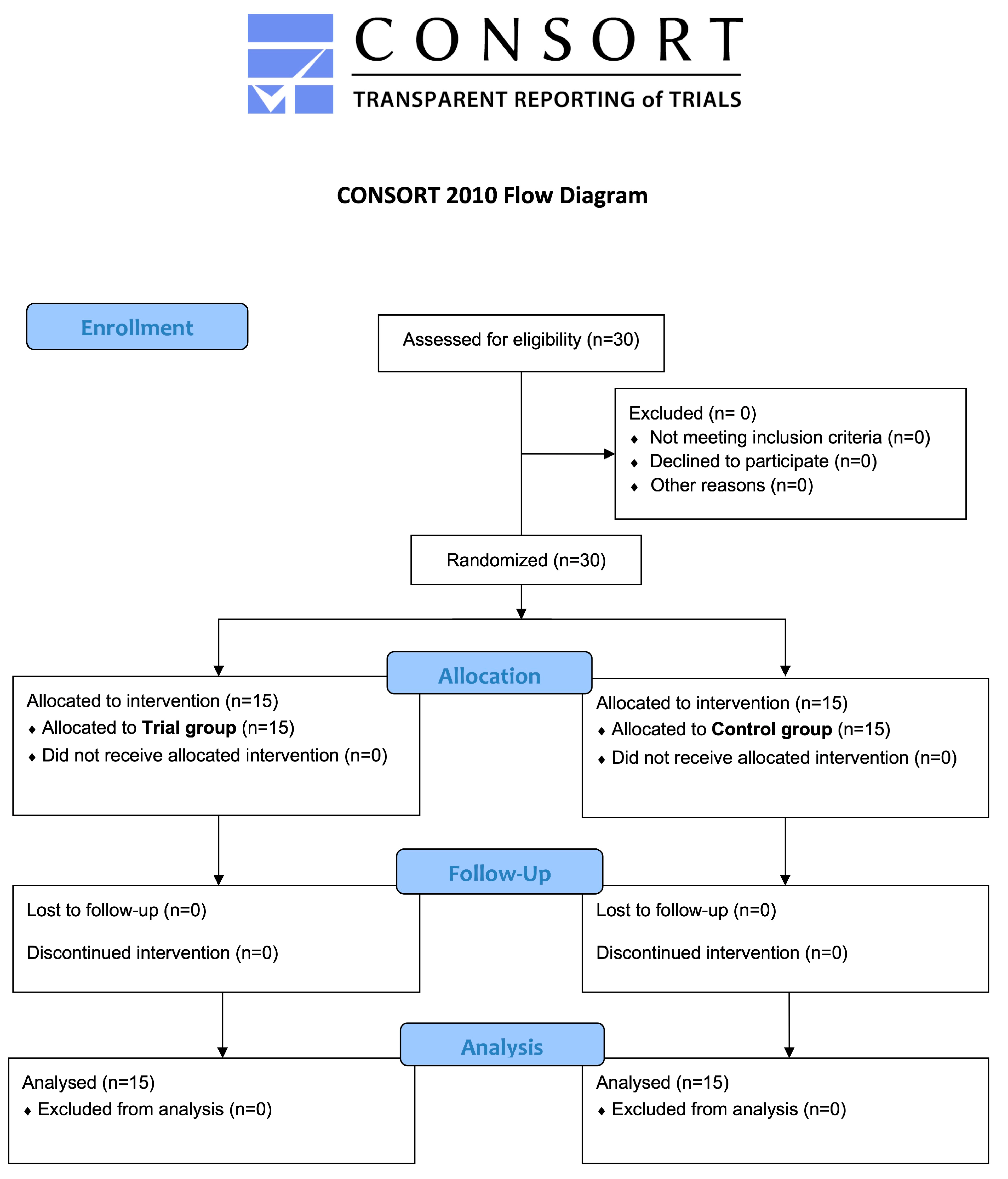
3.1. Participants Flow and Baseline Data
3.2. BEWE
3.3. Schiff Air Index
3.4. Bleeding Score
3.5. Plaque Index
3.6. Ph-Metry
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baroudi, K.; Hassan, N.A. The effect of light-activation sources on tooth bleaching. Niger. Med. J. 2014, 55, 363–368. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Loke, C.; Lee, J.; Sander, S.; Mei, L.; Farella, M. Factors affecting intra-oral pH—A review. J. Oral. Rehabil. 2016, 43, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Ganss, C.; Lussi, A.; Schlueter, N. The histological features and physical properties of eroded dental hard tissues. Monogr. Oral Sci. 2014, 25, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Kanzow, P.; Wegehaupt, F.J.; Attin, T.; Wiegand, A. Etiology and pathogenesis of dental erosion. Quintessence Int. 2016, 47, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Chikte, U.M.; Naidoo, S.; Kolze, T.J.; Grobler, S.R. Patterns of tooth surface loss among winemakers. SADJ 2005, 60, 370–374. [Google Scholar] [PubMed]
- Lussi, A.; Jaeggi, T.; Zero, D. The role of diet in the aetiology of dental erosion. Caries Res. 2004, 38 (Suppl. S1), 34–44. [Google Scholar] [CrossRef] [PubMed]
- Otsu, M.; Hamura, A.; Ishikawa, Y.; Karibe, H.; Ichijyo, T.; Yoshinaga, Y. Factors affecting the dental erosion severity of patients with eating disorders. Biopsychosoc. Med. 2014, 8, 25. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Monda, M.; Costacurta, M.; Maffei, L.; Docimo, R. Oral manifestations of eating disorders in adolescent patients. A review. Eur. J. Paediatr. Dent. 2021, 22, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Pereira Rde, S. Regression of gastresophageal reflux disease symptoms using dietary supplementation with melatonin, vitamins and aminoacids: Comparison with omeprazole. J. Pineal Res. 2006, 41, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Katz, P.O.; Gerson, L.B.; Vela, M.F. Guidelines for the diagnosis and management of gastresophageal reflux disease. Am. J. Gastroenterol. 2013, 108, 308–328. [Google Scholar] [CrossRef] [PubMed]
- Bredenoord, A.J.; Weusten, B.L.; Curvers, W.L.; Timmer, R.; Smout, A.J. Determinants of perception of heartburn and regurgitation. Gut 2006, 55, 313–318. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zerbib, F.; des Varannes, S.B.; Roman, S.; Pouderoux, P.; Artigue, F.; Chaput, U.; Mion, F.; Caillol, F.; Verin, E.; Bommelaer, G.; et al. Normal values and day-to-day variability of 24-h ambulatory esophageal impedance-pH monitoring in a Belgian-French cohort of healthy subjects. Aliment. Pharmacol. Ther. 2005, 22, 1011–1021. [Google Scholar] [CrossRef] [PubMed]
- Wilder-Smith, C.H.; Wilder-Smith, P.; Kawakami-Wong, H.; Voronets, J.; Osann, K.; Lussi, A. Quantification of dental erosions in patients with GERD using optical coherence tomography before and after double-blind, randomized treatment with esomeprazole or placebo. Am. J. Gastroenterol. 2009, 104, 2788–2795. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vinesh, E.; Masthan, K.; Kumar, M.S.; Jeyapriya, S.M.; Babu, A.; Thinakaran, M. A Clinicopathologic Study of Oral Changes in Gastroesophageal Reflux Disease, Gastritis, and Ulcerative Colitis. J. Contemp. Dent. Pract. 2016, 17, 943–947. [Google Scholar]
- Zhang, B.; Zhao, M.; Duan, S.; Tian, J.; Lei, L.; Huang, R. An economic evaluation of pit and fissure sealants and fluoride varnishes in preventing dental caries: A systematic review. J. Clin. Pediatr. Dent. 2023, 47, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Buldur, B.; Taskaya, B. Clinical effectiveness and parental acceptance of silver diamine fluoride in preschool children: A non-randomized trial. J. Clin. Pediatr. Dent. 2024, 48, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Butera, A.; Gallo, S.; Pascadopoli, M.; Montasser, M.A.; Abd El Latief, M.H.; Modica, G.G.; Scribante, A. Home Oral Care with Biomimetic Hydroxyapatite vs. Conventional Fluoridated Toothpaste for the Remineralization and Desensitizing of White Spot Lesions: Randomized Clinical Trial. Int. J. Environ. Res. Public Health 2022, 19, 8676. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Akbeyaz Sivet, E.; Yilmazkasapoglu Turkay, E.; Akbeyaz, I.H.; Kargul, B. Knowledge, attitudes, and practice of general pediatricians and pediatric subspecialists towards oral health in children: A survey in Turkey. J. Clin. Pediatr. Dent. 2024, 48, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Shuja, M.E.; Jeelani, W.; Ahmed, M.; Khalid, A. Management of orthodontically induced white spot lesions: A survey of the orthodontic practitioners of Pakistan. J. Pak. Med. Assoc. 2024, 74, 922–929. [Google Scholar] [CrossRef] [PubMed]
- Al-Qahtani, A.S.; Almutairi, B.; Al-Hamdan, R.S.; Alzahrani, K.M. Remineralizing pretreatment using casein phosphopeptide-amorphous calcium phosphate fluoride, self-assembling peptide, and nanohydroxyapatite gel activation via invisible infrared light on the dentin microhardness and micro shear bond strength to the composite restoration. Photodiagnosis Photodyn. Ther. 2024, 47, 104210. [Google Scholar] [CrossRef] [PubMed]
- Gokce, A.N.P.; Kelesoglu, E.; Sagır, K.; Kargul, B. Remineralization potential of a novel varnish: An in vitro comparative evaluation. J. Clin. Pediatr. Dent. 2024, 48, 173–180. [Google Scholar] [CrossRef]
- Patel, M.K.; Milano, M.; Messer, R.L. Acceptance and awareness of southeastern and western private practice pediatric dentists of fluoride-free toothpastes: A survey study. J. Clin. Pediatr. Dent. 2023, 47, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Scribante, A.; Pascadopoli, M.; Bergomi, P.; Licari, A.; Marseglia, G.L.; Bizzi, F.M.; Butera, A. Evaluation of two different remineralising toothpastes in children with drug-controlled asthma and allergic rhinitis: A randomised clinical trial. Eur. J. Paediatr. Dent. 2024, 25, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Tonguc-Altin, K.; Selvi-Kuvvetli, S.; Topcuoglu, N.; Kulekci, G. Antibacterial effects of dentifrices against Streptococcus mutans in children: A comparative in vitro study. J. Clin. Pediatr. Dent. 2024, 48, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Butera, A.; Maiorani, C.; Gallo, S.; Pascadopoli, M.; Quintini, M.; Lelli, M.; Tarterini, F.; Foltran, I.; Scribante, A. Biomimetic Action of Zinc Hydroxyapatite on Remineralization of Enamel and Dentin: A Review. Biomimetics. 2023, 8, 71. [Google Scholar] [CrossRef]
- Nam, O.H.; Park, T.Y.; Jeong, S.R.; Shin, J.; Jih, M.K. Antimicrobial effect of two fluoride-releasing adhesive tapes on Streptococcus mutansbiofilm. J. Clin. Pediatr. Dent. 2024, 48, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Vishwanathaiah, S.; Maganur, P.C.; Syed, A.A.; Kakti, A.; Hussain Jaafari, A.H.; Albar, D.H.; Renugalakshmi, A.; Jeevanandan, G.; Khurshid, Z.; Ali Baeshen, H.; et al. Effectiveness of silver diamine fluoride (SDF) in arresting coronal dental caries in children and adolescents: A systematic review. J. Clin. Pediatr. Dent. 2024, 48, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, A.D.P.; Mora, V.S.A.; Dávila, M.; Montesinos-Guevara, C. Dental caries prevention in pediatric patients with molar incisor hypomineralization: A scoping review. J. Clin. Pediatr. Dent. 2023, 47, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Butera, A.; Maiorani, C.; Morandini, A.; Simonini, M.; Morittu, S.; Barbieri, S.; Bruni, A.; Sinesi, A.; Ricci, M.; Trombini, J.; et al. Assessment of Genetical, Pre, Peri and Post Natal Risk Factors of Deciduous Molar Hypomineralization (DMH), Hypomineralized Second Primary Molar (HSPM) and Molar Incisor Hypomineralization (MIH): A Narrative Review. Children 2021, 8, 432. [Google Scholar] [CrossRef]
- Bartlett, D.; Ganss, C.; Lussi, A. Basic Erosive Wear Examination (BEWE): A new scoring system for scientific and clinical needs. Clin. Oral Investig. 2008, 12 (Suppl. S1), S65–S68. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Arshad, S.; Zaidi, S.J.A.; Farooqui, W.A. Comparative efficacy of BioMin-F, Colgate Sensitive Pro-relief and Sensodyne Rapid Action in relieving dentin hypersensitivity: A randomized controlled trial. BMC Oral. Health. 2021, 21, 498. [Google Scholar] [CrossRef]
- O’leary, T.J. The plaque control record. J. Periodontol. 1972, 43, 38–42. [Google Scholar] [CrossRef]
- Mombelli, A.; van Oosten, M.A.; Schurch, E., Jr.; Land, N.P. The microbiota associated with successful or failing osseointegrated titanium implants. Oral Microbiol. Immunol. 1987, 2, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Pepelassi, E.; Rahiotis, C.; Peponi, E.; Kakaboura, A.; Vrotsos, I. Effectiveness of an in-office arginine-calcium carbonate paste on dentine hypersensitivity in periodontitis patients: A double-blind, randomized controlled trial. J. Clin. Periodontol. 2015, 42, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Piepho, H.P. An Algorithm for a Letter-Based Representation of All-Pairwise Comparisons. J. Comput. Graph. Stat. 2004, 13, 456–466. [Google Scholar] [CrossRef]
- Gyawali, C.P.; Yadlapati, R.; Fass, R.; Katzka, D.; Pandolfino, J.; Savarino, E.; Sifrim, D.; Spechler, S.; Zerbib, F.; Fox, M.R.; et al. Updates to the modern diagnosis of GERD: Lyon consensus 2.0. Gut 2024, 73, 361–371. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schroeder, P.L.; Filler, S.J.; Ramirez, B.; Lazarchik, D.A.; Vaezi, M.F.; Richter, J.E. Dental erosion and acid reflux disease. Ann. Intern. Med. 1995, 122, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A.; Anjankar, A.P. Association of Gastresophageal Reflux Disease with Dental Erosion. Cureus 2022, 14, e30381. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Picos, A.; Chisnoiu, A.; Dumitrasc, D.L. Dental erosion in patients with gastresophageal reflux disease. Adv. Clin. Exp. Med. 2013, 22, 303–307. [Google Scholar] [PubMed]
- Chen, H.; Hill, R.; Baysan, A. The effect of different concentrations of fluoride in toothpastes with or without bioactive glass on artificial root caries. J. Dent. 2023, 133, 104499. [Google Scholar] [CrossRef] [PubMed]
- Buzalaf, M.A.; Hannas, A.R.; Kato, M.T. Saliva and dental erosion. J. Appl. Oral. Sci. 2012, 20, 493–502. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cochrane, N.J.; Saranathan, S.; Cai, F.; Cross, K.J.; Reynolds, E.C. Enamel subsurface lesion remineralisation with casein phosphopeptide stabilised solutions of calcium, phosphate and fluoride. Caries Res. 2008, 42, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, E.C. Casein phosphopeptide-amorphous calcium phosphate: The scientific evidence. Adv. Dent. Res. 2009, 21, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Turkay, E.Y.; Kargul, B.; Aydinoglu, A.K.; Yoruc, A.B.H. Evaluation of different remineralization agents in the treatment of natural caries-affected dentin in permanent teeth. Biomed. Mater. Eng. 2023, 34, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Memarpour, M.; Jafari, S.; Rafiee, A.; Alizadeh, M.; Vossoughi, M. Protective effect of various toothpastes and mouthwashes against erosive and abrasive challenge on eroded dentin: An in vitro study. Sci. Rep. 2024, 14, 9387. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Uskokovic, V.; Tang, S. A review on biomimetic hydroxyapatite nanoparticles for remineralization of dental tissues. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2019, 107, 1469–1483. [Google Scholar]
- Kani, T.; Kani, M.; Isozaki, A.; Shintani, H.; Ohashi, T. Effect of hydroxyapatite toothpaste on reduction of dentin hypersensitivity: An 8-week clinical study. J. Clin. Periodontol. 2014, 41, 1057–1064. [Google Scholar]
- Huang, S.; Gao, S.; Cheng, L.; Yu, H. Remineralization potential of nano-hydroxyapatite on initial enamel lesions: An in vitro study. Caries Res. 2011, 45, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Lombardini, M.; Ceci, M.; Colombo, M.; Bianchi, S.; Poggio, C. Preventive effect of different toothpastes on enamel erosion: AFM and SEM studies. Scanning. 2014, 36, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Scribante, A.; Cosola, S.; Pascadopoli, M.; Genovesi, A.; Battisti, R.A.; Butera, A. Clinical and Technological Evaluation of the Remineralising Effect of Biomimetic Hydroxyapatite in a Population Aged 6 to 18 Years: A Randomized Clinical Trial. Bioengineering 2025, 12, 152. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jung, K.; Kerzel, P.; Hara, A.T.; Luka, B.; Schlueter, N.; Ganss, C. Hydroxyapatite in Oral Care Products: In vitro Effects on Erosion/Abrasion and Analysis of Formulation Components. Caries Res. 2024, 2024, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Orilisi, G.; Vitiello, F.; Notarstefano, V.; Furlani, M.; Riberti, N.; Monterubbianesi, R.; Bellezze, T.; Campus, G.; Carrouel, F.; Orsini, G.; et al. Multidisciplinary evaluation of the remineralization potential of three fluoride-based toothpastes on natural white spot lesions. Clin. Oral. Investig. 2023, 27, 7451–7462. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Esparza-Villalpando, V.; Fernandez-Hernandez, E.; Rosales-Berber, M.; Torre-Delgadillo, G.; Garrocho-Rangel, A.; Pozos-Guillén, A. Clinical Efficacy of Two Topical Agents for the Remineralization of Enamel White Spot Lesions in Primary Teeth. Pediatr. Dent. 2021, 43, 95–101. [Google Scholar] [PubMed]
- González-Cabezas, C.; Fernández, C.E. Recent Advances in Remineralization Therapies for Caries Lesions. Adv. Dent. Res. 2018, 29, 55–59. [Google Scholar] [CrossRef] [PubMed]
| Inclusion Criteria |
|
| Exclusion Criteria |
|
| Zinc hydroxyapatite-based toothpaste (Biorepair Plus Total Protection) | Aqua, Zinc Hydroxyapatite (microRepair®) 20%, Glycerin, Hydrated Silica, Sorbitol, Silica, Aroma, Cellulose Gum, Sodium Myristoyl Sarcosinate, Sodium Methyl Cocoyl Taurate, Citric Acid, Tetrapotassium Pyrophosphate, Zinc PCA 13%, Sodium Saccharin, Phenoxyethanol, Benzyl Alcohol, Sodium Benzoate |
| Zinc-hydroxyapatite-based paste (Biorepair Plus Enamel Repair Intensive Treatment) | Aqua, Zinc Hydroxyapatite (microRepair®), Hydrated Silica, Silica, Sodium Myristoyl Sarcosinate, Sodium Methyl Cocoyl Taurate, Sodium Bicarbonate, Aroma, Sodium Saccharin, Phenoxyethanol, Benzyl Alcohol, Sodium Benzoate, Citric Acid, Menthol |
| Mean | SD | Min | Mdn | Max | Significance * | ||
|---|---|---|---|---|---|---|---|
| Control | T0 | 1.33 | 0.49 | 1.00 | 1.00 | 2.00 | A |
| T1 | 1.27 | 0.46 | 1.00 | 1.00 | 2.00 | A | |
| T2 | 1.20 | 0.41 | 1.00 | 1.00 | 2.00 | A | |
| T3 | 1.13 | 0.35 | 1.00 | 1.00 | 2.00 | A | |
| T4 | 1.13 | 0.35 | 1.00 | 1.00 | 2.00 | A | |
| T5 | 1.13 | 0.35 | 1.00 | 1.00 | 2.00 | A | |
| Trial | T0 | 1.40 | 0.51 | 1.00 | 1.00 | 2.00 | A |
| T1 | 1.33 | 0.49 | 1.00 | 1.00 | 2.00 | A | |
| T2 | 1.13 | 0.35 | 1.00 | 1.00 | 2.00 | A | |
| T3 | 1.00 | 0.00 | 1.00 | 1.00 | 1.00 | A | |
| T4 | 1.00 | 0.00 | 1.00 | 1.00 | 1.00 | A | |
| T5 | 1.00 | 0.00 | 1.00 | 1.00 | 1.00 | A |
| Mean | SD | Min | Mdn | Max | Significance * | ||
|---|---|---|---|---|---|---|---|
| Control | T0 | 1.07 | 0,80 | 0.00 | 1.00 | 3.00 | A,B |
| T1 | 0.93 | 0.70 | 0.00 | 1.00 | 2.00 | A,B | |
| T2 | 0.67 | 0.72 | 0.00 | 1.00 | 2.00 | A,B | |
| T3 | 0.40 | 0.63 | 0.00 | 0.00 | 2.00 | B | |
| T4 | 0.47 | 0.64 | 0.00 | 0.00 | 2.00 | B | |
| T5 | 0.47 | 0.64 | 0.00 | 0.00 | 2.00 | B | |
| Trial | T0 | 1.53 | 0.92 | 0.00 | 2.00 | 3.00 | A |
| T1 | 1.20 | 1.01 | 0.00 | 1.00 | 3.00 | A,B | |
| T2 | 1.00 | 0.85 | 0.00 | 1.00 | 3.00 | A,B | |
| T3 | 0.93 | 0.88 | 0.00 | 1.00 | 3.00 | A,B | |
| T4 | 0.93 | 0.88 | 0.00 | 1.00 | 3.00 | A,B | |
| T5 | 0.93 | 0.92 | 0.00 | 1.00 | 3.00 | A,B |
| Mean | SD | Min | Mdn | Max | Significance * | ||
|---|---|---|---|---|---|---|---|
| Control | T0 | 0.45 | 0.20 | 0.15 | 0.50 | 1.00 | A,B |
| T1 | 0.42 | 0.16 | 0.12 | 0.50 | 0.67 | A,B | |
| T2 | 0.34 | 0.18 | 0.10 | 0.35 | 0.67 | A,B | |
| T3 | 0.35 | 0.20 | 0.10 | 0.30 | 0.67 | A,B | |
| T4 | 0.31 | 0.18 | 0.10 | 0.30 | 0.67 | A,B | |
| T5 | 0.31 | 0.18 | 0.10 | 0.30 | 0.67 | A,B | |
| Trial | T0 | 0.46 | 0.20 | 0.22 | 0.50 | 1.00 | A |
| T1 | 0.36 | 0.20 | 0.11 | 0.32 | 0.93 | A,B | |
| T2 | 0.35 | 0.21 | 0.10 | 0.30 | 0.95 | A,B | |
| T3 | 0.34 | 0.21 | 0.10 | 0.30 | 0.90 | A,B | |
| T4 | 0.31 | 0.21 | 0.08 | 0.30 | 0.90 | B | |
| T5 | 0.33 | 0.21 | 0.08 | 0.30 | 0.90 | B |
| Mean | SD | Min | Mdn | Max | Significance * | ||
|---|---|---|---|---|---|---|---|
| Control | T0 | 0.65 | 0.19 | 0.30 | 0.62 | 1.00 | A |
| T1 | 0.59 | 0.20 | 0.35 | 0.50 | 1.00 | A,B | |
| T2 | 0.53 | 0.20 | 0.30 | 0.50 | 1.00 | A,B | |
| T3 | 0.41 | 0.12 | 0.17 | 0.45 | 0.56 | B | |
| T4 | 0.39 | 0.12 | 0.17 | 0.40 | 0.56 | B | |
| T5 | 0.39 | 0.13 | 0.17 | 0.45 | 0.56 | B | |
| Trial | T0 | 0.57 | 0.22 | 0.25 | 0.50 | 1.00 | A,B |
| T1 | 0.45 | 0.22 | 0.20 | 0.50 | 1.00 | A,B | |
| T2 | 0.52 | 0.22 | 0.20 | 0.50 | 1.00 | A,B | |
| T3 | 0.49 | 0.19 | 0.20 | 0.50 | 1.00 | A,B | |
| T4 | 0.47 | 0.19 | 0.20 | 0.50 | 1.00 | A,B | |
| T5 | 0.47 | 0.20 | 0.20 | 0.50 | 1.00 | A,B |
| Mean | SD | Min | Mdn | Max | Significance * | |
|---|---|---|---|---|---|---|
| Control | 0.16 | 0.40 | 0.00 | 0.00 | 1.00 | A |
| Trial | 0.33 | 0.51 | 0.00 | 0.00 | 1.00 | A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scribante, A.; Pardo, A.; Pascadopoli, M.; Biagi, F.; Schiepatti, A.; Giammona, V.; Vecchio, M.; Alquati, C.; Modica, G.G.; Casu, C.; et al. Remineralizing Treatments for Dental Erosion and Sensitivity in Patients Suffering from Gastroesophageal Reflux Disease (GERD): Randomized Clinical Trial. J. Clin. Med. 2025, 14, 3525. https://doi.org/10.3390/jcm14103525
Scribante A, Pardo A, Pascadopoli M, Biagi F, Schiepatti A, Giammona V, Vecchio M, Alquati C, Modica GG, Casu C, et al. Remineralizing Treatments for Dental Erosion and Sensitivity in Patients Suffering from Gastroesophageal Reflux Disease (GERD): Randomized Clinical Trial. Journal of Clinical Medicine. 2025; 14(10):3525. https://doi.org/10.3390/jcm14103525
Chicago/Turabian StyleScribante, Andrea, Alessia Pardo, Maurizio Pascadopoli, Federico Biagi, Annalisa Schiepatti, Valentina Giammona, Marco Vecchio, Christian Alquati, Gioia Giada Modica, Cinzia Casu, and et al. 2025. "Remineralizing Treatments for Dental Erosion and Sensitivity in Patients Suffering from Gastroesophageal Reflux Disease (GERD): Randomized Clinical Trial" Journal of Clinical Medicine 14, no. 10: 3525. https://doi.org/10.3390/jcm14103525
APA StyleScribante, A., Pardo, A., Pascadopoli, M., Biagi, F., Schiepatti, A., Giammona, V., Vecchio, M., Alquati, C., Modica, G. G., Casu, C., & Butera, A. (2025). Remineralizing Treatments for Dental Erosion and Sensitivity in Patients Suffering from Gastroesophageal Reflux Disease (GERD): Randomized Clinical Trial. Journal of Clinical Medicine, 14(10), 3525. https://doi.org/10.3390/jcm14103525








